Abstract
Iron storage in yeast requires the activity of the vacuolar iron transporter Ccc1. Yeast with an intact CCC1 are resistant to iron toxicity, but deletion of CCC1 renders yeast susceptible to iron toxicity. We used genetic and biochemical analysis to identify suppressors of high iron toxicity in Δccc1 cells to probe the mechanism of high iron toxicity. All genes identified as suppressors of high iron toxicity in aerobically grown Δccc1 cells encode organelle iron transporters including mitochondrial iron transporters MRS3, MRS4, and RIM2. Overexpression of MRS3 suppressed high iron toxicity by decreasing cytosolic iron through mitochondrial iron accumulation. Under anaerobic conditions, Δccc1 cells were still sensitive to high iron toxicity, but overexpression of MRS3 did not suppress iron toxicity and did not result in mitochondrial iron accumulation. We conclude that Mrs3/Mrs4 can sequester iron within mitochondria under aerobic conditions but not anaerobic conditions. We show that iron toxicity in Δccc1 cells occurred under both aerobic and anaerobic conditions. Microarray analysis showed no evidence of oxidative damage under anaerobic conditions, suggesting that iron toxicity may not be solely due to oxidative damage. Deletion of TSA1, which encodes a peroxiredoxin, exacerbated iron toxicity in Δccc1 cells under both aerobic and anaerobic conditions, suggesting a unique role for Tsa1 in iron toxicity.
Keywords: Iron, Mitochondria, Oxidative Stress, Transport Metals, Yeast
Introduction
Iron is a required element but may be toxic at high concentrations. The toxicity of iron is ascribed to its ability to participate in Fenton reactions generating oxygen radicals that damage a wide range of macromolecules. There is evidence in model systems and in some in vitro cell systems that iron may induce oxidative damage (1, 2). Iron overload disease in humans, due to mutations in genes encoding proteins involved in hepcidin-mediated iron acquisition (HFE, TfR2, hepcidin, and hemojuvelin), can lead to tissue pathology, although the level and mechanism of injury are subject to debate (3). Similar mutations in mice also result in tissue iron loading but with little resulting pathology. The budding yeast offers a facile system to study the effects of iron deprivation or excess on metabolism as iron acquisition and/or storage can be experimentally manipulated. Hyperaccumulation of iron by dysregulated iron acquisition resulted in a growth defect, which was ascribed to an inhibition of the cell cycle (4). Yeast store iron in the vacuole, and mutations in the vacuolar H+-ATPase show decreased cell growth and increased oxidative stress (5). Many vacuolar transport activities rely on the vacuolar H+ gradient established by the H+-ATPase. It is unclear, however, whether the oxidative stress is a result of increased cytosolic metals reacting with oxygen metabolites or is due to metabolic effects downstream from compromised vacuolar function.
To examine the mechanism underlying iron toxicity, we took advantage of the observation that deletion of CCC1, which encodes for the vacuolar iron transporter, results in increased sensitivity to high concentrations of iron (6) to identify high copy suppressors of iron toxicity. The results of this study show that the iron sensitivity of Δccc1 cells can be suppressed by overexpression of mitochondrial iron transporter Mrs3 or Mrs4 or the mitochondrial pyrimidine phosphate transporter Rim2, a member of the mitochondrial carrier family that is homologous to Mrs3 and Mrs4. Overexpression of either of these transporters rescues cells from iron toxicity by decreasing cytosolic iron levels through mitochondrial iron sequestration. We determined that Mrs3/Mrs4 can sequester iron within mitochondria under aerobic conditions but not anaerobic conditions. We also show that deletion of TSA1, which encodes a thioredoxin peroxidase, exacerbated iron toxicity in Δccc1 cells under both aerobic and anaerobic conditions. Microarray data, however, show no evidence of “oxidative” damage due to iron under anaerobic conditions, suggesting a unique role for Tsa1 in modulating iron toxicity.
EXPERIMENTAL PROCEDURES
Yeast Strains, Media, and Plasmids
All Saccharomyces cerevisiae strains used in this study are derived from the W303 or S288C background and are listed in Table 1. Most deletion strains were created by PCR amplifying the KanMX cassette from specific strains in the diploid homozygous deletion collection (Research Genetics) and then using that amplicon to delete genes by homologous recombination in vivo. The primers used for amplification are available upon request. Multiple deletion strains were generated by combining traditional mating, sporulation, and dissection or by direct chromosome recombination of PCR knock-out constructs.
TABLE 1.
Yeast strains used in this study
| Strain | Genotype | Note |
|---|---|---|
| DY150 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) | Wild type |
| Δccc1 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δccc1::HIS3 | Ref. 9 |
| Δmrs3Δmrs4 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δmrs3::KanMX Δmrs4::KanMX | Ref. 9 |
| Δrim2 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δrim2::HIS3 | This study |
| Δmrs3Δmrs4Δrim2 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δmrs3Δmrs4::KanMX Δrim2::HIS3 | This study |
| Δtsa1 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δtsa1::KanMX | This study |
| Δccc1Δtsa1 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δccc1::HIS3 Δtsa1::KanMX | This study |
| Δyap1 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δyap1::KanMX | This study |
| Δccc1Δyap1 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δccc1::HIS3 Δyap1::KanMX | This study |
| Δtrx2 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δtrx2::KanMX | This study |
| Δccc1Δtrx2 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δccc1::HIS3 Δtrx2::KanMX | This study |
| Δgrx4 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δgrx4::KanMX | This study |
| Δccc1Δgrx4 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δccc1::HIS3 Δgrx4::KanMX | This study |
| Δtrr1 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δtrr1::HIS3 | Ref. 8 |
| Δccc1Δtrr1 | MATa ade2-1 his3-11 leu2-3.112 trp1-1 ura3-52 can1-100(oc) Δccc1::LEU2 Δtrr1::HIS3 | This study |
Cells were grown in YPD (1% yeast extract, 2% peptone, 2% dextrose) or CM (0.67% yeast nitrogen base, 0.12% drop-out amino acid mixture, 2% dextrose). GE medium (2% glycerol, 2% ethanol) is a carbon source in CM. Media were made iron deficient by addition of 80 μm bathophenanthroline sulfonate (BPS)3, and specific concentrations of ferrous sulfate were added back. The concentration of added iron in μm is denoted as BPSx. Anaerobic experiments in which cells were grown on plates were carried out using a BBLTM GasPakTM Plus anaerobic system (BD Biosciences). For anaerobic experiments involving cells grown in liquid medium, cells were grown in an anaerobic glove box (Model HE-493, Vacuum Atmospheres Co., Hawthorne, CA). The level of oxygen was found to be less than 0.2%.
All plasmids used in this study are listed in Table 2. MRS3 or MRS4 with a FLAG tag at the carboxyl terminus were cloned into pCM195 under the tetracycline-repressible promoter (Tet-Off). The proteins were fused to a FLAG tag at the carboxyl terminus for immunodetection.
TABLE 2.
Plasmids used in this study
Subcellular Fractionation and Western Blot Analysis
Cells were homogenized using glass beads as described previously (6). Proteins were analyzed by 12% SDS-PAGE followed by Western blot analysis using Western Lightning (PerkinElmer Life Sciences). Antisera used for probing Westerns included rabbit anti-Ccc1 (1:500), rabbit anti-HA (Invitrogen; 1:5000), mouse anti-Pgk (Invitrogen; 1:10,000), mouse anti-Cpy (Invitrogen; 1:2000), and mouse anti-porin (Invitrogen; 1:1000). Secondary antibodies were either peroxidase-conjugated goat anti-rabbit IgG or peroxidase-conjugated goat anti-mouse IgG (Jackson ImmunoResearch Laboratories; 1:5000).
OxyBlot and 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFDA) Assay
An OxyBlot protein oxidation detection kit was purchased from Millipore. About 5 × 107 yeast cells were homogenized with glass beads, and the lysate was collected by low speed centrifugation. One volume of lysate was mixed with 1 volume of 12% SDS, boiled for 3 min, allowed to cool down to room temperature, and centrifuged at 14,000 × g for 5 min. A 20-μg aliquot of the supernatant was derivatized with 2,4-dinitrophenylhydrazine as described by the manufacturer and analyzed by Western blot using the anti-2,4-dinitrophenyl antibody provided. Protein levels of each sample were determined by Coomassie Blue staining. Carbonyl groups assayed by antibodies to 2,4-dinitrophenyl and protein levels determined by Coomassie were measured by densitometry.
The production of reactive oxygen species was measured using DCFDA from Invitrogen. Cells in log phase were incubated with 15 μm DCFDA (final concentration) in culture media for 1 h. Two × 108 cells were harvested and washed once with water and twice with ice-cold phosphate-buffered saline. Cells were homogenized by vortexing with glass beads at 4 °C for 10 min, and the supernatant was collected by centrifugation at 14,000 × g for 10 min. Fluorescence was measured using an excitation wavelength of 504 nm and an emission wavelength of 524 nm in a PerkinElmer Life Sciences LS55 luminescence spectrometer. The fluorescence intensity was normalized to protein concentrations, which were determined by the bicinchoninic acid method (Pierce) using bovine serum albumin as a standard.
Measurement of Whole Cell and Mitochondrial Iron
Mitochondria were isolated from cells as described previously (35). Cells or mitochondrial pellets were digested with 200 μl of nitric acid at 80 °C for 1 h. The metal content was determined using a PerkinElmer Life Sciences inductively coupled plasma optical emission spectrometer with a standard curve generated from mixed metal standards. The results were normalized to mitochondrial protein concentrations or cell number.
Miscellaneous Procedures
Aconitase activity was determined as described previously (9). Microarray analysis was performed in the Huntsman Cancer Institute Microarray Core Facility (9). Immunofluorescence was performed, and images were captured on an Olympus BX051 epifluorescence microscope using a 100× 1.4 aperture objective as described previously (7). Measurement of CCC1-lacZ activity was performed as described (8). Specific activity was normalized to protein concentration and is presented as nmol/min/mg of protein.
RESULTS
Expression of Mitochondrial Iron Transporters Suppresses Iron Toxicity in Δccc1 Cells
We initially identified the mitochondrial iron transporters MRS3 and MRS4 as high copy suppressors of the high iron-sensitive growth defect of Δccc1 cells (9). We recently identified a third genomic clone that could also suppress that growth defect. Subcloning the plasmid identified RIM2 as the responsible gene. A genomic clone of RIM2 under the control of its own promoter in a high copy vector suppressed the high iron growth defect of Δccc1 (Fig. 1). RIM2 encodes a member of the mitochondrial carrier family and is homologous to MRS3 and MRS4. Rim2 had been identified as a mitochondrial pyrimidine phosphate transporter, leading us to examine whether Rim2 had effects on mitochondrial iron homeostasis. As shown previously (7), high copy RIM2 expression also suppressed the low iron growth defect of Δmrs3Δmrs4 cells. Δmrs3Δmrs4 cells have alterations in transition metal homeostasis due to activation of the vacuolar iron transporter Ccc1 (7, 9). For example, relative to control cells, Δmrs3Δmrs4 cells show decreased cadmium and copper resistance and increased cobalt resistance. Expression of RIM2 in Δmrs3Δmrs4 cells restored metal homeostasis to wild type (7). Thus, expression of RIM2 can suppress both the low iron growth defect and the altered transition metal metabolism in Δmrs3Δmrs4 cells.
FIGURE 1.
Expression of Rim2 rescues iron toxicity in Δccc1 cells. Cells deleted for CCC1 were transformed with a plasmid containing a genomic copy of MRS3, CCC1, RIM2, or a control plasmid. The cells were plated on CM with or without iron and grown at 30 °C for 3 days.
These results prompted us to examine the effect of deletion of RIM2. We verified that loss of RIM2 results in loss of mitochondrial respiration due to loss of the mitochondrial genome (10). Deletion of one genomic copy of RIM2 in diploids followed by sporulation showed that deletion of RIM2 led to the generation of small colonies (Fig. 2A) that were unable to grow on glycerol-ethanol (Fig. 2B). Respiratory growth of Δrim2 cells could not be recovered by transformation with a RIM2 plasmid, suggesting that the Δrim2 cells were rho0. Deletion of RIM2 did not lead to low iron growth sensitivity as Δrim2 cells grew as well on low iron plates as wild type cells made rho0 by growth in ethidium bromide (data not shown). Deletion of RIM2, however, did exacerbate the low iron growth phenotype of Δmrs3Δmrs4 cells (Fig. 2C). The low iron sensitivity of Δmrs3Δmrs4 cells is due to impaired mitochondrial iron transport. Measurement of the activity of the mitochondrial Fe-S cluster-containing enzyme aconitase showed lower levels of activity in Δrim2 cells compared with wild type rho0 but higher levels than Δmrs3Δmrs4 rho0 cells (Fig. 2D). The triple deletion Δmrs3Δmrs4Δrim2 showed a further loss of aconitase activity. In summary, these results indicate that Rim2 can effect mitochondrial iron transport.
FIGURE 2.
Deletion of Rim2 affects mitochondrial functions. A, sporulation of a heterozygous diploid deletion, Δrim2, showed large and small colonies when grown on YPD. B, wild type, wild type rho0, Δrim2, and Δrim2 transformed with a RIM2-expressing plasmid were spotted on glycerol-ethanol-containing (G/E) medium and grown at 30 °C for 3 days. C, wild type rho0, Δmrs3Δmrs4 rho0, Δrim2, or Δmrs3Δmrs4Δrim2 cells were streaked on CM medium or BPS2 and grown for 3 days. D, aconitase activity was assayed in cells from C. Protein concentration was determined, and aconitase activity was normalized to protein concentrations.
Overexpression of Mitochondrial Iron Transporters Protects Δccc1 Cells from Iron Toxicity by Lowering Cytosolic Iron
To examine whether the mitochondrial localization of Mrs3/Mrs4 is critical for their suppression of iron toxicity, we generated plasmids expressing MRS3 or MRS4 under the control of the regulated tetracycline promoter. The expressed proteins were tagged with a FLAG epitope at the carboxyl terminus. The tagged proteins were able to support the growth of Δmrs3Δmrs4 cells under low iron conditions and suppress the toxic effect of high concentrations of iron in Δccc1 cells (data not shown). These results confirm that the epitope-tagged Mrs proteins are functional. The expression levels of Mrs3-FLAG were not affected by the iron concentration of the growth medium (Fig. 3A). Mrs3-FLAG colocalized with mitochondrial porin protein when assayed by immunofluorescence, and the localization was not changed with environmental iron concentrations (Fig. 3B). Similarly, Mrs4-FLAG localized to mitochondria as determined by immunofluorescence (Fig. 3C). These results show that overexpression of Mrs3 or Mrs4 or the iron content of cells did not change the mitochondrial localization of Mrs3 and Mrs4.
FIGURE 3.
Overexpressed Mrs3-FLAG localizes to mitochondria. A, Δccc1 cells were transformed with a control vector or MRS3-FLAG. Cells were incubated in BPS2, CM medium, or CM medium containing 250 μm iron overnight. The levels of Mrs3-FLAG or mitochondrial porin were assayed by Western blot. B, Δccc1 cells transformed with a control vector or MRS3-FLAG were grown in CM medium in the presence orabsence of 250 μm iron overnight. The expression of Mrs3-FLAG and mitochondrial porin wasexamined by immunofluorescence. C, cells transformed with a control plasmid or MRS4-FLAG were grown overnight in CM medium, and the localization of Mrs4-FLAG and mitochondrial porin was examined by immunofluorescence.
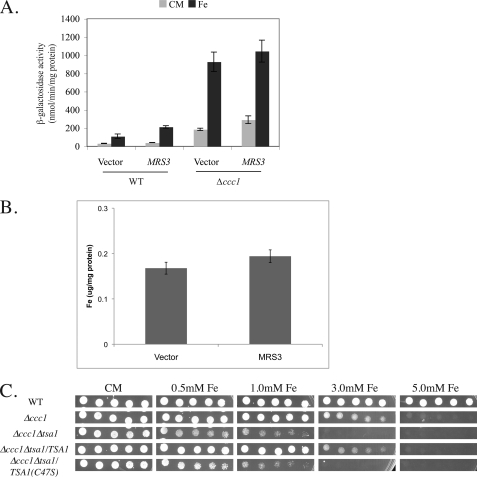
Expression of Mrs3-FLAG in Δccc1 cells had no effect on cellular iron accumulation when cells were grown in medium containing 250 μm iron (Fig. 4A). This result indicates that overexpression of MRS3 did not affect iron homeostasis of Δccc1 cells through decreased cellular iron accumulation. It is possible that overexpression of Mrs3/Mrs4 decreased the toxic effect of iron in the cytosol by reducing cytosolic iron through mitochondrial iron accumulation. To test this hypothesis, we assayed cytosolic iron levels using a reporter construct, CCC1-lacZ, that is sensitive to high cytosolic iron. CCC1 transcription is regulated by the Yap5 transcription factor in response to high iron (8). In Δccc1 cells, iron remained in the cytosol, inducing the expression of CCC1-lacZ (Fig. 4B). Overexpression of MRS3 in either wild type or Δccc1 cells led to a small but measurable increase in CCC1-lacZ activity. In the presence of added iron, overexpression of MRS3 was able to decrease the expression level of CCC1-lacZ, indicating decreased cytosolic iron. We examined whether the suppression of iron toxicity by overexpressed MRS3 was due to increased mitochondrial iron accumulation. Mitochondria were isolated from Δccc1 cells transformed with an empty vector or an MRS3-FLAG-containing plasmid. Overexpression of MRS3 was able to increase mitochondrial iron compared with vector control (Fig. 4C). This result indicates that expressed Mrs3-FLAG increased iron accumulation in mitochondria even under high iron conditions. In Δccc1 cells, increased medium iron resulted in increased mitochondrial iron, and expression of mitochondrial iron transporters led to a further increase in mitochondrial iron.
FIGURE 4.
Effect of overexpression of Mrs3-FLAG on whole cell and mitochondrial iron. A, wild type or Δccc1 cells transformed with a control vector or MRS3-FLAG were incubated in CM containing 250 μm iron. About 2 × 108 cells were harvested and digested with nitric acid at 80 °C for 1 h. Whole cell iron was determined by an inductively coupled plasma optical emission spectrometer. B, wild type and Δccc1 cells were transformed with a plasmid expressing CCC1-lacZ and either a control vector or MRS3-FLAG. Cells were incubated overnight in CM medium or CM medium with 250 μm iron, and β-galactosidase activity was determined. C, Δccc1 cells were transformed with a control vector or MRS3-FLAG. The cells were incubated in CM medium or CM medium with 50 or 250 μm iron overnight. Mitochondria were isolated, and the amount of mitochondrial iron was determined by an inductively coupled plasma optical emission spectrometer and normalized to mitochondrial protein concentrations. Error bars represent the S.E. of three separate experiments.
Determination of Mechanism of Iron Toxicity
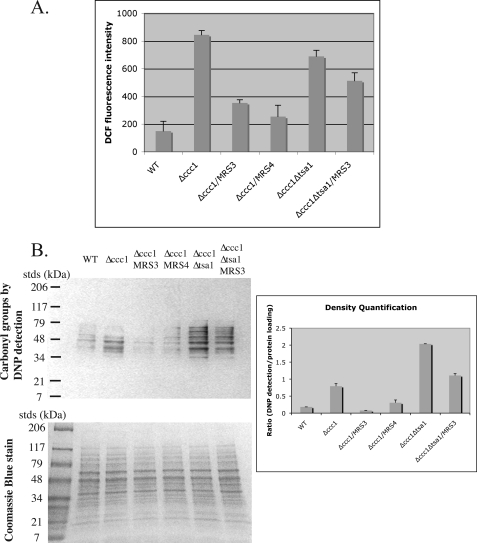
All genes identified to date that suppress high iron toxicity in Δccc1 cells encode organelle transporters, resulting in organelle iron sequestration and reduced cytosolic iron (9, 11, 12). It is thought that iron leads to damage through the generation of oxygen radicals; however, our genetic screens (high copy suppressor and UV and transposon mutagenesis) did not identify genes that either regulate or encode antioxidant systems as suppressors of high iron toxicity. Furthermore, transformation of Δccc1 cells with high copy plasmids expressing either SOD1, SOD2, or catalase (CTT1) did not lead to reduction of iron sensitivity in Δccc1 cells (data not shown). These findings led us to determine whether increased iron was correlated with an increase in oxidative damage. We used two measures to determine whether increased cytosolic iron resulted in increased oxidants. We measured the fluorescence of DCF as a measure of oxidant generation. The non-fluorescent reagent DCFDA is metabolized by oxidants to a fluorescent compound, DCF, the level of which is a measure of oxidants (13). Wild type cells showed low levels of DCF fluorescence, which was increased in Δccc1 cells. Overexpression of MRS3 or MRS4 reduced DCF fluorescence back to near base-line levels (Fig. 5A). We then assayed oxidative damage to proteins through the use of 2,4-dinitrophenylhydrazine, a compound that reacts with carbonyl groups of proteins (14). Carbonyl groups are common by-products of oxidative damage to amino acid side chains. Antibodies against 2,4-dinitrophenyl allow the immunodetection of this compound bound to proteins by Western blot techniques. In cells incubated with high iron (3 mm) for 3 h, the degree of protein oxidation was higher in Δccc1 cells than in wild type cells (Fig. 5B). Overexpression of MRS3 or MRS4 in Δccc1 cells reduced the degree of protein oxidation to that seen in wild type cells. These results show that overexpression of MRS3 or MRS4 in Δccc1 cells, by lowering cytosolic iron, can reduce oxidative damage of proteins.
FIGURE 5.
Iron induces oxidative damage in Δccc1 cells. A, wild type (DY150), Δccc1, and Δccc1Δtsa1 cells were transformed with empty vector, MRS3-FLAG, or MRS4-FLAG. Cells were incubated with 3 mm iron for 3 h. DCFDA was added after the 2nd h of incubation, and cells were incubated for an additional 1 h. The cells were lysed, and fluorescence was determined. The data were normalized to protein concentration, and error bars represent the S.E. of n = 3 experiments. B, wild type (DY150), Δccc1, and Δccc1Δtsa1 cells were transformed with empty vector, MRS3-FLAG, or MRS4-FLAG. Cells were incubated with 3 mm iron for 3 h. Cells were harvested and homogenized with glass beads. The oxidant modification of proteins was examined by Western blot following derivatization of protein carbonyl groups by 2,4-dinitrophenylhydrazine and incubation with antibodies specific to 2,4-dinitrophenyl (DNP). The upper left panel is an OxyBlot, and the lower left panel is a Coomassie Blue-stained gel.
Tsa1 Has a Novel Role in Reducing Cytosolic Iron Toxicity
We used microarray analysis to compare transcripts in Δccc1 cells with those in wild type cells. Cells were incubated with 3 mm iron for 3 h, and total RNA was analyzed using an Affymetrix microarray. High iron induced changes in transcripts for several processes including cell cycle progression, DNA repair, and oxidative response. There was significant up-regulation of genes that encode enzymes to combat oxidant damage, such as thiol-specific peroxiredoxins Ahp1 and Tsa1, thioredoxin Trx2, glutaredoxin Grx4, and Yap1, which is a transcriptional activator of antioxidant response (Table 3).
TABLE 3.
Changes in gene expression in Δccc1 cells exposed to high iron
Wild type cells and Δccc1 cells grown in CM under aerobic or anaerobic conditions were incubated for 3 h with 3 mm ferrous ammonium sulfate. RNA was isolated and analyzed using an Affymetrix microarray. The data are the average of two independent experiments, and the comparison is the change in transcripts in Δccc1 cells grown in 3 mm iron for 3 h versus wild type yeast grown in 3 mm iron for 3 h. TFIIH, transcription factor IIH.
| Gene | Aerobic-fold | Description | Anaerobic-fold |
|---|---|---|---|
| Oxidative damage | |||
| HSP12 | 7.12 | Heat shock protein | −9.77 |
| GTT2 | 4.71 | Glutathione transferase | −4.34 |
| AHP1 | 4.48 | Thiol-specific peroxidase | −2.84 |
| TSA1 | 4.27 | Thioredoxin peroxidase | −1.95 |
| TRX2 | 3.41 | Cytoplasmic thioredoxin | −3.00 |
| YAP1 | 3.34 | Transcription factor required for oxidative stress tolerance | 1.44 |
| SOD2 | 3.17 | Mitochondrial superoxide dismutase | −5.18 |
| SOD1 | 1.90 | Cytosolic superoxide dismutase | −2.13 |
| GRX4 | 2.33 | Glutathione-dependent oxidoreductase | 4.26 |
| Cell cycle | |||
| BUB1 | 2.89 | Mitotic cell cycle spindle assembly checkpoint | 1.16 |
| RAD53 | 2.47 | Protein kinase required for cell cycle arrest in response to DNA damage | 1.44 |
| MEC1 | 2.87 | Mitosis entry checkpoint | 1.20 |
| CLB1 | −12.77 | B-type cyclin involved in cell cycle progression | −1.13 |
| CLB2 | −12.24 | B-type cyclin involved in cell cycle progression | −1.24 |
| CLN1 | −9.25 | G1 cyclin involved in regulation of the cell cycle | −1.84 |
| CLN2 | −9.23 | G1 cyclin involved in regulation of the cell cycle | −1.90 |
| PCL1 | −18.96 | Cyclin, redundant with CLN1 and CLN2 | −5.16 |
| FAR1 | −5.28 | Cyclin-dependent kinase inhibitor | −1.25 |
| DNA repair | |||
| HUG1 | 9.53 | Responds to DNA damage, transcription is induced by DNA damage | 2.15 |
| RAD2 | 3.10 | DNA repair | −1.23 |
| RAD7 | 3.37 | DNA repair | 1.41 |
| RAD16 | 3.42 | DNA repair | 1.50 |
| RAD51 | 2.85 | DNA repair | 1.49 |
| RAD54 | 3.29 | DNA repair | 1.25 |
| RAD28 | 3.48 | DNA repair | −1.21 |
| RPN4 | 3.65 | Transcription factor | −1.22 |
| OGG1 | 1.43 | DNA glycosylase, DNA repair in mitochondria | 2.76 |
| NTG2 | 2.27 | DNA glycosylase, DNA repair in nucleus | −1.51 |
| MAG1 | 3.87 | DNA glycosylase, base excision repair | −1.17 |
| MLH3 | 1.94 | DNA mismatch repair | −1.01 |
| BDF2 | 4.38 | Protein involved in transcription initiation | 1.02 |
| DUN1 | 2.18 | DNA damage checkpoint | 1.29 |
| SSL2 | 2.50 | Core TFIIH complex, nucleotide excision repair factor 3 complex | 1.71 |
| POL4 | 3.21 | Mediates repair of DNA | 1.47 |
| NSE1 | 2.24 | DNA repair | 1.61 |
| DDR48 | 3.65 | DNA damage-responsive protein | −2.89 |
Deletion of genes that encode subunits of the vacuolar H+-ATPase leads to increased transcription of genes involved in oxidative damage repair. Deletion of some of the oxidative damage repair genes leads to a synthetic growth defect when combined with deletion of genes that encode vacuolar H+-ATPase subunits (5). Those results suggested that loss of vacuolar function resulted in chronic oxidative stress. To determine whether these genes play a role in suppressing iron toxicity in Δccc1 cells, we selected a subset of genes and examined the effects of gene deletions on iron toxicity. We focused on genes that were known to have little deleterious effect when deleted singly. Single deletions of the selected antioxidant genes did not show deficient growth compared with wild type or Δccc1 cells (Fig. 6A). These results show that the ability of Ccc1 to transport iron into the vacuole prevents cytosolic iron accumulation and iron toxicity. Surprisingly, deletion of YAP1, which controls the transcriptional response to H2O2 (15), had no effect on the growth phenotype of Δccc1 cells in high iron medium. Similarly, deletion of GRX4 (an iron-induced Yap5-dependent gene4) (Fig. 6B) or TRX2 had no effect on high iron toxicity (Fig. 6A). In contrast, deletion of TSA1 led to a dramatic increase in sensitivity to iron toxicity in Δccc1 cells. Tsa1 has been reported to be a bifunctional protein (16). One function is to act as a peroxiredoxin involved in the breakdown of hydrogen peroxide and organic hydroperoxides. A second function is to act as a chaperone affecting protein folding. Mutation of cysteine 47 abolished the peroxiredoxin activity but not the chaperone activity. To determine whether the increased sensitivity to the loss of TSA1 was due to loss of chaperone activity or peroxiredoxin activity, we transformed Δccc1Δtsa1 cells with plasmids expressing wild type Tsa1 or Tsa1(C47S). Expression of Tsa1 suppressed the increased sensitivity of Δccc1Δtsa1 cells to iron, but expression of Tsa1(C47S) did not (Fig. 6C). Deletion of TSA1 did not affect DCF fluorescence (Fig. 5A), indicating that Tsa1 was not affecting the generation of oxygen radicals. In contrast, deletion of TSA1 had a large effect on the level of protein carbonyl groups (Fig. 5B), indicating that it can modify oxidative damage.
FIGURE 6.
Deletion of antioxidant genes affects high iron sensitivity. Wild type (DY150), Δccc1, Δyap1, Δtrx2, Δtsa1, and double knock-out strains Δccc1Δyap1, Δccc1Δtsa1, and Δccc1Δtrx2 (A) or Δgrx4 and Δccc1Δgrx4 cells (B) were grown in CM medium. Serial dilutions were spotted onto CM or CM plates containing the specified concentrations of iron. Plates were incubated at 30 °C for 2 days. C, DY150, Δccc1, and Δccc1Δtsa1 cells transformed with empty vector, TSA1, or TSA1(C47S) plasmids were spotted on CM-Ura plates with different concentrations of iron. Plates were incubated at 30 °C for 2 days. D, DY150, Δccc1, Δtrr1, or Δccc1Δtrr1 cells were spotted onto iron plates and grown at 30 °C for 2 days. E, cells as described in D were spotted on plates with addition of H2O2 and grown for 2 days.
These results suggest that Tsa1 is acting through its peroxiredoxin activity to reduce oxidant damage in Δccc1 cells exposed to high iron, but further analysis suggests that the mechanism by which Tsa1 prevents iron toxicity is less clear. Tsa1 resolves peroxides by reducing them, the reductant being furnished by NADPH, which is transferred to Trr1 and through Trr1 to Trx and then to Tsa1 (17). If this model for peroxiredoxin activity holds true, then loss of Trr1 should abrogate Tsa1 function. Deletion of TRR1 had no effect on the iron sensitivity of Δccc1 cells in marked contrast to loss of TSA1 (Fig. 6, D versus A). The loss of TRR1, however, had a significant effect on the sensitivity of Δccc1 cells to H2O2 (Fig. 6E). Further results presented below suggest that iron toxicity may not be ascribed simply to the generation of Fenton radicals.
Overexpression of MRS3 Does Not Lead to Iron Accumulation under Anaerobic Conditions
Previously we showed that overexpression of MRS3 or MRS4 protected respiratory-incompetent Δccc1 cells from iron toxicity but did not protect Δccc1 cells from high iron under anaerobic conditions (9). In those experiments, MRS3 and MRS4 were under the control of their native promoter, and it was possible that the levels of expression under anaerobic conditions were inadequate to accumulate iron in mitochondria. We took advantage of the regulated MRS3-FLAG construct to test that hypothesis. We first demonstrated that robust amounts of Mrs3-FLAG were produced in wild type and Δccc1 cells grown anaerobically (Fig. 7A). Cells with a double deletion in MRS3 and MRS4 are more sensitive to iron deprivation than wild type cells, and the low iron growth defect of Δmrs3Δmrs4 cells can be suppressed by overexpression of MRS3-FLAG or MRS4-FLAG (Fig. 7B). Mrs3-FLAG is localized to mitochondria under anaerobiosis, and high iron levels did not affect localization (Fig. 7C). These results show that Mrs3-FLAG and Mrs4-FLAG can function anaerobically. Expression of Mrs3-FLAG under anaerobic conditions, however, did not protect Δccc1 cells from high iron toxicity (Fig. 7D). In contrast, expression of a mutant vacuolar zinc transporter, Zrc1(N44I), which has a gain of function mutation that permits it to transport iron into the vacuole (12), can protect Δccc1 cells from high iron toxicity. The inability of Mrs3-FLAG to protect Δccc1 cells anaerobically suggests that it was not able to deplete cytosolic iron through mitochondrial iron accumulation. We examined the ability of Mrs3-FLAG to modulate cytosolic iron using CCC1-lacZ. In marked contrast to what was seen in aerobic conditions (see Fig. 4), there was no difference in the level of β-galactosidase activity induced by iron in anaerobically grown Mrs3-FLAG expressing Δccc1 cells (Fig. 8A). This result suggests that overexpression of Mrs3-FLAG does not lead to mitochondrial iron accumulation under anaerobic conditions. We confirmed this result by measuring mitochondrial iron. The level of mitochondrial iron in Δccc1 cells grown anaerobically was less than that in cells grown aerobically (Fig. 8B versus Fig. 4C); however, mitochondrial iron did not change when Mrs3-FLAG was overexpressed. This result is consistent with reduced expression of cytochromes and heme-containing proteins (18, 19). These results suggest that the ability of Mrs3 to protect against iron toxicity is dependent on its ability to deplete cytosolic iron through mitochondrial iron accumulation.
FIGURE 7.
Overexpression of Mrs3-FLAG does not protect anaerobically grown Δccc1 cells from high iron toxicity. A, cells transformed with empty vector or MRS3-FLAG were grown aerobically or anaerobically in CM media. Cells were harvested and homogenized with glass beads. Protein levels were analyzed by Western blot using antibodies directed against FLAG and porin. B, wild type, Δmrs3Δmrs4, or Δmrs3Δmrs4 cells transformed with either a control plasmid, MRS3-FLAG, or MRS4-FLAG were grown anaerobically in CM or BPS5 for 3 days. C, Δccc1 cells transformed with empty vector or MRS3-FLAG were grown in CM with 250 μm iron aerobically or anaerobically. Cells were examined by immunofluorescence using antibodies against FLAG and porin. D, wild type cells transformed with empty vector or Δccc1 cells transformed with empty vector, MRS3, ZRC1, or ZRC1(N44I) were spotted onto CM plates or CM plates containing 3 mm iron. Plates were incubated aerobically or anaerobically at 30 °C for 2–3 days.
FIGURE 8.
Overexpression of Mrs3-FLAG anaerobically does not reduce cytosolic iron. A, wild type and Δccc1 cells transformed with empty vector or MRS3-FLAG were also transformed with a CCC1-lacZ plasmid. The cells were grown anaerobically and incubated in the presence or absence of 250 μm iron. Cells were harvested, and β-galactosidase activity was determined. Error bars represent the S.E. from n = 3 experiments. B, mitochondria were isolated from Δccc1 cells transformed with empty vector or MRS3-FLAG grown anaerobically and exposed to 50 μm iron. The amount of iron in mitochondria was determined by an inductively coupled plasma optical emission spectrometer and normalized to mitochondrial protein concentrations. Error bars represent the S.E. from n = 3 experiments. C, wild type (DY150), Δccc1, and Δccc1Δtsa1 cells with empty vector and Δccc1Δtsa1 cells with TSA1 or TSA1(C47S) plasmid were spotted on plates with different concentrations of iron and grown for 3 days.
We observed that deletion of TSA1 affected iron sensitivity in Δccc1 cells even under anaerobic conditions (Fig. 8C). Based on this result, we focused on whether there is evidence of oxidative damage due to iron toxicity under anaerobic conditions. Studies suggest that oxidative damage may occur in the transition between aerobic and anaerobic environments (20). To prevent that situation, we adapted cells to anaerobic growth for 24 h before the addition of iron. Evidence that the cultures had adapted to anaerobiosis was obtained by microarray analysis of anaerobic versus aerobic cultures. Anaerobic cultures showed dramatically increased transcripts for genes expressed under anaerobic conditions (Table 4) and decreased expression of genes encoding proteins involved in the aerobic process, such as cytochromes (data not shown). We then examined the effect of 3 h of iron addition to wild type and Δccc1 cells adapted to anaerobiosis. There was no increase in most of the transcripts indicative of oxidative damage or DNA repair (Table 3). We note that transcripts for GRX4 were increased in response to iron in both aerobic and anaerobic cultures. GRX4 is regulated by the transcription factorYap5 in response to iron, independent of oxygen status.4
TABLE 4.
Up-regulation of selected genes in Δccc1 cells grown anaerobically
Δccc1 cells were grown to midlog phase under aerobic or anaerobic conditions (anaerobic chamber). RNA was isolated and analyzed using an Affymetrix microarray. The data are the average of two independent experiments, and the comparison is the change in transcripts in Δccc1 cells grown anaerobically versus Δccc1 cells grown aerobically. Cells were grown anaerobically in an anaerobic glove box in which the O2 concentration was measured to be no greater than 0.2%.
| Gene | -Fold | Description |
|---|---|---|
| DAN1 | 1365 | Cell wall mannoprotein |
| ANB1 | 186 | Translation elongation factor |
| TIR3 | 144 | Cell wall mannoprotein |
| TIR4 | 123 | Cell wall mannoprotein |
| HEM13 | 36 | Heme biosynthetic process |
DISCUSSION
Overexpression of Mitochondrial Iron Transporters Leads to Mitochondrial Iron Accumulation
Increased levels of iron lead to cellular toxicity and organ dysfunction in higher eukaryotes. We have taken advantage of yeast with defined genetic deletions to examine the mechanisms of iron toxicity. Yeast store iron in the vacuole through the iron/manganese transporter Ccc1 (6). In the absence of Ccc1 or its transcriptional activator Yap5 (8), cells become sensitive to high iron. Using a variety of genetic approaches including high copy suppressor screens (9), UV mutagenesis, and transposon mutagenesis (11, 12), we identified a limited number of genes that could suppress iron toxicity. All of the genes encoded transporters that acted by reducing cytosolic iron levels through iron accumulation in subcellular organelles. We reported that mutations in the vacuolar zinc transporter Zrc1, which converted it to a vacuolar iron transporter, would suppress iron toxicity in a Δccc1 strain (11, 12). We also reported that overexpression of MRS3 or MRS4 would also suppress iron toxicity in Δccc1 cells (9). Expanding our screen, we identified RIM2 as a high copy suppressor of iron toxicity. Our data suggest that Rim2 can function as a mitochondrial iron importer as it can suppress the mitochondrial iron deficit of Δmrs3Δmrs4 cells. Of interest is that in vitro studies suggest that Rim2 is a pyrimidine/pyrimidine phosphate antiporter (21). How a pyrimidine/pyrimidine phosphate antiporter can lead to iron transport activity needs to be resolved. It may be possible that pyrimidine phosphates are iron-binding molecules. A second possibility is based on recent studies that suggested that catechols (22) or dihydroxybenzoic acid derivatives (23) are iron-binding moieties. These molecules in the iron-bound form are thought to bind to lipocalin. 2,5-Dihydroxybenzoic acid was shown to deliver iron to isolated mitochondria (23). Based on these results, we speculate that perhaps Rim2 can accept dihydroxybenzoic acid-iron compounds as a substrate and effect their transport into mitochondria. Experiments to test this hypothesis are ongoing.
Focusing on the mechanism by which overexpression of mitochondrial iron transporters protects cells from iron toxicity, we determined that expression of Mrs3 leads to decreased cytosolic iron. Assays for cytosolic iron, such as induction of CCC1-lacZ or direct measurement of mitochondrial iron, showed that overexpression of Mrs3 reduced cytosolic iron and increased mitochondrial iron. There are a number of features regarding Mrs-mediated mitochondrial iron accumulation worth noting. First, in the presence of Ccc1, overexpression of Mrs3 did not lead to increased mitochondrial iron even in cells incubated in high iron medium. We ascribe this result to the fact that Ccc1 controls the level of cytosolic iron, preventing iron accumulation in the cytosol, which is a prerequisite for mitochondrial iron accumulation. We speculate that in those instances in which increased levels of iron are found in mitochondria in cells that have a functional Ccc1 iron accumulation results not from increased mitochondrial iron transport but from processes that affect iron subsequent to its entry into mitochondria. One example would be the precipitation of iron found in mitochondria due to defects in iron-sulfur cluster synthesis (21).
Second, overexpression of Mrs3 led to mitochondrial iron accumulation in Δccc1 cells, but even when Mrs3 was massively overexpressed, the level of mitochondrial accumulation was only 3–4-fold over that seen in cells with an empty vector control. A similar observation was made by Froschauer et al. (24) who measured Mrs-mediated iron transport into isolated mitochondria. Increased levels of Mrs3 had a small effect on mitochondrial iron accumulation. There appears to be a balance between cytosolic iron and mitochondrial iron in which increased cytosolic iron leads to increased mitochondrial iron, and overexpression of Mrs3 can lead to a further increase in mitochondrial iron. This modest increase in iron accumulation occurs in the face of significant overexpression of Mrs3. This result suggests that the pool size of mitochondrial iron is limited either by regulation of Mrs3 transport activity or by increased mitochondrial iron efflux. Discrimination between these hypotheses is difficult because of the lack of a direct in vitro assay for mitochondrial iron transport and the difficulty in performing pulse-chase studies on mitochondrial iron in yeast. Third, although MRS3/MRS4 is required for mitochondrial iron acquisition under anaerobic conditions, overexpression did not result in cytosolic iron depletion or mitochondrial iron accumulation. The mechanism underlying the failure of Mrs3/Mrs4 to accumulate mitochondrial iron is unclear. Mrs3/Mrs4 belong to the family of mitochondrial carrier facilitators, most of which are anionic antiporters. One possibility is that the substrate for Mrs3/Mrs4 may not be iron but rather a small organic molecule, and it or a counterion (most members of the mitochondrial carrier family are antiporters) may be rate-limiting under anaerobic conditions.
Iron Toxicity Results from Accumulation of Cytosolic Iron
Our data support the view that iron toxicity is due to accumulation of iron in the cytosol as depletion of cytosolic iron by either storage in the vacuole or in the mitochondria protects cells from iron toxicity. The finding that mitochondrial iron storage can protect cells from iron toxicity was unexpected as mitochondria are thought to be a major source of toxic iron metabolites. It is thought that H2O2 is produced through the generation of superoxide anion resulting from electron loss in mitochondrial respiration, yet toxicity either in response to high iron or deletion of the vacuolar H+-ATPase is not decreased by the loss of respiration (5). There is a complex relationship between mitochondrial iron and oxidative damage. Accumulation of mitochondrial iron because of mutations in Fe-S cluster enzymes can suppress the growth deficit due to loss of Sod1. Growth of Δsod1 cells is improved by these mutants (seo mutants); however, measures of oxidative damage, such as carbonyl generation, are increased (25). In contrast, deletion of YFH1 results in iron accumulation and the generation of respiratory-incompetent cells. Restriction of mitochondrial iron accumulation in Δyfh1 cells preserves mitochondrial respiration (26). It is thought that the loss of respiration results from toxicity due to iron-induced oxidative damage. Iron that accumulates in mitochondria because of defects in Fe-S cluster synthesis is most often found in the form of iron precipitates, which are expected to be Fe(III) (27, 28). We suggest that iron accumulated by overexpression of Mrs3/Mrs4/Rim2 is not precipitated and is in a form that renders it insensitive to oxidative stress. Whatever that form is, its location or concentration does not lead to mismetallation of Sod2.5 Iron incorporation into the normal manganese site on Sod2 is often seen in conditions that lead to mitochondrial iron accumulation, such as loss of proteins involved in Fe-S cluster synthesis (29, 30).
Iron Toxicity May Not Be Due to Oxidative Damage
Increased cytosolic iron in Δccc1 cells does lead to increased oxidant damage as assessed by OxyBlot, DCFDA, and microarray analyses. There are clearly increased transcripts for genes involved in antioxidant defense, but deletion of a number of those genes had no effect on iron toxicity. A caveat to this conclusion is that we restricted our analysis to genes whose loss did not affect viability under steady state growth conditions. For example, we excluded SOD1 as deletion of SOD1 results in severely compromised growth under aerobic conditions. With that caveat, we were surprised to find that deletion of YAP1, a gene that encodes a regulator of oxidative damage response (31), had no effect on iron sensitivity. Furthermore, deletion of TRR1 also had no effect on iron toxicity. Trr1 is a key enzyme in redox reactions as it reduces oxidized thioredoxin with electrons donated by NADPH. As shown by published studies (32) and the data in Fig. 6, Trr1 plays a role in mitigating damage due to H2O2 but not to iron. The finding that deletion of TRR1 had no effect on iron toxicity suggests that the Tsa1 may be acting independently of electron transfer. A caveat to this conclusion is that there is a homologue of Trr1, which might provide functional redundancy. Trr2, however, is localized to mitochondria and would not be expected to participate in cytosolic reactions.
Evidence that suggests that iron toxicity may not be due to oxidant damage is that iron is toxic under anaerobic conditions. Similar to aerobically grown cells, the toxicity of iron under anaerobic conditions is due to accumulation of cytosolic iron as sequestration in vacuoles reduces toxicity. Iron toxicity under anaerobic conditions is also affected by the loss of Tsa1 as Δccc1Δtsa1 cells are much more iron-sensitive than cells with a single deletion. The findings that deletion of TRR1 did not affect iron sensitivity under aerobic conditions, that iron is toxic under anaerobic conditions, that overexpression of SOD1, SOD2, or CTT1 was unable to protect Δccc1 cells from high iron toxicity, and that there is no evidence of induction of antioxidant transcripts under anaerobic conditions cast doubt on whether iron toxicity is mediated solely or in part by the generation of oxygen radicals. Under anaerobic conditions, there is decreased expression of DNA repair genes. This result suggests that DNA damage is due to oxidative damage. Reduced expression of DNA repair genes does not exclude DNA damage as a cause of toxicity. DNA damage may result from conditions other than oxidation. An alternate hypothesis is that iron toxicity results from mismetallation of enzymes in which iron is incorporated instead of other transition metals. Finally, the finding that deletion of TSA1 had profound effects on iron sensitivity, whereas deletion of TRR1 or TRX2 did not, suggests a unique role for TSA1 in modulating iron toxicity. The effect of Tsa1 is independent of the electrons transferred by Trr1; however, it does require cysteine 47 of Tsa1. Mutation of cysteine 47 prevents Tsa1 from participating in mixed disulfide reduction but not from acting as a chaperone (16). Peroxiredoxins have multiple functions; in addition to chaperone and antioxidant activity, peroxiredoxins are also involved in signaling activities as the binding of Tsa1 to Yap1 (33) or the binding of the peroxiredoxin Ahp1 to Cad1 (34) leads to a transcriptional response. The fact that deletion of TSA1 makes Δccc1 less tolerant to iron toxicity even under anaerobic conditions suggests a new function of Tsa1 in modulating iron toxicity independently of oxidative damage.
Acknowledgment
We express our appreciation to members of the Kaplan laboratory for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK030534 and DK052830.
L. Li, unpublished data.
H. Lin, unpublished data.
- BPS
- bathophenanthroline sulfonate
- DCFDA
- 2′,7′-dichlorodihydrofluorescein diacetate
- DCF
- 2′,7′-dichlorodihydrofluorescein.
REFERENCES
- 1. Touati D. (2000) Arch. Biochem. Biophys. 373, 1–6 [DOI] [PubMed] [Google Scholar]
- 2. Valko M., Morris H., Cronin M. T. (2005) Curr. Med. Chem. 12, 1161–1208 [DOI] [PubMed] [Google Scholar]
- 3. Pietrangelo A., Trautwein C. (2004) Nat. Clin. Pract. Gastroenterol. Hepatol. 1, 39–45 [DOI] [PubMed] [Google Scholar]
- 4. Philpott C. C., Rashford J., Yamaguchi-Iwai Y., Rouault T. A., Dancis A., Klausner R. D. (1998) EMBO J. 17, 5026–5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Milgrom E., Diab H., Middleton F., Kane P. M. (2007) J. Biol. Chem. 282, 7125–7136 [DOI] [PubMed] [Google Scholar]
- 6. Li L., Chen O. S., McVey Ward D., Kaplan J. (2001) J. Biol. Chem. 276, 29515–29519 [DOI] [PubMed] [Google Scholar]
- 7. Li L., Murdock G., Bagley D., Jia X., Ward D. M., Kaplan J. (2010) J. Biol. Chem. 285, 10232–10242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Li L., Bagley D., Ward D. M., Kaplan J. (2008) Mol. Cell. Biol. 28, 1326–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Li L., Kaplan J. (2004) J. Biol. Chem. 279, 33653–33661 [DOI] [PubMed] [Google Scholar]
- 10. Van Dyck E., Jank B., Ragnini A., Schweyen R. J., Duyckaerts C., Sluse F., Foury F. (1995) Mol. Gen. Genet. 246, 426–436 [DOI] [PubMed] [Google Scholar]
- 11. Lin H., Burton D., Li L., Warner D. E., Phillips J. D., Ward D. M., Kaplan J. (2009) Biochem. J. 422, 273–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin H., Kumánovics A., Nelson J. M., Warner D. E., Ward D. M., Kaplan J. (2008) J. Biol. Chem. 283, 33865–33873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eruslanov E., Kusmartsev S. (2010) Methods Mol. Biol. 594, 57–72 [DOI] [PubMed] [Google Scholar]
- 14. Cabiscol E., Piulats E., Echave P., Herrero E., Ros J. (2000) J. Biol. Chem. 275, 27393–27398 [DOI] [PubMed] [Google Scholar]
- 15. Delaunay A., Isnard A. D., Toledano M. B. (2000) EMBO J. 19, 5157–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jang H. H., Lee K. O., Chi Y. H., Jung B. G., Park S. K., Park J. H., Lee J. R., Lee S. S., Moon J. C., Yun J. W., Choi Y. O., Kim W. Y., Kang J. S., Cheong G. W., Yun D. J., Rhee S. G., Cho M. J., Lee S. Y. (2004) Cell 117, 625–635 [DOI] [PubMed] [Google Scholar]
- 17. Arnér E. S., Holmgren A. (2000) Eur. J. Biochem. 267, 6102–6109 [DOI] [PubMed] [Google Scholar]
- 18. Kwast K. E., Burke P. V., Poyton R. O. (1998) J. Exp. Biol. 201, 1177–1195 [DOI] [PubMed] [Google Scholar]
- 19. Zitomer R. S., Lowry C. V. (1992) Microbiol. Rev. 56, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dirmeier R., O'Brien K. M., Engle M., Dodd A., Spears E., Poyton R. O. (2002) J. Biol. Chem. 277, 34773–34784 [DOI] [PubMed] [Google Scholar]
- 21. Marobbio C. M., Di Noia M. A., Palmieri F. (2006) Biochem. J. 393, 441–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bao G., Clifton M., Hoette T. M., Mori K., Deng S. X., Qiu A., Viltard M., Williams D., Paragas N., Leete T., Kulkarni R., Li X., Lee B., Kalandadze A., Ratner A. J., Pizarro J. C., Schmidt-Ott K. M., Landry D. W., Raymond K. N., Strong R. K., Barasch J. (2010) Nat. Chem. Biol. 6, 602–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Devireddy L. R., Hart D. O., Goetz D. H., Green M. R. (2010) Cell 141, 1006–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Froschauer E. M., Schweyen R. J., Wiesenberger G. (2009) Biochim. Biophys. Acta 1788, 1044–1050 [DOI] [PubMed] [Google Scholar]
- 25. Jensen L. T., Sanchez R. J., Srinivasan C., Valentine J. S., Culotta V. C. (2004) J. Biol. Chem. 279, 29938–29943 [DOI] [PubMed] [Google Scholar]
- 26. Radisky D. C., Babcock M. C., Kaplan J. (1999) J. Biol. Chem. 274, 4497–4499 [DOI] [PubMed] [Google Scholar]
- 27. Li J., Kogan M., Knight S. A., Pain D., Dancis A. (1999) J. Biol. Chem. 274, 33025–33034 [DOI] [PubMed] [Google Scholar]
- 28. Miao R., Kim H., Koppolu U. M., Ellis E. A., Scott R. A., Lindahl P. A. (2009) Biochemistry 48, 9556–9568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Naranuntarat A., Jensen L. T., Pazicni S., Penner-Hahn J. E., Culotta V. C. (2009) J. Biol. Chem. 284, 22633–22640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang M., Cobine P. A., Molik S., Naranuntarat A., Lill R., Winge D. R., Culotta V. C. (2006) EMBO J. 25, 1775–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kuge S., Jones N. (1994) EMBO J. 13, 655–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Trotter E. W., Grant C. M. (2005) Eukaryot. Cell 4, 392–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tachibana T., Okazaki S., Murayama A., Naganuma A., Nomoto A., Kuge S. (2009) J. Biol. Chem. 284, 4464–4472 [DOI] [PubMed] [Google Scholar]
- 34. Iwai K., Naganuma A., Kuge S. (2010) J. Biol. Chem. 285, 10597–10604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L., Kaplan J. (1997) J. Biol. Chem. 272, 28485–28493 [DOI] [PubMed] [Google Scholar]