Abstract
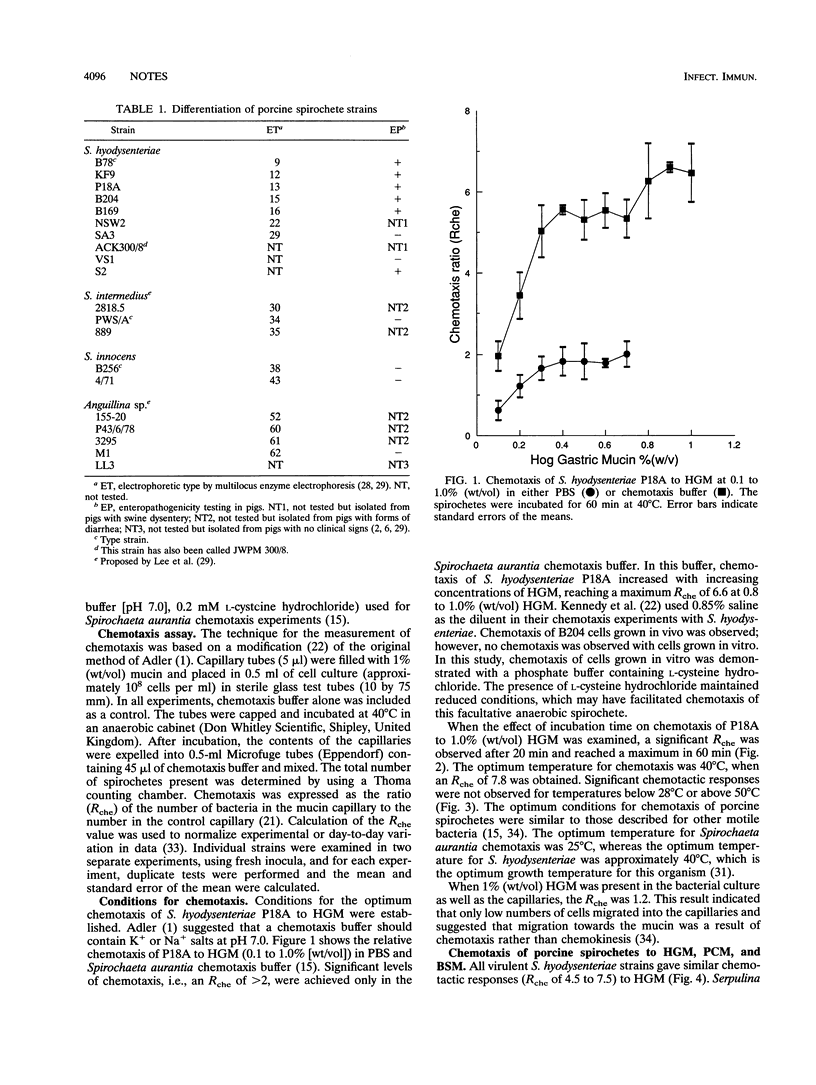
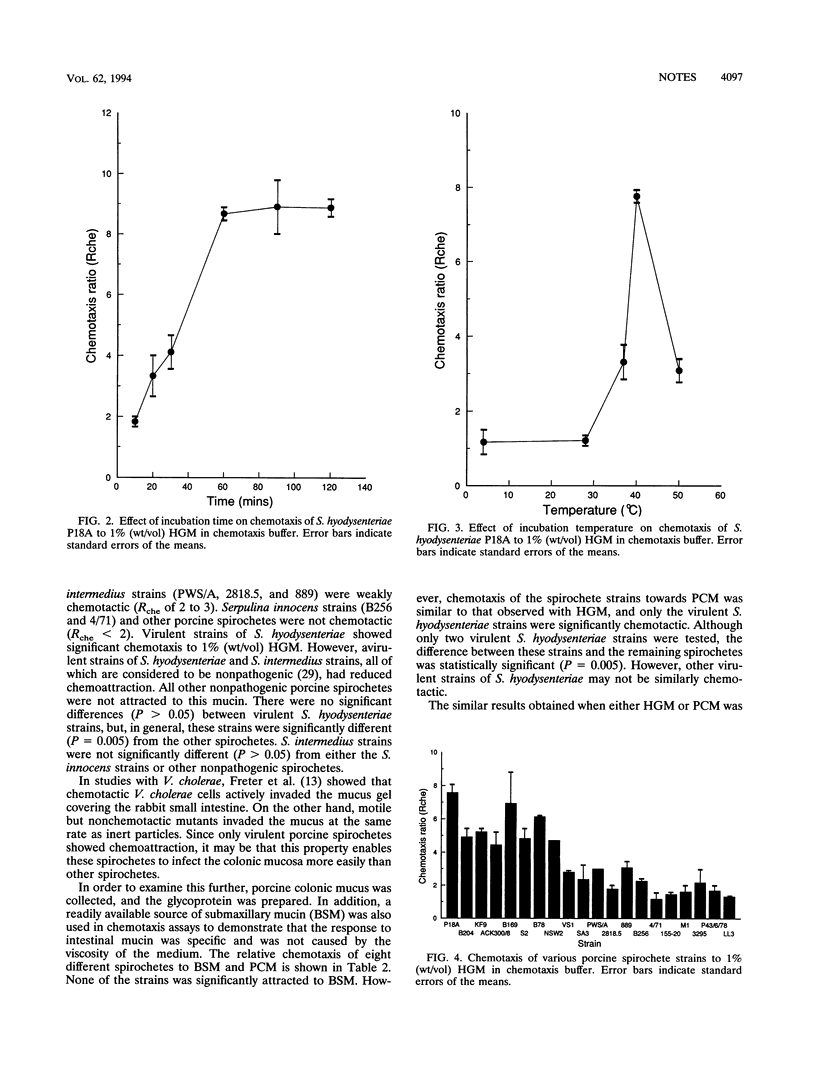
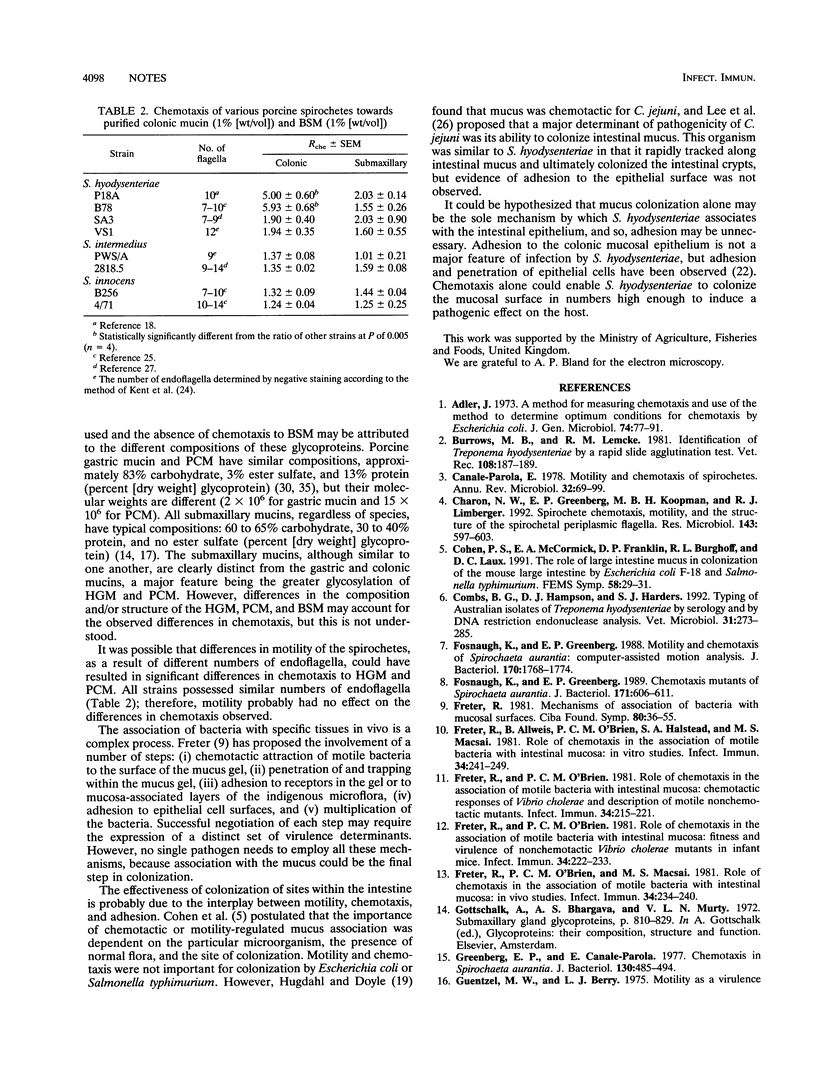
Chemotaxis of porcine spirochetes towards a variety of mucins was measured quantitatively by a capillary method. A chemotaxis buffer consisting of 0.01 M potassium phosphate buffer (pH 7.0) and 0.2 mM L-cysteine hydrochloride was necessary for chemotaxis of spirochetes. The optimum incubation time and incubation temperature were 1 h and 40 degrees C, respectively. The mucin concentration also affected the chemotaxis observed, and a concentration of 1% (wt/vol) was near the optimum. Virulent Serpulina hyodysenteriae strains were chemotactic towards 1% (wt/vol) hog gastric mucin and 1% (wt/vol) porcine colonic mucin but not towards 1% (wt/vol) bovine submaxillary mucin. Virulent S. hyodysenteriae strains were significantly more chemotactic than avirulent strains of S. hyodysenteriae (SA3 and VS1), Serpulina intermedius, and Serpulina innocens. Other spirochetes belonging to the proposed group of spirochetes Anguillina coli were also not chemotactic. Pathogenicity of S. hyodysenteriae strains that cause swine dysentery may, in part, be attributed to their attraction to porcine intestinal mucus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Burrows M. R., Lemcke R. M. Identification of Treponema hyodysenteriae by a rapid slide agglutination test. Vet Rec. 1981 Feb 28;108(9):187–189. doi: 10.1136/vr.108.9.187. [DOI] [PubMed] [Google Scholar]
- Canale-Parola E. Motility and chemotaxis of spirochetes. Annu Rev Microbiol. 1978;32:69–99. doi: 10.1146/annurev.mi.32.100178.000441. [DOI] [PubMed] [Google Scholar]
- Charon N. W., Greenberg E. P., Koopman M. B., Limberger R. J. Spirochete chemotaxis, motility, and the structure of the spirochetal periplasmic flagella. Res Microbiol. 1992 Jul-Aug;143(6):597–603. doi: 10.1016/0923-2508(92)90117-7. [DOI] [PubMed] [Google Scholar]
- Combs B. G., Hampson D. J., Harders S. J. Typing of Australian isolates of Treponema hyodysenteriae by serology and by DNA restriction endonuclease analysis. Vet Microbiol. 1992 Jun 1;31(2-3):273–285. doi: 10.1016/0378-1135(92)90085-8. [DOI] [PubMed] [Google Scholar]
- Fosnaugh K., Greenberg E. P. Chemotaxis mutants of Spirochaeta aurantia. J Bacteriol. 1989 Jan;171(1):606–611. doi: 10.1128/jb.171.1.606-611.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosnaugh K., Greenberg E. P. Motility and chemotaxis of Spirochaeta aurantia: computer-assisted motion analysis. J Bacteriol. 1988 Apr;170(4):1768–1774. doi: 10.1128/jb.170.4.1768-1774.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R. Mechanisms of association of bacteria with mucosal surfaces. Ciba Found Symp. 1981;80:36–55. doi: 10.1002/9780470720639.ch4. [DOI] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: chemotactic responses of Vibrio cholerae and description of motile nonchemotactic mutants. Infect Immun. 1981 Oct;34(1):215–221. doi: 10.1128/iai.34.1.215-221.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect Immun. 1981 Oct;34(1):222–233. doi: 10.1128/iai.34.1.222-233.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg E. P., Canale-Parola E. Chemotaxis in Spirochaeta aurantia. J Bacteriol. 1977 Apr;130(1):485–494. doi: 10.1128/jb.130.1.485-494.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentzel M. N., Berry L. J. Motility as a virulence factor for Vibrio cholerae. Infect Immun. 1975 May;11(5):890–897. doi: 10.1128/iai.11.5.890-897.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill H. D., Jr, Reynolds J. A., Hill R. L. Purification, composition, molecular weight, and subunit structure of ovine submaxillary mucin. J Biol Chem. 1977 Jun 10;252(11):3791–3798. [PubMed] [Google Scholar]
- Hovind-Hougen K., Høgh P., Birch-Andersen A. Morphological heterogeneity among spirochetes isolated from cases of swine dysentery. Zentralbl Bakteriol. 1990 Oct;274(1):1–15. doi: 10.1016/s0934-8840(11)80970-9. [DOI] [PubMed] [Google Scholar]
- Kennedy M. J., Lawless J. G. Role of chemotaxis in the ecology of denitrifiers. Appl Environ Microbiol. 1985 Jan;49(1):109–114. doi: 10.1128/aem.49.1.109-114.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. J., Rosnick D. K., Ulrich R. G., Yancey R. J., Jr Association of Treponema hyodysenteriae with porcine intestinal mucosa. J Gen Microbiol. 1988 Jun;134(6):1565–1576. doi: 10.1099/00221287-134-6-1565. [DOI] [PubMed] [Google Scholar]
- Kent K. A., Lemcke R. M., Lysons R. J. Production, purification and molecular weight determination of the haemolysin of Treponema hyodysenteriae. J Med Microbiol. 1988 Nov;27(3):215–224. doi: 10.1099/00222615-27-3-215. [DOI] [PubMed] [Google Scholar]
- Kent K. A., Sellwood R., Lemcke R. M., Burrows M. R., Lysons R. J. Analysis of the axial filaments of Treponema hyodysenteriae by SDS-PAGE and immunoblotting. J Gen Microbiol. 1989 Jun;135(6):1625–1632. doi: 10.1099/00221287-135-6-1625. [DOI] [PubMed] [Google Scholar]
- Lee A., O'Rourke J. L., Barrington P. J., Trust T. J. Mucus colonization as a determinant of pathogenicity in intestinal infection by Campylobacter jejuni: a mouse cecal model. Infect Immun. 1986 Feb;51(2):536–546. doi: 10.1128/iai.51.2.536-546.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. I., Hampson D. J., Combs B. G., Lymbery A. J. Genetic relationships between isolates of Serpulina (Treponema) hyodysenteriae, and comparison of methods for their subspecific differentiation. Vet Microbiol. 1993 Jan;34(1):35–46. doi: 10.1016/0378-1135(93)90005-r. [DOI] [PubMed] [Google Scholar]
- Lee J. I., Hampson D. J., Lymbery A. J., Harders S. J. The porcine intestinal spirochaetes: identification of new genetic groups. Vet Microbiol. 1993 Mar;34(3):273–285. doi: 10.1016/0378-1135(93)90017-2. [DOI] [PubMed] [Google Scholar]
- Marshall T., Allen A. The isolation and characterization of the high-molecular-weight glycoprotein from pig colonic mucus. Biochem J. 1978 Aug 1;173(2):569–578. doi: 10.1042/bj1730569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morooka T., Umeda A., Amako K. Motility as an intestinal colonization factor for Campylobacter jejuni. J Gen Microbiol. 1985 Aug;131(8):1973–1980. doi: 10.1099/00221287-131-8-1973. [DOI] [PubMed] [Google Scholar]
- Moulton R. C., Montie T. C. Chemotaxis by Pseudomonas aeruginosa. J Bacteriol. 1979 Jan;137(1):274–280. doi: 10.1128/jb.137.1.274-280.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paster B. J., Gibbons R. J. Chemotactic response to formate by Campylobacter concisus and its potential role in gingival colonization. Infect Immun. 1986 May;52(2):378–383. doi: 10.1128/iai.52.2.378-383.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scawen M., Allen A. The action of proteolytic enzymes on the glycoprotein from pig gastric mucus. Biochem J. 1977 May 1;163(2):363–368. doi: 10.1042/bj1630363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz L. C., Inoue T., Irimura T., Damen J. E., Greenberg A. H., Wright J. A. Relationships between heparanase activity and increasing metastatic potential of fibroblasts transfected with various oncogenes. Cancer Lett. 1990 Jun 15;51(3):187–192. doi: 10.1016/0304-3835(90)90101-3. [DOI] [PubMed] [Google Scholar]
- Yuri K., Takamoto Y., Okada M., Hiramune T., Kikuchi N., Yanagawa R. Chemotaxis of leptospires to hemoglobin in relation to virulence. Infect Immun. 1993 May;61(5):2270–2272. doi: 10.1128/iai.61.5.2270-2272.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]


