Abstract
Background
Allergic eye diseases are complex inflammatory conditions of the conjunctiva that are becoming increasingly prevalent and present an increasing economic burden because of direct and indirect health expenditures.
Objective
We sought to identify factors that may synergize with antigen-induced allergic inflammation and lead to allergic conjunctivitis. We used a murine model of allergic conjunctivitis to test the effect of oxidative stress generated by pollen oxidases using nicotinamide adenine dinucleotide (reduced) or nicotinamide adenine dinucleotide phosphate (reduced) (NAD[P]H) as an electron donor present in pollen grains.
Methods
Reactive oxygen species (ROS) generation by hydrated Ambrosia artemisiifolia pollen (short ragweed pollen; RWP) grains was determined by using 2′-7′-dihydro-dichlorofluorescein diacetate, nitroblue tetrazolium reduction, and Amplex Red assay. The RWP-induced changes in intracellular ROS levels were examined in A549 cells, human primary bronchial epithelial cells, and murine conjunctiva.
Results
Ragweed pollen grains contain NAD(P)H oxidase activity, which is diphenyleneiodonium-sensitive and quinacrine-sensitive and sodium azide-resistant. These NAD(P)H oxidases generate a superoxide anion that can be converted to H2O2 by pollen grain–associated superoxide dismutase. These diffusible oxygen radicals from pollen grains increase intracellular ROS levels in cultured epithelial cells and murine conjunctiva. Similar phenomena were observed in sensitized and naive mice, indicating that the RWP-induced oxidative stress in conjunctival epithelium is independent of adaptive immunity. Inactivation of NAD(P)H oxidase activity in RWP decreases the immediate-type hypersensitivity and inflammatory cell infiltration into the conjunctiva.
Conclusion
Our data suggest that ROS generated by NAD(P)H oxidases in pollen grains intensify immediate allergic reactions and recruitment of inflammatory cells in murine conjunctiva.
Keywords: Pollen NAD(P)H oxidase, oxidative stress, epithelium, conjunctivitis
Seasonal allergic conjunctivitis, or hay fever, one of the most common allergic diseases, results in significant morbidity and presents an increasing economic burden because of direct health expenditures, as well as less evident cost factors, such as lost work time. The immediate hypersensitivity associated with this disease is characterized by allergen-mediated cross-linking of IgE on mast cells, leading to degranulation and release of mediators, including histamine, tryptase, leukotrienes, cytokines, and platelet-activating factors.1 These mediators stimulate nerve endings, dilate blood vessels, and recruit inflammatory cells to the reaction site, causing clinical symptoms1 such as itching, erythema, and palpebral and conjunctival edema. The late phase of this disease is associated with an accumulation of inflammatory cells in the conjunctiva.2
Antigenic components of pollen grains have been implicated in mediating allergic inflammation. However, recent studies have shown that pollens also release proteolytic enzymes3 and eicosanoid-like lipid mediators,4 which may influence the course of allergic reactions. Other evidence suggests that reactive oxygen species (ROS) play a prominent role in the pathogenesis of allergic diseases.5-9 In allergic rhinitis caused by house dust mites, nasal eosinophils generate H2O2, which causes tissue injury and augmentation of the allergic reaction.10 Another source of ROS is exposure to environmental air pollutants. Ozone, diesel exhaust, and cigarette smoke can generate oxidative stress in the airways and participate in the worsening of disease symptoms.11-13 An earlier report suggests the existence of a linear relationship between nasal symptoms and ozone levels during periods of high atmospheric pollen in patients with pollen allergy.14 Ozone exposure augments antigen-induced rhinitis, sneezing, nasal secretion, hyperresponsiveness, and eosinophil infiltration in guinea pigs.15
Our study reports for the first time that pollen grains contain oxidases using nicotinamide adenine dinucleotide (reduced) or nicotinamide adenine dinucleotide phosphate (reduced) (NAD[P]H) as an electron donor, which produce ROS and lead to oxidative stress in cultured human epithelial cells and murine conjunctiva. Inhibition of NAD(P)H oxidase activity significantly decreased clinical manifestations and late-phase events in a murine model of allergic conjunctivitis. Our data indicate that oxidative stress generated by NAD(P)H oxidase in pollen grains augments immediate-type hypersensitivity reactions and pollen antigen-driven allergic conjunctivitis.
METHODS
Cell cultures and pollen grains
Primary normal human bronchial epithelial (NHBE) cells (catalog #CC-2641) were cultured in BEGM BulletKit medium supplied by the manufacturer (Cambrex Bio Science, Walkersville, Md). The A549 bronchial epithelial cells (American Type Culture Collection, Manassas, Va) were cultured in Ham’s F-12 medium supplemented with 10% heat-inactivated FBS, L-glutamine (2 mmol/L), penicillin (100 U/mL), and streptomycin (100 μg/mL). Pollen grains from weeds (short/common ragweed, Ambrosia artemisiifolia; English plantain, Plantago lanceolata; redroot pigweed, Amaranthus retroflexus; Russian thistle, Salsola kali), grasses (Bermuda grass, Cynodon dactylon; redtop, Agrostis gigantea; timothy grass, Phleum pratense), and trees (white birch, Betula populifolia; white oak, Quercus alba) were purchased from Greer Laboratory (Lenoir, NC).
Animal sensitization and conjunctival challenge
Female BALB/c mice 6 to 8 weeks old from Harlan Sprague-Dawley (San Diego, Calif) were used for these studies. Mice were sensitized intraperitoneally on days 0 and 4 with 150 μg/mouse ragweed pollen extract (Greer Laboratory) mixed with alum, as previously described.16 On day 10, conjunctivitis was induced by topical application of 10 μg ragweed pollen (RWP), suspended in 5 μL PBS (pH 7.4), into each eye. Animal experiments were performed according to the National Institutes of Health Guide for Care and Use of Experimental Animals and approved by UTMB’s Animal Care and Use Committee.
Measurement of ROS
Pollen grains were hydrated in PBS for 10 minutes, and then 20 μmol/L 2′-7′-dihydro-dichlorofluorescein diacetate (H2DCF-DA; Molecular Probes, Eugene, Ore) was added.17,18 A change in dichlorofluorescein-mediated fluorescence intensity was assessed in a FLx800 micro plate reader (Bio-Tek Instruments, Winooski, Vt) at 488 nm excitation and 530 nm emission. In parallel experiments, dichlorofluorescein fluorescence was visualized by a NIKON Eclipse TE 200 UV microscope (excitation at 485 nm) (Lewisville, Tex). Images were taken with a Photometrix CoolSNAP Fx digital camera and analyzed with Metamorph software (version 5; Universal Imaging, Downingtown, Pa). NHBE or A549 were cells grown to 70% confluence and loaded with 50 μmol/L H2DCF-DA at 37°C for 15 minutes. After removing excess probe, the cells were exposed to RWP, heat-treated RWP (RWPH, 72°C for 30 min), RWP with superoxide dismutase (SOD; 50 U/mL), or RWP pretreated with Tiron (5 mmol/L; Sigma Inc, St Louis, Mo). The change in fluorescence intensity was assessed as described.
The conjunctival epithelia of BALB/c mice were topically loaded with 250 μmol/L carboxy–H2DCF-DA (Molecular Probes) for 15 minutes. After excess carboxy–H2DCF-DA was washed out, the conjunctiva of mice were challenged with 5 μL PBS, 100 μg RWP, or 100 μg RWP pretreated with Tiron (10 mmol/L) in 5 μL volume. After 15 minutes, mice were anesthetized, and the eyes were enucleated with the attached lids and intact conjunctiva. Tissues were embedded in optimal cutting temperature medium (Sakura Finetek, Torrance, Calif), frozen, and sectioned. Dichlorofluorescein fluorescence was analyzed as described.
Nitroblue tetrazolium assay
Pollen grains (100 μg/assay) were hydrated in PBS for 10 minutes and mixed with 2 mmol/L nitroblue tetrazolium (NBT) ± nicotinamide adenine dinucleotide (reduced) (NADH) (100 μmol/L) or nicotinamide adenine dinucleotide phosphate (reduced) (NADPH) (100 μmol/L). Mixtures were then incubated for 15 minutes at 37°C. NBT was completely removed by repeated washing steps, and the formazan precipitate was dissolved in methanol.19 Absorbance was determined at 530 nm (A530nm) on a spectrophotometer (DU 530; Beckman Instruments, Fullerton, Calif).
Measurement of hydrogen peroxide
The H2O2 level was measured spectrophotometrically at 560 nm (A560nm) by using an Amplex Red assay kit (Molecular Probes). The reaction mixture (100 μg pollen in 200 μL PBS + 100 μL Amplex Red reagent/horseradish peroxidase solution) was incubated at 37°C for 30 minutes before to the experiment. The H2O2 concentrations were calculated by comparison with assays of standard serial dilutions of H2O2. The addition of catalase (400 U/mL), but not SOD, decreased A560nm by ~90%.20
Clinical evaluation
Twenty minutes after the topical administration of RWP, animals were examined for signs of immediate hypersensitivity, and clinical scores (chemosis, conjunctival redness, lid edema, tearing, and discharge) were determined as previously described.21
Histology
The enucleated eyes with the attached lids and intact conjunctiva were immediately fixed in 10% buffered formaldehyde for 24 hours. The tissue was paraffin-embedded, serially sectioned through the central sagittal plane, and stained with Giemsa or hematoxylin and eosin. Three to 6 sections were made from each conjunctivum, and their identities were encoded and analyzed for inflammatory cell recruitment or mast cell degranulation, respectively, by an observer trained in pathology.
Statistics
Data were analyzed by ANOVA, followed by Fisher post hoc analyses for least significant difference. Differences were considered significant at P < .05.
RESULTS
Pollen grains produce ROS by intrinsic NAD(P)H oxidases
We tested the ability of RWP, which is one of the most important aeroallergens in North America, to generate ROS. Here we show that hydrated RWP grains converted the redox-sensitive 2′-7′-dihydro-dichlorofluorescein (H2DCF) into fluorescent dichlorofluorescein within 15 minutes, whereas RWPH did not (Fig 1). We also analyzed pollen grains from 42 different plant species by dichlorofluorescein assays, some of which are shown in Fig 2, A. Addition of H2DCF to these hydrated pollens induced intense fluorescence, indicating that they were able to produce ROS (data not shown), suggesting that ROS-inducing activity is universally present across many pollen grains.
FIG 1.
RWP grains oxidize H2DCF into fluorescent dichlorofluorescein. The dichlorofluorescein fluorescence of RWP suspension (RWP) and heat-treated RWP suspension (RWPH) was visualized by fluorescent microscopy. Right panels are phase-contrast images of pollen grains (100× magnification). Images are representative of 3 independent experiments.
FIG 2.

Generation of superoxide anion by various pollen grains. A, Reduction of NBT to formazan by pollen grains. ■, Pollen grain suspension; □, pollen grain suspension + NADH;  , pollen grain suspension + NADPH. B, Microscopic visualization of formazan deposits in RWP grains. Left, Hydrated RWP. Middle, Hydrated RWP + NBT. Right, Hydrated RWP + NBT + NADPH (100× magnification). C, H2O2 production in RWP grains detected by Amplex Red assay. *P < .05; **P < .01.
, pollen grain suspension + NADPH. B, Microscopic visualization of formazan deposits in RWP grains. Left, Hydrated RWP. Middle, Hydrated RWP + NBT. Right, Hydrated RWP + NBT + NADPH (100× magnification). C, H2O2 production in RWP grains detected by Amplex Red assay. *P < .05; **P < .01.
Reactive oxygen species is an umbrella term that includes different types of oxygen radicals, all of which can oxidize H2DCF with subsequent changes in fluorescence. Therefore, we sought to determine which particular oxygen radical was primarily involved in oxidizing H2DCF. RWP rapidly reduced NBT to formazan in a dose-dependent manner (data not shown), suggesting that it generates superoxide anions (O2·-). Pretreating RWP with the O2·- anion scavenger Tiron22 or SOD significantly decreased formation of formazan (Table I). Similarly, other tested pollens also reduced NBT to formazan; this activity strongly increased after the addition of excess NADH or NADPH (Fig 2, A and B). Pollen-mediated NBT reduction was inhibited by the NAD(P)H oxidase inhibitors diphenyleneiodonium and quinacrine,23 whereas azide, an inhibitor of plant peroxidases, had no effect,24-26 suggesting the presence of NAD(P)H oxidase in pollen grains.
TABLE I.
Superoxide anion generation by RWP grain
| Formazan production (A530 nm ± SD) |
||
|---|---|---|
| Inhibitors | Basal level | +NADH (100 μmol/L) |
| None | 0.119 ± 0.017 (100%) | 0.776 ± 0.045 (100%) |
| Tiron (100 μmol/L) | 0.107 ± 0.016 (90%) | 0.713 ± 0.017 (92%) |
| Tiron (1 mmol/L) | 0.059 ± 0.011 (50%)** | 0.481 ± 0.034 (62%)** |
| Tiron (10 mmol/L) | 0.043 ± 0.004 (36%)** | 0.263 ± 0.005 (34%)** |
| SOD (50 U/mL) | 0.085 ± 0.006 (71%)* | 0.541 ± 0.044 (70%)* |
| Quinacrine (5 mmol/L) | 0.089 ± 0.014 (75%)* | 0.495 ± 0.003 (64%)** |
| Diphenyleneiodium (100 μmol/L) | 0.061 ± 0.011 (51%)** | 0.425 ± 0.019 (55%)** |
| NaN3 (10 mmol/L) | 0.122 ± 0.013 (102%) | 0.807 ± 0.024 (104%) |
| NADPH (100 μmol/L) | 0.538 ± 0.029 (452%)**** | — |
P < .05;
P < .01;
P < .0001 vs control.
The O2·- anion is rapidly converted to H2O2, non-enzymatically or by SOD.27,28 Because pollen grains contain SOD,29 we performed an Amplex Red assay20 to detect H2O2 in the RWP suspension. As shown in Fig 2, C, RWP produced significant amounts of H2O2 that were further increased by addition of NADH or NADPH. Generation of H2O2 was inhibited by pretreatment of RWP with Tiron. Similar results were observed in suspensions of pollen grains from weeds, grasses, and trees (data not shown). These data suggest that NADPH oxidases in pollen grains generate O2·-, which is converted to H2O2 by intrinsic SOD activity.
Pollen grains induce oxidative stress in cultured epithelial cells and conjunctival epithelium
To determine whether reactive oxygen radicals generated by pollen NAD(P)H oxidases have an effect on intracellular ROS levels, we performed a series of experiments by using NHBE and A549 cells. Cells were loaded with H2DCF-DA, and changes in fluorescence intensity after RWP exposure were monitored fluorometrically or visualized by fluorescent microscopy. RWP increased the conversion of the intracellular H2DCF into fluorescent dichlorofluorescein in a dose-dependent manner in cultured NHBE cells, and it was augmented by addition of SOD to pollen grains (Fig 3, A). Pretreatment with Tiron, an O2·- scavenger, attenuated RWP-mediated dichlorofluorescein fluorescence intensity in the cell cultures (Fig 3, A). We also examined a change in dichlorofluorescein fluorescence in A549 cell cultures after RWP exposure by fluorescence microscopy. Superimposition of light and fluorescence images showed that oxidative stress occurred in epithelial cells that are in direct contact with pollen grains (Fig 3, B).
FIG 3.

Exposure to RWP grains increases ROS levels in cultured epithelial cells. A, Dose-dependent increase in dichlorofluorescein (DCF) fluorescence in RWP-exposed cultured primary NHBE cells. ■, RWP; □, RWP + SOD;  , RWP + Tiron. AU, Arbitrary units. B, Direct contact of pollen grains with A549 cells increases their intracellular ROS levels. Left, DCF fluorescence in the A549 cells. Middle, Phase-contrast images of cells and RWP grains. Right, Superimposed fluorescent and phase-contrast images (200×). *P < .05; **P < .01; ***P < .001.
, RWP + Tiron. AU, Arbitrary units. B, Direct contact of pollen grains with A549 cells increases their intracellular ROS levels. Left, DCF fluorescence in the A549 cells. Middle, Phase-contrast images of cells and RWP grains. Right, Superimposed fluorescent and phase-contrast images (200×). *P < .05; **P < .01; ***P < .001.
To study the effects of pollen NAD(P)H oxidases on intracellular ROS levels in vivo, conjunctival epithelia of naive or sensitized mice were loaded with H2DCF-DA and challenged with PBS or RWP. At 15 minutes postchallenge, eyes were enucleated, embedded in optimal cutting temperature medium, snap-frozen, sectioned, and analyzed by fluorescent microscopy. In contrast with PBS, RWP enhanced ROS levels in murine conjunctival epithelium (Fig 4). Pretreatment of RWP with Tiron blocked ROS production and the increase in intracellular ROS levels in conjunctival epithelium (Fig 4). Similarphenomena were observed in naive mice, which suggest that RWP-induced ROS generation in conjunctival epithelium is independent of adaptive immunity.
FIG 4.
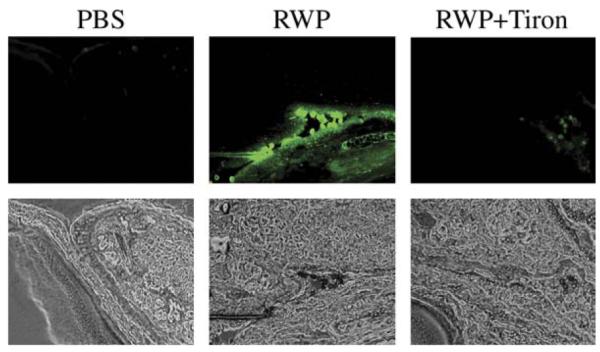
Change in ROS levels in murine conjunctiva after topical challenge with RWP grains. Upper panels, Dichlorofluorescein fluorescence in conjunctiva of mice challenged with PBS, RWP, or RWP + Tiron. Lower panels, Corresponding phase contrast images. Images are representative of serial sections from conjunctiva of 4 mice in each group.
RWP-induced oxidative stress augments allergic conjunctivitis in mice
To test whether the oxidative stress generated by pollen NAD(P)H oxidases is involved in an immediate response in allergic conjunctivitis, RWP-sensitized mice were challenged with PBS, RWP, RWPH, or RWP pretreated with Tiron. Allergic symptoms (chemosis, conjunctival redness, lid edema, tearing, and discharge) in these mice were assessed 20 minutes postchallenge as previously described21 and are presented here as a clinical score (Table II). Mice challenged with RWP had a mean clinical score of 8.7 ± 0.6 (mean ± SEM); this value was significantly decreased by pretreating the RWP with heat (2.2 ± 0.75) or Tiron (2.4 ± 0.5; Table II). To validate these findings, mice were challenged with RWPH plus surrogate O2·- generator hypoxanthine (X) and xanthine oxidase (XO). As shown in Table II, oxidative stress generated by X+XO augmented the clinical score in RWPH-treated mice from 2.2 ± 0.75 to 6.3 ± 0.5. However, administration of X+XO by itself failed to induce an immediate response in the eye (Table II). Taken together, these data show that oxidative stress generated by NAD(P)H oxidases in RWP augments antigen-induced early hypersensitivity and clinical symptoms in a murine model of allergic conjunctivitis.
TABLE II.
Clinical scores and mast cell degranulation
| Groups of sensitized BALB/c mice challenged with |
||||||
|---|---|---|---|---|---|---|
| PBS (n = 6) |
RWP (n = 6) |
RWPH (n = 6) |
RWPH + (X+XO) (n = 6) |
RWP + Tiron (n = 5) |
X+XO (n = 6) |
|
| Chemosis (mean ± SEM) | 0 | 1.7 ± 0.4** | 0.4 ± 0.2 | 1 ± 0.4** | 0.4 ± 0.2 | 0 |
| Conjunctival redness (mean ± SEM) |
0 | 2.3 ± 0.2*** | 0.5 ± 0.3 | 1 ± 0.3** | 0.6 ± 0.2* | 0 |
| Lid edema (mean ± SEM) | 0 | 2.2 ± 0.2*** | 0.3 ± 0.2 | 2 ± 0.3*** | 0.8 ± 0.3* | 0 |
| Tearing and scratching (mean ± SEM) |
0 | 2.5 ± 0.2*** | 1 ± 0.3** | 2.3 ± 0.2*** | 0.6 ± 0.2* | 0.5 ± 0.2 |
| Total score (mean ± SEM; the maximum possible total clinical score is 12) |
0 | 8.7 ± 0.6**** | 2.2 ± 0.75** | 6.3 ± 0.5**** | 2.4 ± 0.5** | 0.5 ± 0.2 |
| Mast cells in conjunctiva (cells/section, mean ± SEM) |
5.8 ± 0.1 | 5.7 ± 0.13 | 5.9 ± 0.27 | 6.1 ± 0.07 | 6.0 ± 0.1 | 5.8 ± 0.2 |
| Mast cell degranulation in conjunctiva (cells/section, mean ± SEM) |
0 | 5.1 ± 0.4**** | 1.5 ± 0.17** | 4.9 ± 0.13**** | 2.1 ± 0.07** | 0 |
P < .05;
P < .01;
P < .001;
P < .0001 vs PBS control.
Next, we investigated the effect of RWP-generated oxidative stress on mast cell degranulation and cellular infiltration into the conjunctiva. The best time points to examine early-phase (degranulation) and late-phase (eosinophil infiltration) events in the eyes of the animals were determined previously.21 The numbers of degranulating mast cells in conjunctival tissue sections were counted 3 hours after the challenge. Topical challenge with RWP induced mast cell degranulation that was significantly decreased in mice challenged with either RWPH or RWP pretreated with Tiron (Fig 5; Table II). Coadministration of X+XO with RWPH increased the number of degranulated mast cells. Even though the number of mast cells was similar across all treatment groups, mast cell degranulation was significantly higher after treatments that induced ROS (RWP, RWPH + [X+XO]; Table II).
FIG 5.
Effects of RWP-induced oxidative stress on mast cell degranulation. Mice were topically challenged with PBS, RWP, or RWPH. The mice were killed 3 hours postchallenge, their eyes were enucleated and processed, and sections were stained with Giemsa. Images are representative of serial sections from conjunctivum of 5 to 6 mice in each group.
Conjunctival inflammatory cell infiltration was examined 48 hours after topical application of RWP suspension. This challenge induced robust recruitment of inflammatory cells, predominantly eosinophils, into the conjunctiva (Fig 6). In contrast, there was less inflammatory cell infiltration in mice challenged with RWPH (Fig 6) or RWP pretreated with Tiron (data not shown). Coadministration of X+XO with RWPH restored eosinophil recruitment into the conjunctiva (Fig 6). These findings suggest that RWP-induced ROS augments mast cell degranulation and inflammatory cell recruitment into conjunctiva, correlating with the clinical signs.
FIG 6.
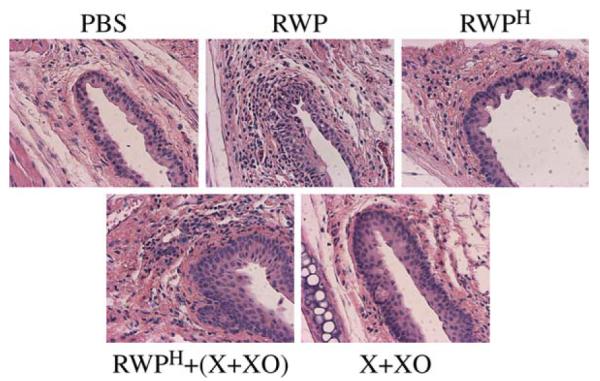
Oxidative stress generated by RWP augments inflammatory cell recruitment in murine conjunctiva. Mice were topically challenged with PBS, RWP, RWPH, RWPH + (X+XO), or (X+XO), and killed 48 hours later. Their eyes were removed and processed, and sections were stained with hematoxylin and eosin. Images are representative of serial sections from conjunctivum of 6 mice in each group.
DISCUSSION
Seasonal allergic conjunctivitis is a Type 1 hypersensitivity reaction induced by airborne allergens. Here we show for the first time that in addition to the antigenic components, ROS-generating NAD(P)H oxidases in pollen grains have a significant role in the development and augmentation of allergic conjunctivitis. Pollen grains are complex biological entities with the important function of fertilizing the female gametophyte. Because O2·- and H2O2 play a critical role in such vital physiological functions as seed germination24,30 and cell expansion,31,32 we hypothesized that ROS generation is among the first events during pollen hydration. Consistent with our hypothesis, we found that all hydrated pollens tested can generate ROS. By using an in vitro NBT assay, we identified O2·- as the primary reactive radical generated in pollens.
Previous studies indicate that plant cells have plasma membrane oxidases that catalyze 1-electron reduction of molecular oxygen to O2·-, in a manner similar to the action the NADPH oxidases in the plasma membrane of mammalian phagocytes.19,23,33 These superoxide-generating enzymes in plant cells use NADH or NADPH as an electron donor, contrary to the phagocyte NAD(P)H oxidase, which is characterized by a nearly absolute specificity to NADPH.24 Because O2·- generation of pollens increased in the presence of excess NADH or NADPH,26 and the latter were sensitive to inhibitors of the mammalian NADPH oxidase, we concluded that NAD(P)H oxidases are involved in O2·- production in pollen. The superoxide anion has a limited ability to penetrate membranes at physiological pH.27 However, we were able to detect H2O2 resulting from the superoxide dismutase activity of pollen grains. H2O2 diffuses readily through cell membrane bilayers and can increase intracellular ROS levels in cultured epithelial cells and conjunctival epithelia of mice within minutes after exposure.
Mast cells play a central role in the early phase of allergic reactions. Cross-linking of high-affinity IgE receptors (FceRI) by IgE causes mast cell degranulation and releases various inflammatory mediators, including histamine.34 These mediators are responsible for early-phase allergic reactions and clinical symptoms such as itching, redness, and swelling of conjunctiva. These mediators also perpetuate late-phase inflammation and recruitment of inflammatory cells. Exposure to O2·- or H2O2 induces histamine release by rat peritoneal mast cells in vitro.35,36 Other studies showed that increased oxidative stress augments, whereas antioxidants significantly decrease, mast cell degranulation, and consequently the plasma levels of histamine and tryptase.37-40 Systemic hypoxia, which results in an ROS burst, also results in mast cell degranulation via oxidative burst, which is inhibited by the antioxidant lipoic acid, suggesting involvement of ROS in mast cell activation.41
We presume that ROS produced by pollen NAD(P)H oxidases together with pollen antigens are important in stimulation of mast cell degranulation in the conjunctiva. The RWPH was significantly less effective in provoking symptoms of allergic conjunctivitis because heat treatment destroys the NAD(P)H oxidases in pollens. Heat inactivation may also have denatured antigenic epitopes on proteins, so they may not bind IgE. To exclude this possibility, we show that coadministration of an extrinsic O2·- generator (X+XO) with RWPH restored the ability of the pollen grains to induce an immediate response in the eyes, even though administration of X+XO by itself failed to induce allergic symptoms. On the other hand, pretreating of pollens with the superoxide scavenger Tiron significantly decreased the mast cell degranulation. To the best of our knowledge, Tiron does not inhibit proteases or eicosanoid-like lipid mediators and does not affect the antigenicity of proteins.42-44
We also investigated the effect of oxidative stress on the late-phase consequences of allergic conjunctivitis in sensitized mice. We observed a marked inflammatory cell influx in conjunctiva, consisting predominantly of eosinophils. Eosinophils secrete cytotoxic proteins, including major basic protein, cationic protein, eosinophil-derived neurotoxin, and eosinophil peroxidase, all of which have been shown to aggravate allergic inflammation.45 Our data show that inactivation of pollens’ NAD(P)H oxidase by heat treatment, or scavenging O2·- by Tiron, significantly decreased the accumulation of inflammatory cells into the conjunctiva.
Collectively, these data add a new dimension to our current understanding of the mechanisms of ocular allergy. Our findings suggest that RWP contains NAD(P)H oxidases that can generate ROS in conjunctival epithelium and cause mast cell degranulation. This initial event is independent of an adaptive immune response, as suggested by experiments showing no difference in ROS levels between naive and sensitized mice exposed to RWP. This early-phase oxidative insult contributes to the development of late-phase responses of allergic conjunctivitis, as evidenced by increased inflammatory cell recruitment in RWP-exposed mice. It is likely that inhibition of the initial oxidative insult has a beneficial effect on both immediate symptoms and late-phase allergic inflammation in the conjunctiva. Future experiments will test our hypothesis in the human disease and explore the potential therapeutic benefits of our discovery.
Acknowledgments
Supported by National Institutes of Health grants (R01-HL071163, P01 AI46004, Dr Sur; and CA84461, Dr Boldogh), a University of Texas Medical Branch pilot project grant, Galveston, Tex, and a National Institute of Environmental Health and Sciences Center grant (ES06676, Dr Boldogh).
We thank David Konkel, PhD (Human Biological Chemistry and Genetics, UTMB), and Michael Lett-Brown, PhD (Department of Internal Medicine, Allergy Division, UTMB), for their helpful scientific discussions. Special thanks to Daniel Liebenthal for maintaining and providing cell cultures and to Mardelle Susman for editorial help in assembling the manuscript.
Abbreviations used
- H2DCF-DA
2′-7′-Dihydro-dichlorofluorescein diacetate
- NADH
nicotinamide adenine dinucleotide, reduced
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced
- NAD(P)H
NADH or NADPH
- NBT
Nitroblue tetrazolium
- NHBE
Primary normal human bronchial epithelial
- O2·-
Superoxide anion
- ROS
Reactive oxygen species
- RWP
Ragweed pollen
- RWPH
Heat-treated ragweed pollen
- SOD
Superoxide dismutase
- X+XO
Hypoxanthine and xanthine oxidase
REFERENCES
- 1.Leonardi A. Pathophysiology of allergic conjunctivitis. Acta Ophthalmol Scand. 1999;228(Suppl):21–3. doi: 10.1111/j.1600-0420.1999.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 2.Allansmith MR, Ross RN. Ocular allergy. Clin Allergy. 1988;18:1–13. doi: 10.1111/j.1365-2222.1988.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 3.Bagarozzi DA, Jr, Travis J. Ragweed pollen proteolytic enzymes: possible roles in allergies and asthma. Phytochemistry. 1998;47:593–8. doi: 10.1016/s0031-9422(97)00634-1. [DOI] [PubMed] [Google Scholar]
- 4.Traidl-Hoffmann C, Kasche A, Jakob T, Huger M, Plotz S, Feussner I, et al. Lipid mediators from pollen act as chemoattractants and activators of polymorphonuclear granulocytes. J Allergy Clin Immunol. 2002;109:831–8. doi: 10.1067/mai.2002.124655. [DOI] [PubMed] [Google Scholar]
- 5.Bowler RP, Crapo JD. Oxidative stress in allergic respiratory diseases. J Allergy Clin Immunol. 2002;110:349–56. doi: 10.1067/mai.2002.126780. [DOI] [PubMed] [Google Scholar]
- 6.Calhoun WJ, Reed HE, Moest DR, Stevens CA. Enhanced superoxide production by alveolar macrophages and air-space cells, airway inflammation, and alveolar macrophage density changes after segmental antigen bronchoprovocation in allergic subjects. Am Rev Respir Dis. 1992;145:317–25. doi: 10.1164/ajrccm/145.2_Pt_1.317. [DOI] [PubMed] [Google Scholar]
- 7.Sanders SP, Zweier JL, Harrison SJ, Trush MA, Rembish SJ, Liu MC. Spontaneous oxygen radical production at sites of antigen challenge in allergic subjects. Am J Respir Crit Care Med. 1995;151:1725–33. doi: 10.1164/ajrccm.151.6.7767513. [DOI] [PubMed] [Google Scholar]
- 8.Evans DJ, Lindsay MA, O’Connor BJ, Barnes PJ. Priming of circulating human eosinophils following late response to allergen challenge. Eur Respir J. 1996;9:703–8. doi: 10.1183/09031936.96.09040703. [DOI] [PubMed] [Google Scholar]
- 9.Vachier I, Chanez P, Le Doucen C, Damon M, Descomps B, Godard P. Enhancement of reactive oxygen species formation in stable and unstable asthmatic patients. Eur Respir J. 1994;7:1585–92. doi: 10.1183/09031936.94.07091585. [DOI] [PubMed] [Google Scholar]
- 10.Ogasawara H, Yoshimura S, Kumoi T. Hydrogen peroxide generation by eosinophils in allergic rhinitis. Auris Nasus Larynx. 1991;18:133–43. doi: 10.1016/s0385-8146(12)80217-3. [DOI] [PubMed] [Google Scholar]
- 11.Michalec L, Choudhury BK, Postlethwait E, Wild JS, Alam R, Lett-Brown M, et al. CCL7 and CXCL10 orchestrate oxidative stress-induced neutrophilic lung inflammation. J Immunol. 2002;168:846–52. doi: 10.4049/jimmunol.168.2.846. [DOI] [PubMed] [Google Scholar]
- 12.Comhair SA, Thomassen MJ, Erzurum SC. Differential induction of extracellular glutathione peroxidase and nitric oxide synthase 2 in airways of healthy individuals exposed to 100% O(2) or cigarette smoke. Am J Respir Cell Mol Biol. 2000;23:350–4. doi: 10.1165/ajrcmb.23.3.4076. [DOI] [PubMed] [Google Scholar]
- 13.Jung M, Davis WP, Taatjes DJ, Churg A, Mossman BT. Asbestos and cigarette smoke cause increased DNA strand breaks and necrosis in bronchiolar epithelial cells in vivo. Free Radic Biol Med. 2000;28:1295–9. doi: 10.1016/s0891-5849(00)00211-2. [DOI] [PubMed] [Google Scholar]
- 14.Riediker M, Monn C, Koller T, Stahel WA, Wuthrich B. Air pollutants enhance rhinoconjunctivitis symptoms in pollen-allergic individuals. Ann Allergy Asthma Immunol. 2001;87:311–8. doi: 10.1016/S1081-1206(10)62246-6. [DOI] [PubMed] [Google Scholar]
- 15.Iijima MK, Kobayashi T, Kamada H, Shimojo N. Exposure to ozone aggravates nasal allergy-like symptoms in guinea pigs. Toxicol Lett. 2001;123:77–85. doi: 10.1016/s0378-4274(01)00392-7. [DOI] [PubMed] [Google Scholar]
- 16.Sur S, Kita H, Gleich GJ, Chenier TC, Hunt LW. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J Allergy Clin Immunol. 1996;97:1272–8. doi: 10.1016/s0091-6749(96)70195-1. [DOI] [PubMed] [Google Scholar]
- 17.Sweetlove LJ, Heazlewood JL, Herald V, Holtzapffel R, Day DA, Leaver CJ, et al. The impact of oxidative stress on Arabidopsis mitochondria. Plant J. 2002;32:891–904. doi: 10.1046/j.1365-313x.2002.01474.x. [DOI] [PubMed] [Google Scholar]
- 18.Boldogh I, Roy G, Lee MS, Bacsi A, Hazra TK, Bhakat KK, et al. Reduced DNA double strand breaks in chlorambucil resistant cells are related to high DNA-PKcs activity and low oxidative stress. Toxicology. 2003;193:137–52. doi: 10.1016/j.tox.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Murphy TM, Vu H, Nguyen T. The superoxide synthases of rose cells: comparison of assays. Plant Physiol. 1998;117:1301–5. doi: 10.1104/pp.117.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland RP. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–8. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- 21.Magone MT, Chan CC, Rizzo LV, Kozhich AT, Whitcup SM. A novel murine model of allergic conjunctivitis. Clin Immunol Immunopathol. 1998;87:75–84. doi: 10.1006/clin.1997.4507. [DOI] [PubMed] [Google Scholar]
- 22.Zuo L, Christofi FL, Wright VP, Liu CY, Merola AJ, Berliner LJ, et al. Intra- and extracellular measurement of reactive oxygen species produced during heat stress in diaphragm muscle. Am J Physiol Cell Physiol. 2000;279:C1058–66. doi: 10.1152/ajpcell.2000.279.4.C1058. [DOI] [PubMed] [Google Scholar]
- 23.Van Gestelen P, Asard H, Caubergs RJ. Solubilization and separation of a plant plasma membrane NADPH-O2-synthase from other NAD(P)H oxidoreductases. Plant Physiol. 1997;115:543–50. doi: 10.1104/pp.115.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frahry G, Schopfer P. NADH-stimulated, cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta. 2001;212:175–83. doi: 10.1007/s004250000376. [DOI] [PubMed] [Google Scholar]
- 25.Bolwell GP, Butt VS, Davies DR, Zimmerlin A. The origin of the oxidative burst in plants. Free Radic Res. 1995;23:517–32. doi: 10.3109/10715769509065273. [DOI] [PubMed] [Google Scholar]
- 26.Papadakis AK, Roubelakis-Angelakis KA. The generation of active oxygen species differs in tobacco and grapevine mesophyll protoplasts. Plant Physiol. 1999;121:197–206. doi: 10.1104/pp.121.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Messner KR, Imlay JA. In vitro quantitation of biological superoxide and hydrogen peroxide generation. Methods Enzymol. 2002;349:354–61. doi: 10.1016/s0076-6879(02)49351-2. [DOI] [PubMed] [Google Scholar]
- 28.Wojtaszek P. Oxidative burst: an early plant response to pathogen infection. Biochem J. 1997;322:681–92. doi: 10.1042/bj3220681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalinowski A, Radlowski M, Bartkowiak S. Maize pollen enzymes after two-dimensional polyacrylamide gel electrophoresis in the presence or absence of sodium dodecyl sulfate. Electrophoresis. 2002;23:138–43. doi: 10.1002/1522-2683(200201)23:1<138::AID-ELPS138>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 30.Schopfer P, Plachy C, Frahry G. Release of reactive oxygen intermediates (superoxide radicals, hydrogen peroxide, and hydroxyl radicals) and peroxidase in germinating radish seeds controlled by light, gibberellin, and abscisic acid. Plant Physiol. 2001;125:1591–602. doi: 10.1104/pp.125.4.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez AA, Grunberg KA, Taleisnik EL. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol. 2002;129:1627–32. doi: 10.1104/pp.001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–6. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- 33.Doke N, Miura Y, Sanchez LM, Park HJ, Noritake T, Yoshioka H, et al. The oxidative burst protects plants against pathogen attack: mechanism and role as an emergency signal for plant bio-defence—a review. Gene. 1996;179:45–51. doi: 10.1016/s0378-1119(96)00423-4. [DOI] [PubMed] [Google Scholar]
- 34.Williams CM, Galli SJ. The diverse potential effector and immunoregulatory roles of mast cells in allergic disease. J Allergy Clin Immunol. 2000;105:847–59. doi: 10.1067/mai.2000.106485. [DOI] [PubMed] [Google Scholar]
- 35.Akagi M, Katakuse Y, Fukuishi N, Kan T, Akagi R. Superoxide anion-induced histamine release from rat peritoneal mast cells. Biol Pharm Bull. 1994;17:732–4. doi: 10.1248/bpb.17.732. [DOI] [PubMed] [Google Scholar]
- 36.Wolfreys K, Oliveira DB. Alterations in intracellular reactive oxygen species generation and redox potential modulate mast cell function. Eur J Immunol. 1997;27:297–306. doi: 10.1002/eji.1830270143. [DOI] [PubMed] [Google Scholar]
- 37.Peden DB, Dailey L, DeGraff W, Mitchell JB, Lee JG, Kaliner MA, et al. Hydrogen peroxide effects on rat mast cell function. Am J Physiol. 1994;267:L85–93. doi: 10.1152/ajplung.1994.267.1.L85. [DOI] [PubMed] [Google Scholar]
- 38.Yoshimaru T, Suzuki Y, Matsui T, Yamashita K, Ochiai T, Yamaki M, et al. Blockade of superoxide generation prevents high-affinity immunoglobulin E receptor-mediated release of allergic mediators by rat mast cell line and human basophils. Clin Exp Allergy. 2002;32:612–8. doi: 10.1046/j.0954-7894.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- 39.Santos FX, Arroyo C, Garcia I, Blasco R, Obispo JM, Hamann C, et al. Role of mast cells in the pathogenesis of postburn inflammatory response: reactive oxygen species as mast cell stimulators. Burns. 2000;26:145–7. doi: 10.1016/s0305-4179(99)00021-2. [DOI] [PubMed] [Google Scholar]
- 40.Laberge S, Pinsonneault S, Ernst P, Olivenstein R, Ghaffar O, Center DM, et al. Phenotype of IL-16-producing cells in bronchial mucosa: evidence for the human eosinophil and mast cell as cellular sources of IL-16 in asthma. Int Arch Allergy Immunol. 1999;119:120–5. doi: 10.1159/000024186. [DOI] [PubMed] [Google Scholar]
- 41.Steiner DR, Gonzalez NC, Wood JG. Mast cells mediate the microvascular inflammatory response to systemic hypoxia. J Appl Physiol. 2003;94:325–34. doi: 10.1152/japplphysiol.00637.2002. [DOI] [PubMed] [Google Scholar]
- 42.Matsuda F, Miyagawa H, Ueno T. Involvement of reactive oxygen species in the induction of (S)-N-p-coumaroyloctopamine accumulation by beta-1,3-glucooligosaccharide elicitors in potato tuber tissues. Z Naturforsch [C] 2001;56:228–34. doi: 10.1515/znc-2001-3-410. [DOI] [PubMed] [Google Scholar]
- 43.Watkins MT, Patton GM, Soler HM, Albadawi H, Humphries DE, Evans JE, et al. Synthesis of 8-epi-prostaglandin F2alpha by human endothelial cells: role of prostaglandin H2 synthase. Biochem J. 1999;344:747–54. [PMC free article] [PubMed] [Google Scholar]
- 44.Dechend R, Viedt C, Muller DN, Ugele B, Brandes RP, Wallukat G, et al. AT1 receptor agonistic antibodies from preeclamptic patients stimulate NADPH oxidase. Circulation. 2003;107:1632–9. doi: 10.1161/01.CIR.0000058200.90059.B1. [DOI] [PubMed] [Google Scholar]
- 45.Martin LB, Kita H, Leiferman KM, Gleich GJ. Eosinophils in allergy: role in disease, degranulation, and cytokines. Int Arch Allergy Immunol. 1996;109:207–15. doi: 10.1159/000237239. [DOI] [PubMed] [Google Scholar]




