Abstract
Gene expression within the context of eukaryotic chromatin is regulated by enzymes that catalyze histone lysine methylation. Histone lysine methyltransferases that have been identified to date possess the evolutionarily conserved SET or Dot1-like domains. We previously reported the identification of a new multi-subunit histone H3 lysine 4 methyltransferase lacking homology to the SET or Dot1 family of histone lysine methyltransferases. This enzymatic activity requires a complex that includes WRAD (WDR5, RbBP5, Ash2L, and DPY-30), a complex that is part of the MLL1 (mixed lineage leukemia protein-1) core complex but that also exists independently of MLL1 in the cell. Here, we report that the minimal complex required for WRAD enzymatic activity includes WDR5, RbBP5, and Ash2L and that DPY-30, although not required for enzymatic activity, increases the histone substrate specificity of the WRAD complex. We also show that WRAD requires zinc for catalytic activity, displays Michaelis-Menten kinetics, and is inhibited by S-adenosyl-homocysteine. In addition, we demonstrate that WRAD preferentially methylates lysine 4 of histone H3 within the context of the H3/H4 tetramer but does not methylate nucleosomal histone H3 on its own. In contrast, we find that MLL1 and WRAD are required for nucleosomal histone H3 methylation, and we provide evidence suggesting that each plays distinct structural and catalytic roles in the recognition and methylation of a nucleosome substrate. Our results indicate that WRAD is a new H3K4 methyltransferase with functions that include regulating the substrate and product specificities of the MLL1 core complex.
Keywords: Chromatin Histone Modification, Chromatin Regulation, Enzymes, Epigenetics, Histone Methylation, Ash2L, DPY-30, MLL, RbBP5, WDR5
Introduction
Eukaryotic gene expression programs are established and maintained in part by enzymes that methylate the epsilon amino group of histone lysine residues. Histone lysine methylation regulates gene expression by recruiting proteins that stabilize or remodel distinct chromatin states (1–3). Lysine residues can be mono-, di-, or trimethylated with distinct functional consequences, increasing the combinatorial signaling potential of lysine methylation (4, 5). Although it has become increasingly clear that regulation of the degree of lysine methylation plays a functionally significant role in eukaryotic gene regulation, the molecular mechanisms involved are only beginning to be understood.
Recent data suggest several models for the regulation of the degree of methylation by histone lysine methyltransferases. One model suggests that multiple methylation is achieved by distinct histone lysine methyltransferases that have evolved to catalyze the addition of one, two, or three methyl groups to a single lysine side chain. In this model, the addition of each methyl group is sequentially catalyzed by a distinct enzyme and is supported by the existence of several SET domain enzymes that differ in their abilities to use mono- or dimethyllysine as a substrate for further methylation (6), a phenomenon known as “product specificity” (7). In contrast, an alternative model suggests that multiple lysine methylation may be achieved by allosteric control of a single SET domain enzyme. In the allosteric model, the degree of lysine methylation is controlled by proteins that interact with and alter the conformation of the catalytic SET domain, altering its ability to catalyze the addition of each methyl group to a lysine side chain. The regulation of the trimethylation activity of the ESET/SETDB1 protein by interaction with the mAM protein (8) is an example of this type of regulation.
Another often cited example for allosteric control of multiple lysine methylation is the SET1 family of HMTases. SET1 family enzymes assemble into conserved multi-subunit complexes that are important for the maintenance of transcriptionally accessible forms of chromatin through the regulation of the degree of histone H3 lysine 4 (H3K4)2 methylation (9–21). Evidence supporting the allosteric model for multiple lysine methylation stems from sequence alignments predicting that SET1 family SET domains should only monomethylate their substrates (6, 7, 22). However, mono-, di-, and trimethylation activities have been attributed to SET1 family complexes in vivo and in vitro (4, 9–11, 23). These results have led to the suggestion that SET domain-interacting proteins alter the conformation of the SET1 family active site, allowing allosteric control of multiple lysine methylation (24, 25). However, recent biochemical data suggest that SET1 family complexes instead use a sequential mechanism for multiple lysine methylation.
The human MLL1 (mixed lineage leukemia protein 1) is a member of the SET1 family of H3K4 methyltransferases and has been shown to interact with an evolutionarily conserved group of non-SET domain proteins that include WDR5, RbBP5, Ash2L, and DPY-30 (26), components that have previously been shown to play important roles in cellular differentiation (27–29), development (30, 31), transcription (32), dosage compensation (33, 34), and oncogenesis (35, 36). These proteins, when in complex with MLL1, form the MLL1 core complex (10) and are required for the regulation of HOX genes during hematopoiesis and development (37–42). We recently reported that the intrinsic activity of an isolated MLL1 SET domain is indeed that of an H3K4 monomethyltransferase (43). Surprisingly, we discovered that WDR5, RbBP5, Ash2L, and DPY-30 form a subcomplex (called “WRAD”) that possesses an H3K4 methyltransferase activity that is independent of the MLL1 SET domain (26, 43). In addition, contrary to the predictions of the allosteric model for multiple lysine methylation, we demonstrated that WRAD catalyzes H3K4 dimethylation within the context of the MLL1 core complex, providing the first example of a sequential multiple lysine methylation system (43). Because the components of the WRAD complex lack homology to known S-adenosylmethionine-dependent methyltransferase folds, relatively little is understood about how this novel enzyme works. Here we further characterize the enzymatic activity and substrate specificity of WRAD in isolation and in the presence of MLL1.
EXPERIMENTAL PROCEDURES
Protein Expression and Purification
A construct of human MLL1 consisting of amino acid residues 3745–3969 (MLL3745) as well as full-length human proteins WDR5, RbBP5, Ash2L, and DPY30 were individually expressed in Escherichia coli and purified as described previously (43, 44). The WRAD or MWRAD complexes were created by mixing equimolar amounts of each recombinant component. Sedimentation velocity analytical ultracentrifugation was used to assess complex formation as described below. Histones were expressed, purified, refolded, and reconstituted into nucleosomes using the small scale reconstitution procedure as described by Dyer et al. (45) using the 255-base pair 54A54 double-stranded DNA fragment from the murine mammary tumor virus 3′-LTR promoter and flanking sequences as described by Flause et al. (46).
Analytical Ultracentrifugation
Analytical ultracentrifugation experiments were carried out using a Beckman Coulter ProteomeLabTM XL-A analytical ultracentrifuge equipped with absorbance optics and an eight-hole An-50 Ti analytical rotor. Sedimentation velocity experiments were carried out at 10 °C and 50,000 rpm (200,000 × g) using 3-mm two-sector charcoal-filled Epon centerpieces with quartz windows. For each sample, 300 scans were collected with the time interval between scans set to 0. Protein samples in 20 mm Tris-Cl, pH 7.5, 300 mm NaCl, 1 mm TCEP, and 1 μm ZnCl2 were run at various concentrations as described under “Results.” Sedimentation boundaries were analyzed by the continuous distribution (c(s)) method using the program SEDFIT (47). The program SEDNTERP version 1.09 (48) was used to correct the experimental S value (s) to standard conditions at 20 °C in water (S20,w) and to calculate the partial specific volume of each protein.
[3H]Methyltransferase Assays
Histone methyltransferase activity assays were carried out by combining 5 μg of chicken core histones (Millipore) and 1 μCi of [3H]methyl S-adenosylmethionine ([3H]AdoMet; MP Biomedicals) in the presence of the WRA(D) enzyme at a final concentration of 4.3 μm. The reaction buffer contained 50 mm Tris, pH 8.5, 200 mm NaCl, 3 mm DTT, 5 mm MgCl2, and 5% glycerol. The reactions were incubated at 15 °C for 8 h and quenched by dilution with 5× SDS loading buffer. The reactions were separated by 18% Tris-glycine PAGE or 4–12% Bis-Tris PAGE (Invitrogen). The gels were soaked in autoradiography enhancer solution (ENLIGHTNING; PerkinElmer Life Science) for 30 min, dried, and exposed to film at −80 °C overnight or for up to 4 days. For assays comparing the enzymatic activity of WRAD with full-length unmodified or monomethylated recombinant Xenopus laevis histone H3 (Active Motif®), 4 μg of histone H3 was incubated with 1 μCi of [3H]AdoMet in the presence of the WRA(D) enzyme at a final concentration of 4.3 μm. The reactions were processed as described above.
Enzyme Kinetics
Enzymatic reactions (20 μl) were initiated by the addition of enzyme (1–17 μm) to reaction mixtures containing a fixed concentration of histone H3 peptide (residues 1–20) and variable concentrations of [3H]AdoMet (PerkinElmer Life Sciences; specific activity, 12 μCi/mm) or fixed [3H]AdoMet concentrations and variable concentrations of histone H3 peptide in assay buffer (50 mm Tris, pH 8.5, 200 mm NaCl, 3 mm DTT, 5 mm MgCl2, and 5% glycerol). The reactions were incubated at 15 °C for 4 h and then stopped by the addition of 5 μl of 5× SDS loading buffer. Control time course experiments showed that reactions were still linear after 4 h with various substrate and coenzyme combinations. Quenched reactions were separated by 4–12% Bis-Tris PAGE and stained with Coomassie Brilliant Blue R. Peptide bands were excised from the gel and dissolved in 750 μl of SolvableTM (PerkinElmer Life Sciences) at 50 °C for 3 h. The extracted peptide solution was mixed with 5 ml of scintillation mixture (ULTIMA GOLD XR; PerkinElmer Life Sciences) and counted using a Beckman Coulter LS6500 scintillation counter. Initial rates were plotted as a function of the variable substrate and fitted to the Michaelis-Menten equation (Equation 1) using the nonlinear least squares regression analysis in SigmaPlot. To determine the apparent inhibition constant (Ki) for S-adenosyl-homocysteine (AdoHyc), enzymatic assays were conducted by incubating fixed concentrations of [3H]AdoMet (25 μm), histone H3 peptide (500 μm), and WRAD (4.3 μm) with variable concentrations of AdoHyc (0–250 μm). The reactions were allowed to proceed for 4 h, quenched with SDS loading buffer, and separated by PAGE. Peptide bands were excised, dissolved with Solvable, and counted by LSC as described above. The inhibition curve was fitted to Equation 2 using SigmaPlot software.
Western Blotting
Enzymatic assays with reconstituted nucleosomes were carried out as described above. The reactions were quenched by the addition of SDS loading buffer after 8 h and resolved by 18% Tris-glycine PAGE. The proteins were blotted onto nitrocellulose membranes and probed with antibodies specific for H3K4 monomethyllysine (1:500 dilution; Abcam catalogue number ab8895), dimethyllysine (1:1000 dilution, Abcam catalogue number ab32356), or trimethyllysine (1:500 dilution, Active Motif catalogue number 39160).
RESULTS
WRAD Is a Histone H3 Lysine 4-specific Methyltransferase
We previously demonstrated that the isolated human WRAD complex monomethylates but does not dimethylate histone H3 peptides (residues 1–20) at lysine 4 (43). In contrast, we demonstrated that WRAD acquires the ability to monomethylate histone peptides that are unmethylated or monomethylated at lysine 4 when assembled with a catalytically inactive variant of the MLL1 SET domain (43). These results suggest that WRAD is a one-carbon methyl transfer enzyme that is capable of transferring one methyl group to histone H3 on its own or to a previously monomethylated H3K4 substrate but only when in complex with MLL1.
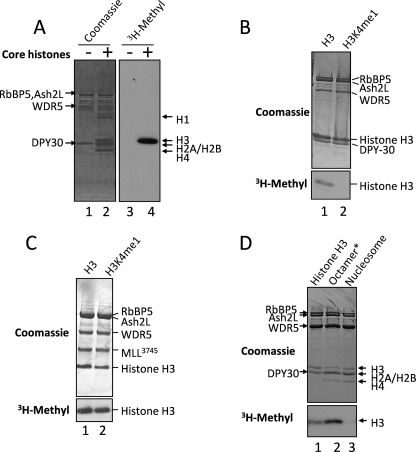
To better understand the substrate specificity of the isolated WRAD complex under our standard in vitro assay conditions, we characterized the methylation activity of recombinant human WRAD with different histone substrates. When purified, chicken core histones and [3H]AdoMet are incubated in the presence of WRAD, [3H]methyl is incorporated into histone H3 but not histones H2A, H2B, H4, or H1 (Fig. 1A). To identify lysine residues methylated by WRAD, we compared activity among recombinant full-length X. laevis histone H3 proteins that were unmethylated or chemically modified to introduce a monomethyllysine analogue at lysine 4 (Active Motif®). The results show that unmethylated histone H3 is a substrate for methylation by WRAD (Fig. 1B, lane 1) but not histone H3 previously monomethylated at lysine 4 (Fig. 1B, lane 2). To determine whether the chemically modified form of histone H3 can be dimethylated, we added to WRAD the wild-type MLL1 SET domain construct (residues 3745–3969 (MLL3745)) to form the fully assembled MLL1 core complex, which we previously demonstrated to be required for dimethylation of H3K4 (43). In these assays, methylation of monomethylated histone H3 is readily observed (Fig. 1C, lane 2), indicating that the monomethyllysine analogue is an adequate mimic of the H3K4 monomethylated state.
FIGURE 1.
WRAD is a histone H3 lysine 4-specific monomethyltransferase. A, enzymatic assays showing the methyltransferase activity of WRAD after a period of 8 h using [3H]methyl-S-adenosylmethionine and purified chicken core histones as substrates. Enzymatic reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (left panel, lanes 1 and 2) or fluorography (right panel, lanes 3 and 4). B, enzymatic activity of WRAD using 4 μg of full-length histone H3 proteins (Active Motif®) that were either unmodified (H3, lane 1) or previously monomethylated at lysine 4 (H3K4me1, lane 2). The reactions were separated by 18% Tris-glycine PAGE and visualized with Coomassie Brilliant Blue (upper panel) and fluorography (3H-Methyl, lower panel). C, enzymatic activity of MLL1 core complex after a period of 8 h using 4 μg of full-length histone H3 proteins that were either unmodified (H3, lane 1) or previously monomethylated at lysine 4 (H3K4me1, lane 2). The reaction products were separated by 4–12% Bis-Tris PAGE and visualized as described in B. D, comparison of the enzymatic activity of WRAD using as substrates recombinant histone H3 (1.5 μg, lane 1), dialyzed histone octamer (6 μg, lane 2), or an equivalent amount of reconstituted nucleosomes (lane 3). The reactions were separated by 18% Tris-glycine SDS-PAGE and visualized as described in B above. Octamer* denotes that the histone octamer dissociates into one H3/H4 tetramer and two H2A/H2B dimers upon dialysis into assay buffer (see text).
These results indicate that histone H3 is the preferred substrate for isolated human WRAD, which, in the absence of MLL1, preferentially monomethylates lysine 4. These results are in agreement with our previous demonstration that the isolated WRAD complex monomethylates H3 peptides in an H3K4-dependent manner (43).
WRAD Preferentially Methylates the Histone H3/H4 Tetramer but Does Not Methylate Nucleosomal Histone H3
We next compared the enzymatic activity of WRAD using as substrates recombinant X. laevis histone H3 in the context of free histone H3, the dialyzed histone octamer (which under the assayed conditions dissociates into an H3/H4 tetramer and two H2B/H2A dimers (49)), and a reconstituted nucleosome. The results show that free recombinant H3 and the H3/H4 tetramer are methylated by WRAD (Fig. 1D, lanes 1 and 2), with the H3/H4 tetramer showing greater methylation. In contrast, reconstituted nucleosomes are not a substrate for WRAD (Fig. 1D, lane 3). It should be noted that the lack of activity with nucleosomes does not appear to be due to inhibition by free DNA, because the addition of a stoichiometric equivalent of free DNA to assays with the histone octamer does not significantly affect methylation by WRAD (data not shown). These results indicate that histone H3 within the context of the histone H3/H4 tetramer is the preferred substrate for WRAD when recombinant histones are used as a substrate.
WRA Is the Minimal Complex Required for Enzymatic Activity
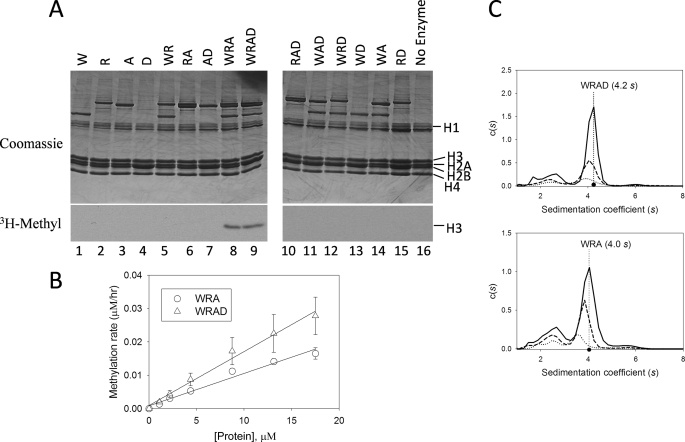
To identify the minimal complex required for methylation by WRAD, we compared activity among the individual components and all possible binary and ternary complexes using purified chicken core histones as a substrate. As shown in Fig. 2A, no activity is observed when the assays are conducted with the individual components of the WRAD complex (lanes 1–4) or with any of the binary complexes (lanes 5–7 and 13–15), similar to that observed in the absence of enzyme (lane 16). Enzymatic activity is observed only with WRA and WRAD complexes (lanes 8 and 9, respectively) but not with any of the other ternary complexes (lanes 10–12).
FIGURE 2.
WDR5, RbBP5, and Ash2L are required for the H3K4 methylation activity of WRAD. A, comparison of enzymatic activity of individual WRAD components and all possible binary, ternary, and quaternary complexes. W, WDR5; R, RbBP5; A, Ash2L; D, DPY-30. Histone methyltransferase assays were conducted for a period of 8 h using [3H]AdoMet and chicken core histones as the substrate. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panels) and fluorography (lower panels). A no-enzyme control is shown in lane 16. B, WRAD and WRA histone methyltransferase activity as a function of enzyme concentration. Methyltransferase activity assays were conducted with 25 μm [3H]AdoMet and 500 μm histone H3 peptide (residues 1–20) with varying concentrations of WRAD (open triangles) or WRA (open circles). Each point corresponds to the average of duplicate measurements with the error bars indicating the standard error of measurement. Linear regression fitting of the data gave slopes of 0.0016 and 0.001, and R2 values of 0.99 and 0.97 for WRAD and WRA complexes, respectively. C, diffusion-free sedimentation coefficient distributions (c(s)) derived from sedimentation velocity analytical ultracentrifugation of WRAD (upper panel) and WRA (lower panel) complexes at concentrations of 2.2 μm (solid line), 1.1 μm (dashed line), and 0.55 μm (dotted line).
WRAD activity depends on the dose of the enzyme, as indicated by the nearly linear dependence of activity on enzyme concentration (Fig. 2B). The addition of DPY-30 to the WRA subcomplex results in a 38% increase in the slope of the dose response curve (Fig. 2B), suggesting that DPY-30 may function to enhance the activity of WRAD. The enhanced activity in the presence of DPY-30 may be due in part to stabilization of the complex, because sedimentation velocity analytical ultracentrifugation experiments show that the WRAD complex is slightly more stable upon dilution than the WRA complex (Fig. 2C). For example, the WRAD complex represents 71% (4.1 s), 69% (4.0 s), and 63% (3.9 s) of all species in the sample run at concentrations of 2.2, 1.1, and 0.6 μm, respectively (Fig. 2C, upper panel). In contrast, in the absence of DPY-30, the WRA subcomplex represents 67% (4.0 s), 65% (3.8 s), and 53% (3.5 s) of all species at the same concentrations, respectively (Fig. 2C, lower panel). DPY-30 promotes complex formation at each protein concentration. Taken together, these results indicate that WDR5, RbBP5, and Ash2L form the minimal complex required for methylation of histone H3 and that DPY-30 functions to enhance complex stability and enzymatic activity.
DPY-30 Increases the Histone Substrate Specificity of the WRAD Complex
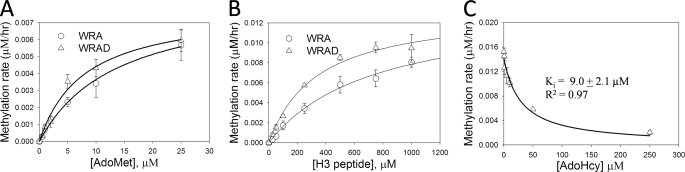
To determine apparent steady-state kinetic parameters for WRA and WRAD complexes, we performed enzymatic reactions with variable amounts of AdoMet at a constant concentration of histone H3 peptide (residues 1–20). Michaelis-Menten kinetics were observed for both WRA and WRAD complexes (Fig. 3). Nonlinear least square regression fitting of the data to the Michaelis-Menten equation shows that AdoMet binds to WRAD and WRA in enzymatic reactions with relatively similar apparent Km values of 8 and 14 μm, respectively (Fig. 3A and Table 1). In contrast, when the histone H3 peptide is the variable substrate, the apparent Km value for histone H3 decreases ∼7-fold from 2.4 to 0.34 mm when DPY-30 is added to the complex (Fig. 3B and Table 1). These data suggest that DPY-30 functions to increase the specificity of WRAD for histone H3, as reflected by the greater increase in the apparent specificity constant (kcat/Km) for histone H3 (3-fold) compared with that of AdoMet (1.6-fold) when DPY-30 is added to the complex (Table 1).
FIGURE 3.
Steady-state kinetics and inhibition analysis of WRA(D). A, comparison of WRA (open circle) and WRAD (open triangle) kinetics with AdoMet as the variable substrate (ranging from 0.5 to 25 μm) and fixed concentrations (1 mm) of histone H3 peptide (residues 1–20). Rates of methylation are the means of duplicate measurements ± standard error of measurement. Apparent kinetic parameters were determined by fitting the data to the Michaelis-Menten equation (Equation 1, “Experimental Procedures”). B, comparison of WRA (open circles) and WRAD (open triangles) kinetics with histone H3 peptide as the variable substrate (ranging from 25 to 5000 μm). The data are represented and fitted as described for A. For clarity, the values for the concentration range from 0 to 1000 μm are shown. C, comparison of the enzymatic activity of WRAD (4.3 μm) with increasing concentrations of AdoHyc (1–250 μm). Activity assays were conducted with fixed concentrations of AdoMet (25 μm) and histone H3 peptide (500 μm). Each point represents the means ± S.E. of measurement from duplicate measurements. The data were fit to Equation 2 (“Experimental Procedures”).
TABLE 1.
Summary of apparent kinetic parameters for WRA and WRAD complexes
| Enzyme | KmAdoMet | KmH3 peptide | KcatAdoMet | KcatH3 peptide | Kcat/KmAdoMet | Kcat/KmH3 peptide |
|---|---|---|---|---|---|---|
| μm | μm | ×10−4h−1 | ×10−4h−1 | ×10−4 μm−1h−1 | ×10−4 μm−1h−1 | |
| WRA | 13.7 ± 3.7 | 2382 ± 269 | 20 | 70 | 1.5 | 0.03 |
| WRAD | 7.9 ± 1.7 | 338 ± 54 | 18 | 30 | 2.3 | 0.09 |
WRAD Is Inhibited by S-Adenosyl-homocysteine
Because the co-factor product AdoHyc is often a potent competitive inhibitor of AdoMet dependent enzymes (50), we compared the enzymatic activity of WRAD with increasing concentrations of AdoHyc. As shown in Fig. 3C, WRAD activity is inhibited in a saturable manner with increasing concentrations of AdoHyc. Fitting the data to equation 2 (materials and methods) reveals an apparent Ki value for AdoHyc of ∼9.0 ± 2.0 μm, which is comparable with that of other AdoMet-dependent methyltransferases (50).
WRAD Requires Zinc for Catalytic Activity
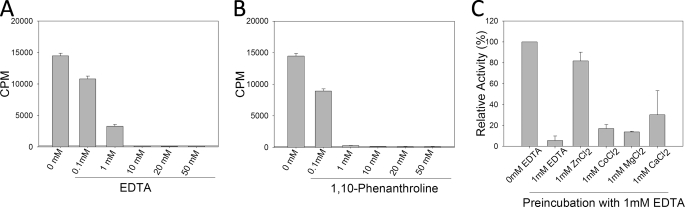
To determine whether the activity of WRAD is metal ion-dependent, we compared methylation of histone H3 peptides by WRAD in the presence of increasing concentrations of chelating agents EDTA or 1,10-phenanthroline. As shown in Fig. 4 (A and B), the enzymatic activity of WRAD is highly sensitive to both chelating agents. In the presence of 1 mm EDTA, the enzymatic activity of WRAD is inhibited by 80% (Fig. 4A). In contrast, the enzymatic activity of WRAD is inhibited >95% in the presence of 1 mm 1,10-phenanthroline (Fig. 4B). To determine which metal ion is required for activity, we preincubated WRAD with 1 mm EDTA and then compared enzymatic activity after the addition of excess amounts of divalent cations of zinc, cobalt, magnesium, or calcium. As shown in Fig. 4C, when 1 mm ZnCl2 is added to EDTA-treated WRAD, most of the catalytic activity is regained. In contrast, the addition of a stoichiometric equivalent of CoCl2, MgCl2, or CaCl2 resulted in relatively small increases in enzymatic activity. These results suggest that WRAD requires zinc for catalytic activity.
FIGURE 4.
Zinc is required for the H3K4 methyltransferase activity of WRAD. A, WRAD enzymatic activity (mean ± S.E.) with increasing concentrations of EDTA. B, WRAD enzymatic activity (mean ± S.E.) with increasing concentrations of 1,10-phenanthroline. C, relative WRAD activity after preincubation with 1 mm EDTA and the addition of different divalent cations: zinc, cobalt, magnesium, or calcium. The data were normalized relative to the untreated control (0 mm EDTA) and represent the means ± the standard error of measurement from two independent experiments.
WRAD Is Required for Methylation of Nucleosomal Histone H3 by the MLL1 Core Complex
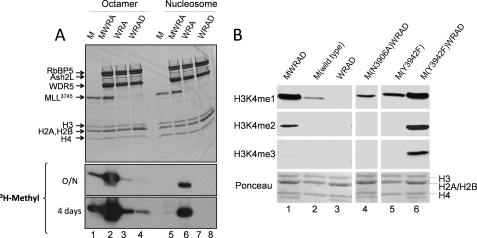
To further characterize the role of WRA(D) in the substrate specificity of the MLL1 core complex, we compared the enzymatic activity of WRA(D) in the presence and absence of an MLL1 SET domain construct MLL3745 (amino acid residues 3745–3969) using recombinant histone octamers or reconstituted nucleosomes as substrates (Fig. 5). We previously demonstrated that MLL3745 is the minimal MLL1 construct required for the assembly and enzymatic activity of the MLL1 core complex in vitro (43, 44). In this investigation, when the histone octamer is used as a substrate, a significantly greater amount of enzymatic activity was observed by the minimal MLL1 core complex (MWRA) (Fig. 5A, lane 2) compared with that of MLL1 alone (Fig. 5A, lane 1) or that of the WRA or WRAD complexes alone (Fig. 5A, lanes 3 and 4, respectively). These results suggest that the histone H3/H4 tetramer is the preferred substrate for the MLL1 core complex. In contrast, when reconstituted nucleosomes are used as a substrate, little activity could be observed when the assays were conducted with the isolated MLL1 SET domain construct (Fig. 5A, lane 5) or the isolated WRA (lane 7) or WRAD (lane 8) complexes. Significant methylation of a nucleosomal substrate was only observed when the assays were conducted with a fully assembled complex containing MLL3745 and WRA (MWRA; Fig. 5A, lane 6) or MLL3745 and WRAD (MWRAD; Fig. 6A, lane 5). These results indicate that a fully assembled MLL1 core complex is required for methylation of nucleosomal histone H3.
FIGURE 5.
MLL1 and WRAD are each required for methylation of nucleosomal histone H3 by the MLL1 core complex. A, comparison of the enzymatic activity of the MLL1 SET domain (M), WRA(D), or the fully assembled MLL1 core complex (MWRA) using the dialyzed histone octamer or reconstituted nucleosomes as substrates. Histone methyltransferase assays were conducted for a period of 8 h. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panels) and fluorography (lower panels, overnight (O/N) and 4-day exposures). B, mono-, di-, and trimethylation of reconstituted nucleosomes were compared using wild-type MLL3745 (M(wild-type)), WRAD, or the fully assembled MLL1 core complex (MWRAD). In addition, nucleosome methylation activities were compared among MLL1 core complexes assembled with a loss-of-function variant of MLL1 (M(N3906A)) or a gain-of-function variant of MLL1 (M(Y3942F)). Western blotting with antibodies specific for the H3K4 mono-, di-, or trimethylated forms of histone H3 was used to detect nucleosome methylation, as described under “Experimental Procedures.”
FIGURE 6.
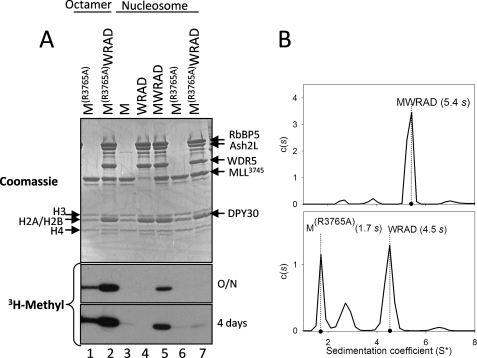
Arginine 3765 of MLL1 is required for the interaction between MLL1 and WRAD and for nucleosomal histone H3 methylation. A, comparison of the enzymatic activity of wild-type (M) and R3765A (MR3765A) MLL1 SET domains in the presence and absence of WRAD using dialyzed histone octamers or reconstituted nucleosomes as substrates. Quenched reactions were separated by 18% Tris-glycine SDS-PAGE and visualized with Coomassie Brilliant Blue (upper panel) and fluorography (3H-Methyl (lower panels), overnight (O/N) and 4-day exposures)). Lanes 1 and 2 are assays conducted with dialyzed histone octamers; lanes 3–7 are assays conducted with a reconstituted nucleosome substrate. B, diffusion-free sedimentation coefficient distribution (c(s)) derived from sedimentation velocity analytical ultracentrifugation of the MLL1 core complex assembled with wild type (upper panel) or the R3765A variant of MLL1 (lower panel). The experimental sedimentation coefficients (s) are indicated.
To determine the product specificity of nucleosome methylation by the MLL1 core complex, we compared relative amounts of mono-, di-, and trimethylation of nucleosomes catalyzed by MLL3745, WRAD, and the fully assembled MLL1 core complex by Western blotting using methylation state-specific antibodies (Fig. 5B). The results show that the fully assembled MLL1 core complex (MWRAD) catalyzes significant mono- and dimethylation of histone H3 when the assays are conducted with reconstituted nucleosomes (Fig. 5B, lane 1). In contrast, dimethylation of H3 is absent and H3K4 monomethylation is significantly reduced when the assays are conducted with isolated MLL3745 (lane 2) or the isolated WRAD complex (lane 3). These results demonstrate that the fully assembled MLL1 core complex is required to catalyze mono- and dimethylation of histone H3 within the context of a nucleosomal substrate.
MLL1 and WRAD Each Play Distinct Catalytic and Structural Roles in the Methylation of the Nucleosome by the MLL1 Core Complex
To begin to understand why MLL1 and WRAD are required for methylation of nucleosomal histone H3, we compared nucleosome methylation among MLL1 SET domain variants that are either catalytically inactive or mutated to catalyze mono-, di-, and trimethylation of histone H3. We previously showed that the N3906A substitution in the MLL1 SET domain abolishes enzymatic activity using histone peptides as a substrate (43). In this investigation, although little nucleosome histone H3 methylation could be observed when the assays were conducted with the isolated WRAD enzyme (Fig. 5B, lane 3), an increase in the monomethylation of nucleosomal histone H3 was observed when WRAD was assembled with the inactive N3906A variant of MLL1 (Fig. 5B, lane 4). These results indicate that MLL1 facilitates nucleosome monomethylation by WRAD, even when MLL1 is catalytically inactive.
To further test this hypothesis, we compared nucleosome methylation by the gain-of-function Y3942F variant of MLL1 in the presence and absence of WRAD. We previously showed that replacement of Tyr-3942 with phenylalanine converts the MLL1 SET domain, in the absence of WRAD, into a processive mono-, di-, and trimethyltransferase when using histone peptides as a substrate (43). However, in this investigation, when nucleosomes are used as the substrate with the isolated Y3942F MLL1 SET domain enzyme, monomethylation is reduced, and di- and trimethylation of histone H3 are not observed (Fig. 5B, lane 5). In contrast, when WRAD is added to the Y3942F MLL1 SET domain, mono-, di-, and trimethylation of histone H3 are significantly increased (Fig. 5B, lane 6). Even though the Y3942F MLL1 SET domain has the ability to mono-, di-, and trimethylate histone H3 peptides on its own, it cannot fully methylate nucleosomal histone H3 without WRAD. Taken together, these results suggest that MLL1 and WRAD, in addition to being required for the mono- and dimethylation activity of the wild-type MLL1 core complex, each play a structural role that allows the complex to use nucleosomes as a substrate.
Arginine 3765 of MLL1 Is Required for the Interaction between MLL1 and WRAD and for Nucleosome Methylation
The results presented above suggest that factors controlling the assembly of the MLL1 core complex may be important for the regulation of nucleosomal histone H3 methylation. We previously demonstrated that the interaction between MLL1 and the WRA complex is dependent on WDR5 recognition of arginine 3765 in MLL1 (44, 51). To test the hypothesis that a fully assembled MLL1 core complex is required for methylation of a nucleosomal substrate, we compared the enzymatic activity of the wild-type and R3765A MLL1 SET domains in the presence and absence of WRAD using recombinant histone octamers or nucleosomes as substrates. As shown in Fig. 6A, like that of wild-type MLL1, the R3765A variant of MLL1 is capable of methylating the histone octamer (Fig. 6A, lane 1) but not a nucleosomal substrate (Fig. 6A, lane 6). In contrast, although WRAD in the presence of wild-type MLL1 activates nucleosome methylation (Fig. 6A, lane 5), WRAD activation of nucleosomal methylation is mostly abolished when Arg-3765 of MLL1 is replaced with alanine (Fig. 6A, lane 7). Sedimentation velocity analytical ultracentrifugation experiments confirm that replacement of Arg-3765 with alanine disrupts the formation of the MLL1 core complex (5.4 s, Fig. 6B, upper panel), resulting in two largely noninteracting species corresponding to free MLL1 at 1.7 s and mostly free WRAD at 4.5 s (Fig. 6B, lower panel). These results demonstrate that nucleosomal histone H3 methylation by the MLL1 core complex is dependent on the interaction between MLL1 and WRAD.
DISCUSSION
Most histone lysine methyltransferases characterized to date possess the evolutionarily conserved SET domain. The SET domain is a ∼130-amino acid motif that possesses a unique β-fold knot-like structure that functions to align the methyl donor S-adenosylmethionine and the peptide methyl acceptor lysine (52). The only other non-SET domain HKMTase identified to date is the Dot1 protein, which methylates lysine 79 in the globular domain of histone H3 within the context of the nucleosome (53–55). The three-dimensional structure of Dot1 shows that it possesses the more classical α/β methyltransferase fold using AdoMet as the methyl donor (56). In this investigation, we describe the characterization of a new histone lysine methyltransferase activity in a multi-subunit complex lacking homology to that of the SET or Dot1 family of histone methyltransferases. This enzyme is composed minimally of three proteins including WDR5 and RbBP5, both possessing WD-40 repeat domains; and Ash2L, which contains a conserved PHD finger and a SPRY domain. To our knowledge, these domains have not previously been shown to be directly involved in S-adenosylmethionine-dependent methyltransferase reactions.
The evidence we present strongly supports the existence of this previously unrecognized enzymatic activity. Using a true biochemical reconstitution system, where each human component is individually expressed and purified to homogeneity from E. coli, we demonstrate that the activity of WRA(D) is linearly dependent on the dose of the enzyme and that it displays Michealis-Menten kinetics with respect to both histone peptides and co-factor AdoMet. We also show that as with other AdoMet-dependent enzymes, the co-factor product S-adenosyl-homocysteine is a potent inhibitor of WRAD methyltransferase activity. Furthermore, we provide evidence suggesting that zinc is required for WRAD activity. It is not clear at present whether zinc plays a direct role in catalysis or whether it is required for subunit association.
We also show that WDR5, RbBP5, and Ash2L are the minimal components required for activity. Interestingly, DPY-30 appears to function by modulating the histone substrate specificity of WRAD. Because the individual components lack catalytic activity on their own, contamination with a bacterial methyltransferase can be ruled out. Whether the active site is entirely contained within one subunit or whether it is shared among subunits is currently unknown.
The complex requirement for the enzymatic activity of WRAD may explain why this activity has not been observed previously. For example, in Saccharomyces cerevisiae, the SET1 protein assembles into a complex homologous to that of the MLL1 core complex, called COMPASS (15–17, 57), and regulates lysine 4 methylation, gene silencing, and gene expression (58–60). Deletion of the SET1 gene in budding yeast greatly reduces lysine 4 methylation (59, 61), which has led to the suggestion that the SET1 protein is the only lysine 4 methyltransferase in yeast, despite possessing homologues of the proteins that make up WRAD: Ash2L (Bre2/CPS60), RbBP5 (Swd1/CPS50), WDR5 (Swd3/CPS30), and DPY-30 (Sdc1/CPS25). However, unlike WRAD in mammalian cells, which exists as an independent complex in the absence of MLL1 (9, 10), the yeast homologue of WRAD apparently does not exist in a complex in the absence of the SET1 protein, because deletion of SET1 prevents co-immunoprecipitation of the Bre2-Sdc1 subcomplex with the Swd1-Swd3 subcomplex (16, 62). It may be that in budding yeast, the SET1 protein is required to stabilize formation of the Bre2-Sdc1-Swd1-Swd3 (WRAD) active site, which is required for enzymatic activity.
We previously demonstrated that WRAD functions within the context of the MLL1 core complex to sequentially methylate H3 substrates previously monomethylated at H3K4 (43). The enzymatic activity of WRAD may also play a functionally significant role independent of MLL1 or other SET1 family enzymes in the cell. Evidence supporting this hypothesis comes from size exclusion chromatography of mammalian nuclear extracts, demonstrating that WDR5, RbBP5, and Ash2L, in addition to co-eluting with MLL1, also co-elutes as a distinct ∼150-kDa complex lacking MLL1 (9). The apparent molecular mass of this complex determined by gel filtration is comparable with the theoretical molecular mass for the WDR5, RbBP5, and Ash2L complex (156 kDa) with 1:1:1 stoichiometry and is consistent with our sedimentation velocity analytical ultracentrifugation characterization of WRA(D). WRA(D) has also been identified in other complexes that appear to lack SET1 family enzymes, such as the CHD8 and NIF1 complexes (63–65). Whether WRAD brings a histone methyltransferase activity to these complexes remains to be determined. Intriguingly, enzymatic assays with an immunoprecipitated NIF1 complex shows histone H3 methylation activity, albeit in a manner that is largely independent of H3K4 (65). It is therefore likely that the majority of enzymatic activity of the NIF1 complex is due to co-immunopurification of other histone lysine or arginine methyltransferases. However, it is also possible that the substrate specificity of WRAD is altered within the context of the NIF1 complex. Further experimentation will be required to distinguish these hypotheses.
A surprising finding from this investigation is the demonstration that WRAD lacks catalytic activity with a reconstituted nucleosome substrate, suggesting that isolated WRAD may play a role prior to or during nucleosome assembly. A similar lack of activity on nucleosomes is observed when the assays are conducted with a purified MLL1 SET domain construct containing residues 3745–3969, likewise suggesting that MLL1 in the absence of other components does not significantly methylate nucleosomal histone H3. Nucleosomal histone H3 methylation is observed only when the assays are conducted with a fully assembled MLL1 core complex containing a stoichiometric equivalent of MLL1 and WRAD that catalyzes H3K4 mono- and dimethylation under our assay conditions. Why the fully assembled MLL1 core complex is required for nucleosome methylation is currently unknown. One possibility is that the histone H3 N-terminal tail may interact with nucleosomal DNA in a way that requires the fully assembled MLL1 core complex to make it available as a substrate for methylation. This mechanism is consistent with the demonstration that histone acetylation facilitates the association of the MLL1 SET domain with nucleosomes in vitro (66). However, a purely charged based mechanism predicts that a sufficient amount of free DNA should also inhibit histone peptide or full-length H3 methylation. The addition of double-stranded DNA to methylation reactions with histone peptides or full-length histone H3 does not significantly inhibit methylation by the isolated MLL3745 or WRAD enzymes (not shown), suggesting that there is something unique about the structure of the nucleosome that is recognized only by the fully assembled MLL1 core complex.
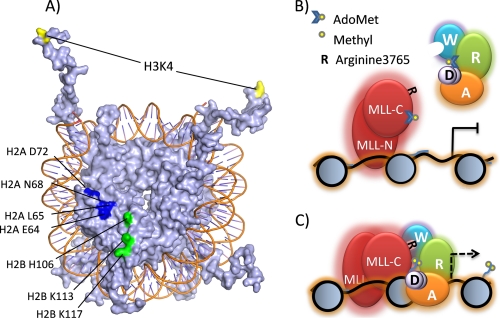
Several lines of evidence suggest that the globular domains of histones within the context of the nucleosome are important for H3K4 methylation by the MLL1 core complex. The globular domains of histones H2A, H2B, H3, and H4 are partially exposed on the face of the nucleosome (67), providing a potential interaction surface that may function to anchor the MLL1 core complex and correctly position the active site for methylation of the histone H3 tail. Consistent with this hypothesis, mutagenesis experiments in yeast revealed a cluster of histone H2A and H2B amino acids required for H3K4 methylation (Fig. 7A) (68, 69). This cluster includes histone H2B residue Lys-123 (Lys-117 in the X. laevis nucleosome structure (70)) that is targeted for ubiquitination (71, 72). It is has been suggested that ubiquitination of histone H2B is required for H3K4 methylation in a potential histone “cross-talk” mechanism (73). However, recent data suggest that the H2B C-terminal helix is recognized by COMPASS (68) and that ubiquitination of H2B K123 stabilizes H2A/H2B dimer association with the nucleosome, perhaps making it a better substrate for lysine 4 methylation by COMPASS (74). Whatever the mechanism, these data are consistent with a model in which the MLL1 core complex recognizes more than just the N-terminal tails of histones and that the structured globular core domain of the nucleosome plays an important role in the regulation of nucleosomal H3K4 methylation.
FIGURE 7.
Proposed model for the regulation of nucleosome methylation by the MLL1 core complex. A, surface model of the X. laevis nucleosome core particle (drawn with Protein Data Bank coordinate 1KX5 (70)). Highlighted are histone H2A and H2B residues required for H3K4 methylation in budding yeast (69). Histone H3K4 is highlighted in yellow, H2A residues are shown in dark blue, and H2B residues are green. B, MLL1 interacts with DNA through AT hooks and CXXC domains located in the MLL-N subunit of the MLL1 core complex. In the absence of WRAD, the histone H3 N-terminal tail interacts with linker DNA and is not a substrate for methylation. Consequently, transcription of MLL1 target genes is repressed. W, WDR5; R, RbBP5; A, Ash2L; and D, DPY-30. MLL-N is the 300-kDa N-terminal portion of MLL1, and MLL-C is the 180-kDa C-terminal portion of MLL1 containing the SET domain. C, the WDR5 component of WRAD interacts with Arg-3765 of MLL1 resulting in the assembly of the MLL1 core complex. The MLL1 complex interacts extensively with the nucleosome core and liberates the histone H3 N-terminal tail, which becomes a substrate for mono- and dimethylation at lysine 4, a critical step in the pathway that is required for transcription of MLL1-dependent genes (indicated by the dashed arrow).
Our demonstration that the fully assembled MLL1 core complex is required for nucleosome methylation suggests a novel mechanism for controlling nucleosomal H3K4 methylation. We suggest that nucleosomal H3K4 methylation may be controlled by factors that regulate the interaction between MLL1 and WRAD. In support of this hypothesis, we demonstrate that nucleosomal methylation by the MLL1 core complex requires Arg-3765 of MLL1, which we have previously shown to be required for the assembly and the H3K4 dimethylation activity of the MLL1 core complex (44, 51). In addition, genome-wide chromatin immunoprecipitation studies show that although Menin, MLL1, and RbBP5 localize to the promoters of thousands of genes in different mammalian cell lines, they are not always found together (75). This result suggests that mechanisms exist in vivo to regulate the assembly of the MLL1 core complex in different genomic contexts and presumably methylation of nucleosomal histone H3.
Based on these observations, we propose the following model (Fig. 7): MLL1 localizes to the promoters of target genes through the DNA-binding domains of MLL1 located in the N-terminal half-of MLL1 (MLL-N, which includes DNA binding AT hooks and CXXC domain (76)) and by interaction with other transcription factors and co-activators (i.e. Menin (20, 39) or CBP (77)) but is inactive in the methylation of promoter-associated nucleosomes in the absence of WRAD (Fig. 7B). Lack of promoter nucleosome methylation by MLL1 prevents the recruitment of other factors required for transcription (i.e. TFIID complex (78) or ATP-dependent nucleosome remodeling complexes (1, 3, 79, 80)). WRAD binding to the nucleosome allows formation of the holo-MLL1 core complex, leading to liberation of the H3 N-terminal tail and mono-, di-, and presumably trimethylation of H3K4 and subsequently gene activation (Fig. 7C). The interaction of WRAD with the MLL1 core complex may be regulated by sequence-specific transcription factors that have been shown to interact with Ash2L, such as Mef2d (81), Ap2δ (32), or Tbx1 (82). In addition, based on previous results demonstrating that arginine methylation modulates the peptidyl arginine binding activity of WDR5 (83), we suggest that methylation of Arg-3765 of MLL1 may be a mechanism to control the assembly of the MLL1 core complex. Confirmation of this hypothesis will depend on the identification of enzymes that methylate Arg-3765 of MLL1 in vivo.
In summary, in this investigation we have characterized the enzymatic activity of WRAD, a new multi-subunit histone methyltransferase lacking a conserved SET domain. We also demonstrate that WRAD, in addition to being required for sequential H3K4 methylation, is also required for methylation of nucleosomal histone H3 by the MLL1 core complex. Identification of amino acid residues involved in the catalytic mechanism of this enzyme will facilitate experiments designed for a greater understanding of the role WRAD plays in vivo.
Acknowledgments
We thank Kyle Fahey, Jeremy French-Lawyer, and Melody Sanders for a critical reading of this manuscript. We thank Tim Richmond for histone plasmids and Andrew Flaus and Tom Owen-Hughes for the murine mammary tumor virus LTR DNA.
This work was supported, in whole or in part, by National Institutes of Health Grant R01CA140522 (to M. S. C.). This work was also supported by Research Scholar Grant RSG-09-245-01-DMC from the American Cancer Society (to M. S. C.).
- H3K4
- H3 lysine 4
- [3H]AdoMet
- [3H]methyl S-adenosylmethionine
- AdoHyc
- S-adenosyl-homocysteine.
REFERENCES
- 1. Santos-Rosa H., Schneider R., Bernstein B. E., Karabetsou N., Morillon A., Weise C., Schreiber S. L., Mellor J., Kouzarides T. (2003) Mol. Cell 12, 1325–1332 [DOI] [PubMed] [Google Scholar]
- 2. Cosgrove M. S., Wolberger C. (2005) Biochem. Cell Biol. 83, 468–476 [DOI] [PubMed] [Google Scholar]
- 3. Wysocka J., Swigut T., Xiao H., Milne T. A., Kwon S. Y., Landry J., Kauer M., Tackett A. J., Chait B. T., Badenhorst P., Wu C., Allis C. D. (2006) Nature 442, 86–90 [DOI] [PubMed] [Google Scholar]
- 4. Santos-Rosa H., Schneider R., Bannister A. J., Sherriff J., Bernstein B. E., Emre N. C., Schreiber S. L., Mellor J., Kouzarides T. (2002) Nature 419, 407–411 [DOI] [PubMed] [Google Scholar]
- 5. Ruthenburg A. J., Allis C. D., Wysocka J. (2007) Mol. Cell 25, 15–30 [DOI] [PubMed] [Google Scholar]
- 6. Cheng X., Collins R. E., Zhang X. (2005) Annu. Rev. Biophys. Biomol. Struct. 34, 267–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang X., Yang Z., Khan S. I., Horton J. R., Tamaru H., Selker E. U., Cheng X. (2003) Mol. Cell 12, 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang H., An W., Cao R., Xia L., Erdjument-Bromage H., Chatton B., Tempst P., Roeder R. G., Zhang Y. (2003) Mol. Cell 12, 475–487 [DOI] [PubMed] [Google Scholar]
- 9. Steward M. M., Lee J. S., O'Donovan A., Wyatt M., Bernstein B. E., Shilatifard A. (2006) Nat. Struct. Mol. Biol. 13, 852–854 [DOI] [PubMed] [Google Scholar]
- 10. Dou Y., Milne T. A., Ruthenburg A. J., Lee S., Lee J. W., Verdine G. L., Allis C. D., Roeder R. G. (2006) Nat. Struct. Mol. Biol. 13, 713–719 [DOI] [PubMed] [Google Scholar]
- 11. Dou Y., Milne T. A., Tackett A. J., Smith E. R., Fukuda A., Wysocka J., Allis C. D., Chait B. T., Hess J. L., Roeder R. G. (2005) Cell 121, 873–885 [DOI] [PubMed] [Google Scholar]
- 12. Cho Y. W., Hong T., Hong S., Guo H., Yu H., Kim D., Guszczynski T., Dressler G. R., Copeland T. D., Kalkum M., Ge K. (2007) J. Biol. Chem. 282, 20395–20406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee J. H., Skalnik D. G. (2005) J. Biol. Chem. 280, 41725–41731 [DOI] [PubMed] [Google Scholar]
- 14. Lee J. H., Tate C. M., You J. S., Skalnik D. G. (2007) J. Biol. Chem. 282, 13419–13428 [DOI] [PubMed] [Google Scholar]
- 15. Miller T., Krogan N. J., Dover J., Erdjument-Bromage H., Tempst P., Johnston M., Greenblatt J. F., Shilatifard A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 12902–12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roguev A., Schaft D., Shevchenko A., Pijnappel W. W., Wilm M., Aasland R., Stewart A. F. (2001) EMBO J. 20, 7137–7148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nagy P. L., Griesenbeck J., Kornberg R. D., Cleary M. L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 90–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura T., Mori T., Tada S., Krajewski W., Rozovskaia T., Wassell R., Dubois G., Mazo A., Croce C. M., Canaani E. (2002) Mol. Cell 10, 1119–1128 [DOI] [PubMed] [Google Scholar]
- 19. Wysocka J., Myers M. P., Laherty C. D., Eisenman R. N., Herr W. (2003) Genes Dev. 17, 896–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yokoyama A., Wang Z., Wysocka J., Sanyal M., Aufiero D. J., Kitabayashi I., Herr W., Cleary M. L. (2004) Mol. Cell. Biol. 24, 5639–5649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strahl B. D., Ohba R., Cook R. G., Allis C. D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 14967–14972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins R. E., Tachibana M., Tamaru H., Smith K. M., Jia D., Zhang X., Selker E. U., Shinkai Y., Cheng X. (2005) J. Biol. Chem. 280, 5563–5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ng H. H., Robert F., Young R. A., Struhl K. (2003) Mol. Cell 11, 709–719 [DOI] [PubMed] [Google Scholar]
- 24. Takahashi Y. H., Lee J. S., Swanson S. K., Saraf A., Florens L., Washburn M. P., Trievel R. C., Shilatifard A. (2009) Mol. Cell. Biol. 29, 3478–3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Southall S. M., Wong P. S., Odho Z., Roe S. M., Wilson J. R. (2009) Mol. Cell 33, 181–191 [DOI] [PubMed] [Google Scholar]
- 26. Cosgrove M. S., Patel A. (2010) FEBS J. 277, 1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gori F., Friedman L., Demay M. B. (2005) J. Musculoskelet. Neuronal Interact. 5, 338–339 [PubMed] [Google Scholar]
- 28. Gori F., Friedman L. G., Demay M. B. (2006) Dev. Biol. 295, 498–506 [DOI] [PubMed] [Google Scholar]
- 29. Zhu E. D., Demay M. B., Gori F. (2008) J. Biol. Chem. 283, 7361–7367 [DOI] [PubMed] [Google Scholar]
- 30. Adamson A. L., Shearn A. (1996) Genetics 144, 621–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wysocka J., Swigut T., Milne T. A., Dou Y., Zhang X., Burlingame A. L., Roeder R. G., Brivanlou A. H., Allis C. D. (2005) Cell 121, 859–872 [DOI] [PubMed] [Google Scholar]
- 32. Tan C. C., Sindhu K. V., Li S., Nishio H., Stoller J. Z., Oishi K., Puttreddy S., Lee T. J., Epstein J. A., Walsh M. J., Gelb B. D. (2008) Proc. Natl. Acad. Sci. U.S.A. 105, 7472–7477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hsu D. R., Chuang P. T., Meyer B. J. (1995) Development 121, 3323–3334 [DOI] [PubMed] [Google Scholar]
- 34. Hsu D. R., Meyer B. J. (1994) Genetics 137, 999–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lüscher-Firzlaff J., Gawlista I., Vervoorts J., Kapelle K., Braunschweig T., Walsemann G., Rodgarkia-Schamberger C., Schuchlautz H., Dreschers S., Kremmer E., Lilischkis R., Cerni C., Wellmann A., Lüscher B. (2008) Cancer Res. 68, 749–758 [DOI] [PubMed] [Google Scholar]
- 36. Wang J., Zhou Y., Yin B., Du G., Huang X., Li G., Shen Y., Yuan J., Qiang B. (2001) J. Mol. Med. 79, 399–405 [DOI] [PubMed] [Google Scholar]
- 37. Milne T. A., Briggs S. D., Brock H. W., Martin M. E., Gibbs D., Allis C. D., Hess J. L. (2002) Mol. Cell 10, 1107–1117 [DOI] [PubMed] [Google Scholar]
- 38. Milne T. A., Dou Y., Martin M. E., Brock H. W., Roeder R. G., Hess J. L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 14765–14770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Milne T. A., Hughes C. M., Lloyd R., Yang Z., Rozenblatt-Rosen O., Dou Y., Schnepp R. W., Krankel C., Livolsi V. A., Gibbs D., Hua X., Roeder R. G., Meyerson M., Hess J. L. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 749–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Terranova R., Agherbi H., Boned A., Meresse S., Djabali M. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6629–6634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu B. D., Hanson R. D., Hess J. L., Horning S. E., Korsmeyer S. J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 10632–10636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu B. D., Hess J. L., Horning S. E., Brown G. A., Korsmeyer S. J. (1995) Nature 378, 505–508 [DOI] [PubMed] [Google Scholar]
- 43. Patel A., Dharmarajan V., Vought V. E., Cosgrove M. S. (2009) J. Biol. Chem. 284, 24242–24256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel A., Vought V. E., Dharmarajan V., Cosgrove M. S. (2008) J. Biol. Chem. 283, 32162–32175 [DOI] [PubMed] [Google Scholar]
- 45. Dyer P. N., Edayathumangalam R. S., White C. L., Bao Y., Chakravarthy S., Muthurajan U. M., Luger K. (2004) Methods Enzymol. 375, 23–44 [DOI] [PubMed] [Google Scholar]
- 46. Flaus A., Rencurel C., Ferreira H., Wiechens N., Owen-Hughes T. (2004) EMBO J. 23, 343–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schuck P. (2000) Biophys. J. 78, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laue T. M., Shah B. D., Ridgeway T. M., Pelletier S. L. (1992) in Analytical Ultracentrifugation in Biochemistry and Polymer Science (Harding S., Rowe A., Horton J. eds) pp. 19–125, Royal Society of Chemistry, Cambridge, UK [Google Scholar]
- 49. Eickbush T. H., Moudrianakis E. N. (1978) Biochemistry 17, 4955–4964 [DOI] [PubMed] [Google Scholar]
- 50. Lawrence F., Robert-Gero M. (1990) in Protein Methylation (Paik W. K., Kim S. eds) pp 305–340, CRC Press Inc., Boca Raton, FL [Google Scholar]
- 51. Patel A., Dharmarajan V., Cosgrove M. S. (2008) J. Biol. Chem. 283, 32158–32161 [DOI] [PubMed] [Google Scholar]
- 52. Dillon S. C., Zhang X., Trievel R. C., Cheng X. (2005) Genome Biol. 6, 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. van Leeuwen F., Gafken P. R., Gottschling D. E. (2002) Cell 109, 745–756 [DOI] [PubMed] [Google Scholar]
- 54. Feng Q., Wang H., Ng H. H., Erdjument-Bromage H., Tempst P., Struhl K., Zhang Y. (2002) Curr. Biol. 12, 1052–1058 [DOI] [PubMed] [Google Scholar]
- 55. Ng H. H., Feng Q., Wang H., Erdjument-Bromage H., Tempst P., Zhang Y., Struhl K. (2002) Genes Dev. 16, 1518–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Min J., Feng Q., Li Z., Zhang Y., Xu R. M. (2003) Cell 112, 711–723 [DOI] [PubMed] [Google Scholar]
- 57. Krogan N. J., Dover J., Khorrami S., Greenblatt J. F., Schneider J., Johnston M., Shilatifard A. (2002) J. Biol. Chem. 277, 10753–10755 [DOI] [PubMed] [Google Scholar]
- 58. Bryk M., Briggs S. D., Strahl B. D., Curcio M. J., Allis C. D., Winston F. (2002) Curr. Biol. 12, 165–170 [DOI] [PubMed] [Google Scholar]
- 59. Briggs S. D., Bryk M., Strahl B. D., Cheung W. L., Davie J. K., Dent S. Y., Winston F., Allis C. D. (2001) Genes Dev. 15, 3286–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nislow C., Ray E., Pillus L. (1997) Mol. Biol. Cell 8, 2421–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schneider J., Wood A., Lee J. S., Schuster R., Dueker J., Maguire C., Swanson S. K., Florens L., Washburn M. P., Shilatifard A. (2005) Mol. Cell 19, 849–856 [DOI] [PubMed] [Google Scholar]
- 62. Dehé P. M., Dichtl B., Schaft D., Roguev A., Pamblanco M., Lebrun R., Rodríguez-Gil A., Mkandawire M., Landsberg K., Shevchenko A., Shevchenko A., Rosaleny L. E., Tordera V., Chávez S., Stewart A. F., Géli V. (2006) J. Biol. Chem. 281, 35404–35412 [DOI] [PubMed] [Google Scholar]
- 63. Thompson B. A., Tremblay V., Lin G., Bochar D. A. (2008) Mol. Cell. Biol. 28, 3894–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yates J. A., Menon T., Thompson B. A., Bochar D. A. (2010) FEBS Lett. 584, 689–693 [DOI] [PubMed] [Google Scholar]
- 65. Garapaty S., Xu C. F., Trojer P., Mahajan M. A., Neubert T. A., Samuels H. H. (2009) J. Biol. Chem. 284, 7542–7552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Krajewski W. A., Vassiliev O. L. (2010) Biochem. Biophys. Res. Commun. 397, 112–116 [DOI] [PubMed] [Google Scholar]
- 67. Luger K., Mäder A. W., Richmond R. K., Sargent D. F., Richmond T. J. (1997) Nature 389, 251–260 [DOI] [PubMed] [Google Scholar]
- 68. Chandrasekharan M. B., Huang F., Chen Y. C., Sun Z. W. Mol. Cell. Biol. 30, 3216–3232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nakanishi S., Sanderson B. W., Delventhal K. M., Bradford W. D., Staehling-Hampton K., Shilatifard A. (2008) Nat. Struct. Mol. Biol. 15, 881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Davey C. A., Sargent D. F., Luger K., Maeder A. W., Richmond T. J. (2002) J. Mol. Biol. 319, 1097–1113 [DOI] [PubMed] [Google Scholar]
- 71. Dover J., Schneider J., Tawiah-Boateng M. A., Wood A., Dean K., Johnston M., Shilatifard A. (2002) J. Biol. Chem. 277, 28368–28371 [DOI] [PubMed] [Google Scholar]
- 72. Sun Z. W., Allis C. D. (2002) Nature 418, 104–108 [DOI] [PubMed] [Google Scholar]
- 73. Lee J. S., Shukla A., Schneider J., Swanson S. K., Washburn M. P., Florens L., Bhaumik S. R., Shilatifard A. (2007) Cell 131, 1084–1096 [DOI] [PubMed] [Google Scholar]
- 74. Chandrasekharan M. B., Huang F., Sun Z. W. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 16686–16691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scacheri P. C., Davis S., Odom D. T., Crawford G. E., Perkins S., Halawi M. J., Agarwal S. K., Marx S. J., Spiegel A. M., Meltzer P. S., Collins F. S. (2006) PLoS Genet 2, e51, 0406–0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cierpicki T., Risner L. E., Grembecka J., Lukasik S. M., Popovic R., Omonkowska M., Shultis D. D., Zeleznik-Le N. J., Bushweller J. H. (2010) Nat. Struct. Mol. Biol. 17, 62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ernst P., Wang J., Huang M., Goodman R. H., Korsmeyer S. J. (2001) Mol. Cell. Biol. 21, 2249–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vermeulen M., Mulder K. W., Denissov S., Pijnappel W. W., van Schaik F. M., Varier R. A., Baltissen M. P., Stunnenberg H. G., Mann M., Timmers H. T. (2007) Cell 131, 58–69 [DOI] [PubMed] [Google Scholar]
- 79. Flanagan J. F., Mi L. Z., Chruszcz M., Cymborowski M., Clines K. L., Kim Y., Minor W., Rastinejad F., Khorasanizadeh S. (2005) Nature 438, 1181–1185 [DOI] [PubMed] [Google Scholar]
- 80. Pray-Grant M. G., Daniel J. A., Schieltz D., Yates J. R., 3rd, Grant P. A. (2005) Nature 433, 434–438 [DOI] [PubMed] [Google Scholar]
- 81. Rampalli S., Li L., Mak E., Ge K., Brand M., Tapscott S. J., Dilworth F. J. (2007) Nat. Struct. Mol. Biol. 14, 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Stoller J. Z., Huang L., Tan C. C., Huang F., Zhou D. D., Yang J., Gelb B. D., Epstein J. A. (2010) Exp. Biol. Med. 235, 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Couture J. F., Collazo E., Trievel R. C. (2006) Nat. Struct. Mol. Biol. 13, 698–703 [DOI] [PubMed] [Google Scholar]