Abstract
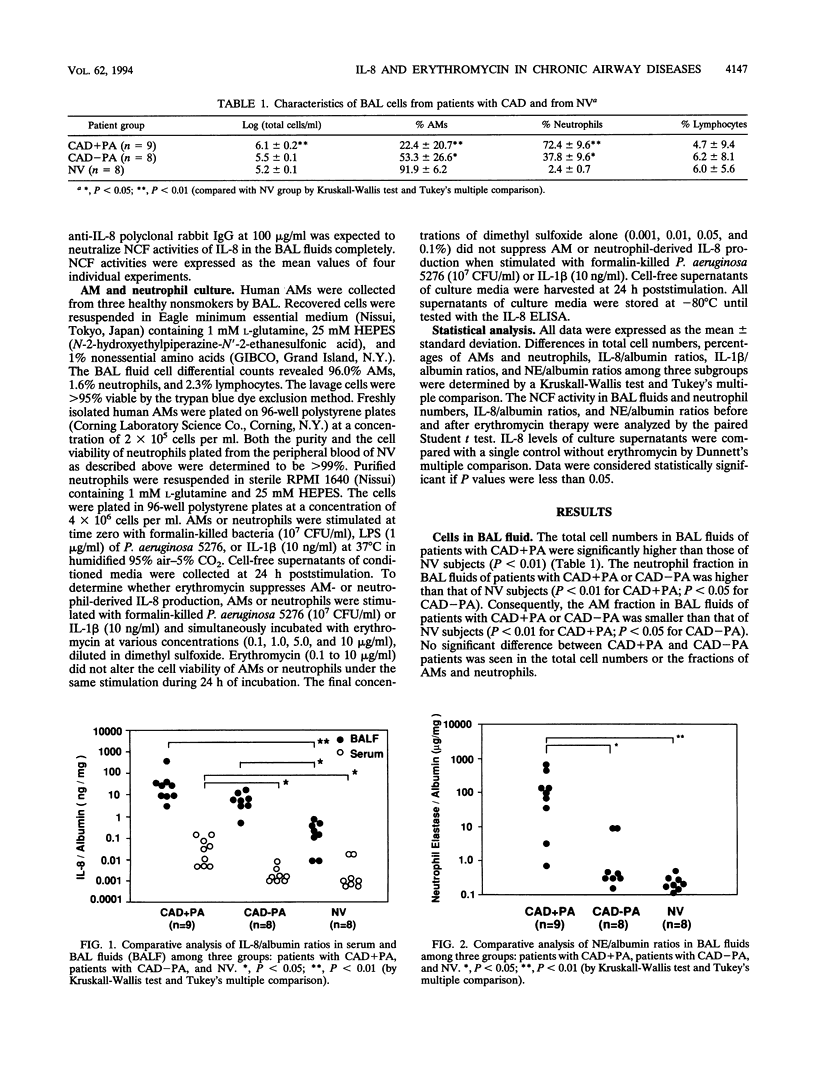
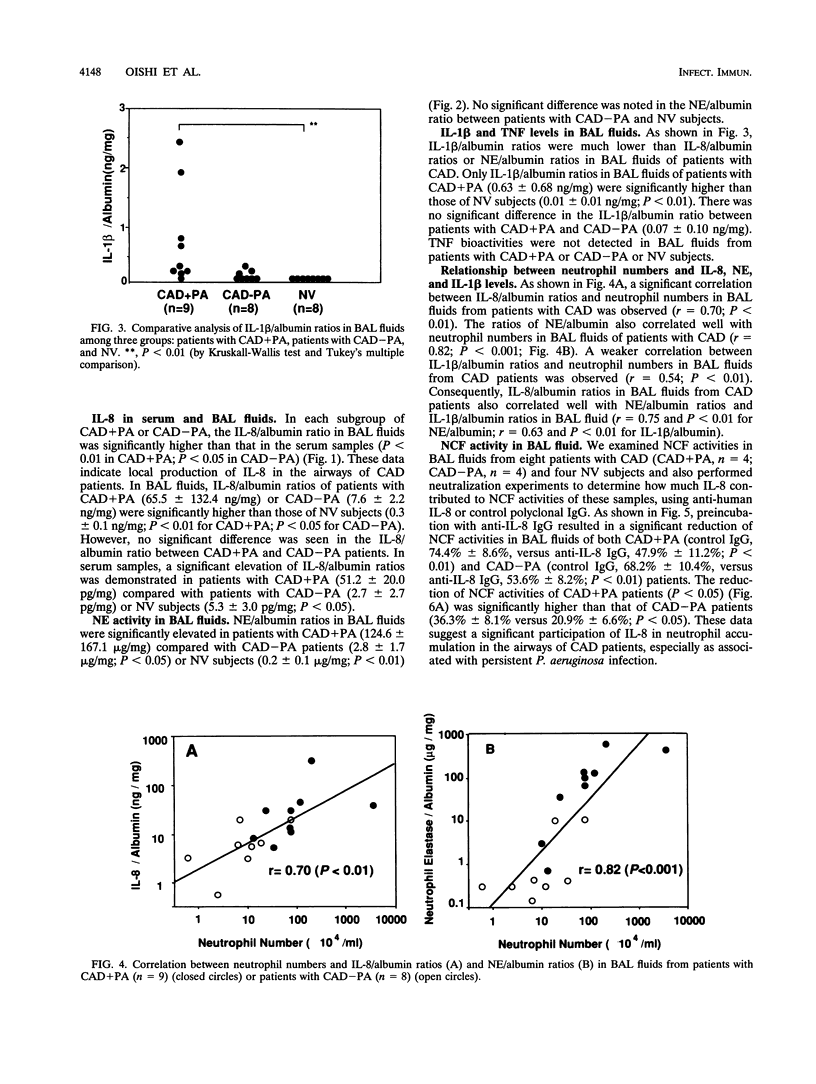
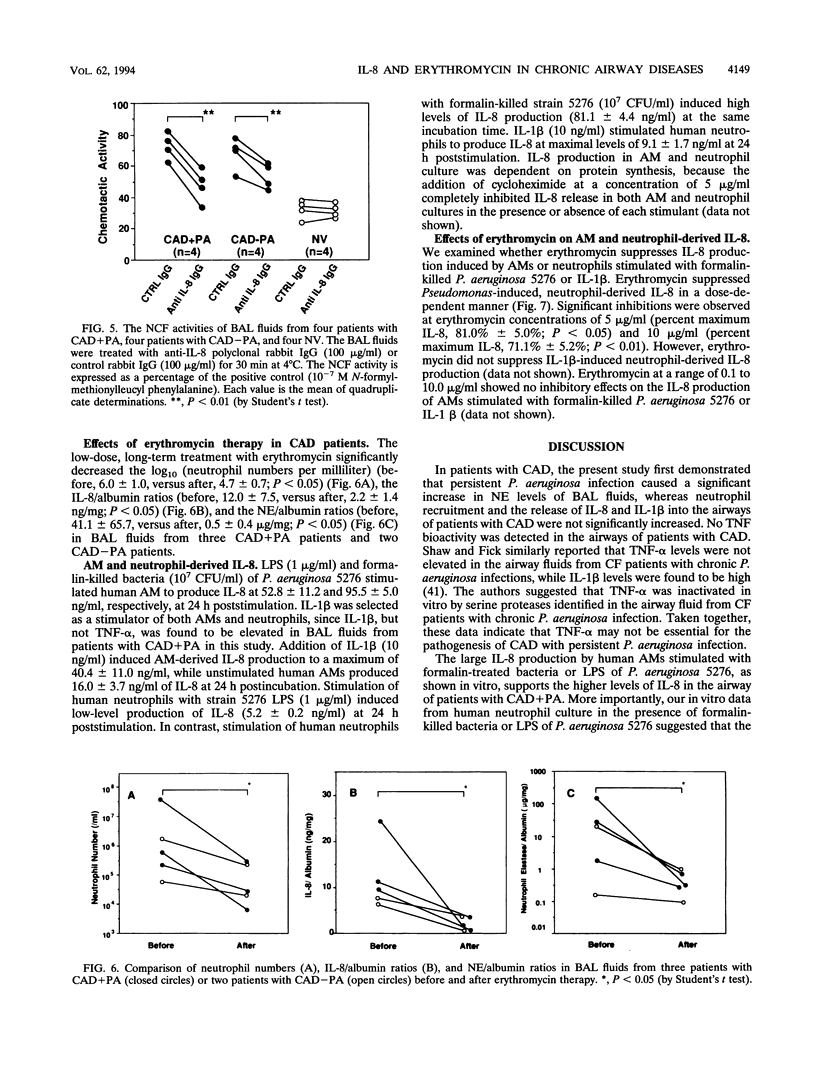
To evaluate of the role of interleukin-8 (IL-8), a chemotactic cytokine, in the continuous neutrophil accumulation in the airways of patients with chronic airway disease (CAD) and persistent Pseudomonas aeruginosa infection, we investigated the cell population, IL-8 levels, IL-1 beta levels, tumor necrosis factor (TNF) activities, and neutrophil elastase (NE) activities of bronchoalveolar lavage (BAL) fluids in 17 CAD patients (with P. aeruginosa infections [CAD+PA], n = 9; without any bacterial infections [CAD-PA], n = 8) and 8 normal volunteers. We found significant elevations of neutrophil numbers, IL-8/albumin ratios, and NE/albumin ratios in BAL fluids from CAD patients, in the following rank order: CAD+PA > CAD-PA > normal volunteers. IL-1 beta/albumin ratios were elevated only in CAD+PA, while no TNF bioactivity was detected in BAL fluids. The neutrophil numbers correlated significantly with the IL-8/albumin ratios and NE/albumin ratios in the BAL fluids of CAD patients. When anti-human IL-8 immunoglobulin G was used for neutralizing neutrophil chemotactic factor (NCF) activities in BAL fluids, the mean reduction rate of NCF activities in CAD+PA patients was significantly higher than that in CAD-PA patients. We also evaluated the effects of low-dose, long-term erythromycin therapy in BAL fluids from three CAD+PA and two CAD-PA patients. Treatment with erythromycin caused significant reductions of neutrophil numbers, IL-8/albumin ratios, and NE/albumin ratios in BAL fluids from these patients. To elucidate the mechanism of erythromycin therapy, we also examined whether erythromycin suppressed IL-8 production by human alveolar macrophages and neutrophils in vitro. We demonstrated a moderate inhibitory effect of erythromycin on IL-8 production in Pseudomonas-stimulated neutrophils but not in alveolar macrophages. Our data support the view that persistent P. aeruginosa infection enhances IL-8 production and IL-8-derived NCF activity, causing neutrophil accumulation in the airways and the progressive lung injuries observed in patients with CAD. The clinical efficacy of erythromycin therapy for CAD patients might be partly mediated through a reduced IL-8 production, diminishing neutrophil accumulation and NE release in the airways.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed K., Dai T. C., Ichinose A., Masaki H., Nagatake T., Matsumoto K. Neutrophil response to Pseudomonas aeruginosa in respiratory infection. Microbiol Immunol. 1993;37(7):523–529. doi: 10.1111/j.1348-0421.1993.tb01672.x. [DOI] [PubMed] [Google Scholar]
- Amitani R., Wilson R., Rutman A., Read R., Ward C., Burnett D., Stockley R. A., Cole P. J. Effects of human neutrophil elastase and Pseudomonas aeruginosa proteinases on human respiratory epithelium. Am J Respir Cell Mol Biol. 1991 Jan;4(1):26–32. doi: 10.1165/ajrcmb/4.1.26. [DOI] [PubMed] [Google Scholar]
- DeForge L. E., Fantone J. C., Kenney J. S., Remick D. G. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992 Nov;90(5):2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr, Naegel G. P., Squier S. U., Wood R. E., Gee J. B., Reynolds H. Y. Proteins of the cystic fibrosis respiratory tract. Fragmented immunoglobulin G opsonic antibody causing defective opsonophagocytosis. J Clin Invest. 1984 Jul;74(1):236–248. doi: 10.1172/JCI111407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick R. B., Jr Pathogenesis of the pseudomonas lung lesion in cystic fibrosis. Chest. 1989 Jul;96(1):158–164. doi: 10.1378/chest.96.1.158. [DOI] [PubMed] [Google Scholar]
- Fick R. B., Jr, Robbins R. A., Squier S. U., Schoderbek W. E., Russ W. D. Complement activation in cystic fibrosis respiratory fluids: in vivo and in vitro generation of C5a and chemotactic activity. Pediatr Res. 1986 Dec;20(12):1258–1268. doi: 10.1203/00006450-198612000-00014. [DOI] [PubMed] [Google Scholar]
- Hack C. E., Hart M., van Schijndel R. J., Eerenberg A. J., Nuijens J. H., Thijs L. G., Aarden L. A. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992 Jul;60(7):2835–2842. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma H., Yamanaka A., Tanimoto S., Tamura M., Chijimatsu Y., Kira S., Izumi T. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest. 1983 Jan;83(1):63–69. doi: 10.1378/chest.83.1.63. [DOI] [PubMed] [Google Scholar]
- Hopkins H., Stull T., Von Essen S. G., Robbins R. A., Rennard S. I. Neutrophil chemotactic factors in bacterial pneumonia. Chest. 1989 May;95(5):1021–1027. doi: 10.1378/chest.95.5.1021. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Lawley T. J., Crystal R. G. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981 Jul;68(1):259–269. doi: 10.1172/JCI110242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y., Ninomiya H., Koga H., Tanaka M., Kinoshita M., Tokunaga N., Yano T., Oizumi K. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am Rev Respir Dis. 1992 Jul;146(1):196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]
- Johnson K. G., Perry M. B. Improved techniques for the preparation of bacterial lipopolysaccharides. Can J Microbiol. 1976 Jan;22(1):29–34. doi: 10.1139/m76-004. [DOI] [PubMed] [Google Scholar]
- Kadota J., Sakito O., Kohno S., Sawa H., Mukae H., Oda H., Kawakami K., Fukushima K., Hiratani K., Hara K. A mechanism of erythromycin treatment in patients with diffuse panbronchiolitis. Am Rev Respir Dis. 1993 Jan;147(1):153–159. doi: 10.1164/ajrccm/147.1.153. [DOI] [PubMed] [Google Scholar]
- Knowles M. R., Church N. L., Waltner W. E., Yankaskas J. R., Gilligan P., King M., Edwards L. J., Helms R. W., Boucher R. C. A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med. 1990 Apr 26;322(17):1189–1194. doi: 10.1056/NEJM199004263221704. [DOI] [PubMed] [Google Scholar]
- Ko Y. C., Mukaida N., Ishiyama S., Tokue A., Kawai T., Matsushima K., Kasahara T. Elevated interleukin-8 levels in the urine of patients with urinary tract infections. Infect Immun. 1993 Apr;61(4):1307–1314. doi: 10.1128/iai.61.4.1307-1314.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko Y., Mukaida N., Panyutich A., Voitenok N. N., Matsushima K., Kawai T., Kasahara T. A sensitive enzyme-linked immunosorbent assay for human interleukin-8. J Immunol Methods. 1992 May 18;149(2):227–235. doi: 10.1016/0022-1759(92)90254-q. [DOI] [PubMed] [Google Scholar]
- Kragballe K., Gjertsen B. T., De Hoop D., Karlsmark T., van de Kerkhof P. C., Larkö O., Nieboer C., Roed-Petersen J., Strand A., Tikjøb G. Double-blind, right/left comparison of calcipotriol and betamethasone valerate in treatment of psoriasis vulgaris. Lancet. 1991 Jan 26;337(8735):193–196. doi: 10.1016/0140-6736(91)92157-w. [DOI] [PubMed] [Google Scholar]
- Kudoh S., Uetake T., Hagiwara K., Hirayama M., Hus L. H., Kimura H., Sugiyama Y. [Clinical effects of low-dose long-term erythromycin chemotherapy on diffuse panbronchiolitis]. Nihon Kyobu Shikkan Gakkai Zasshi. 1987 Jun;25(6):632–642. [PubMed] [Google Scholar]
- Larsen C. G., Kristensen M., Paludan K., Deleuran B., Thomsen M. K., Zachariae C., Kragballe K., Matsushima K., Thestrup-Pedersen K. 1,25(OH)2-D3 is a potent regulator of interleukin-1 induced interleukin-8 expression and production. Biochem Biophys Res Commun. 1991 May 15;176(3):1020–1026. doi: 10.1016/0006-291x(91)90384-j. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Morishita K., Yoshimura T., Lavu S., Kobayashi Y., Lew W., Appella E., Kung H. F., Leonard E. J., Oppenheim J. J. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988 Jun 1;167(6):1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElvaney N. G., Nakamura H., Birrer P., Hébert C. A., Wong W. L., Alphonso M., Baker J. B., Catalano M. A., Crystal R. G. Modulation of airway inflammation in cystic fibrosis. In vivo suppression of interleukin-8 levels on the respiratory epithelial surface by aerosolization of recombinant secretory leukoprotease inhibitor. J Clin Invest. 1992 Oct;90(4):1296–1301. doi: 10.1172/JCI115994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N., Matsushima K. Regulation of IL-8 production and the characteristics of the receptors for IL-8. Cytokines. 1992;4:41–53. [PubMed] [Google Scholar]
- Mukaida N., Shiroo M., Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989 Aug 15;143(4):1366–1371. [PubMed] [Google Scholar]
- Nagai H., Shishido H., Yoneda R., Yamaguchi E., Tamura A., Kurashima A. Long-term low-dose administration of erythromycin to patients with diffuse panbronchiolitis. Respiration. 1991;58(3-4):145–149. doi: 10.1159/000195915. [DOI] [PubMed] [Google Scholar]
- Nelson S., Summer W. R., Terry P. B., Warr G. A., Jakab G. J. Erythromycin-induced suppression of pulmonary antibacterial defenses. A potential mechanism of superinfection in the lung. Am Rev Respir Dis. 1987 Nov;136(5):1207–1212. doi: 10.1164/ajrccm/136.5.1207. [DOI] [PubMed] [Google Scholar]
- Oishi K., Sonoda F., Iwagaki A., Kobayashi S., Nagatake T., Matsumoto K. Effects of the combination of lipopolysaccharide-specific monoclonal antibodies and sparfloxacin against Pseudomonas aeruginosa pneumonia in neutropenic mice. Antimicrob Agents Chemother. 1992 Jul;36(7):1352–1357. doi: 10.1128/aac.36.7.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington J. E., Reynolds H. Y., Carbone P. P. Pseudomonas pneumonia. A retrospective study of 36 cases. Am J Med. 1973 Aug;55(2):155–160. doi: 10.1016/0002-9343(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Richman-Eisenstat J. B., Jorens P. G., Hébert C. A., Ueki I., Nadel J. A. Interleukin-8: an important chemoattractant in sputum of patients with chronic inflammatory airway diseases. Am J Physiol. 1993 Apr;264(4 Pt 1):L413–L418. doi: 10.1152/ajplung.1993.264.4.L413. [DOI] [PubMed] [Google Scholar]
- Rolfe M. W., Kunkel S. L., Rowens B., Standiford T. J., Cragoe E. J., Jr, Strieter R. M. Suppression of human alveolar macrophage-derived cytokines by amiloride. Am J Respir Cell Mol Biol. 1992 Jun;6(6):576–582. doi: 10.1165/ajrcmb/6.6.576. [DOI] [PubMed] [Google Scholar]
- Rolfe M. W., Kunkel S. L., Standiford T. J., Chensue S. W., Allen R. M., Evanoff H. L., Phan S. H., Strieter R. M. Pulmonary fibroblast expression of interleukin-8: a model for alveolar macrophage-derived cytokine networking. Am J Respir Cell Mol Biol. 1991 Nov;5(5):493–501. doi: 10.1165/ajrcmb/5.5.493. [DOI] [PubMed] [Google Scholar]
- Ruff M. R., Gifford G. E. Purification and physico-chemical characterization of rabbit tumor necrosis factor. J Immunol. 1980 Oct;125(4):1671–1677. [PubMed] [Google Scholar]
- Sawaki M., Mikami R., Mikasa K., Kunimatsu M., Ito S., Narita N. [The long term chemotherapy with erythromycin in chronic lower respiratory tract infections--second report: including cases with Pseudomonas infections]. Kansenshogaku Zasshi. 1986 Jan;60(1):45–50. doi: 10.11150/kansenshogakuzasshi1970.60.45. [DOI] [PubMed] [Google Scholar]
- Schröder J. M., Mrowietz U., Morita E., Christophers E. Purification and partial biochemical characterization of a human monocyte-derived, neutrophil-activating peptide that lacks interleukin 1 activity. J Immunol. 1987 Nov 15;139(10):3474–3483. [PubMed] [Google Scholar]
- Sibille Y., Lwebuga-Mukasa J. S., Polomski L., Merrill W. W., Ingbar D. H., Gee J. B. An in vitro model for polymorphonuclear-leukocyte-induced injury to an extracellular matrix. Relative contribution of oxidants and elastase to fibronectin release from amnionic membranes. Am Rev Respir Dis. 1986 Jul;134(1):134–140. doi: 10.1164/arrd.1986.134.1.134. [DOI] [PubMed] [Google Scholar]
- Sommerhoff C. P., Nadel J. A., Basbaum C. B., Caughey G. H. Neutrophil elastase and cathepsin G stimulate secretion from cultured bovine airway gland serous cells. J Clin Invest. 1990 Mar;85(3):682–689. doi: 10.1172/JCI114492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, November 1986. Am Rev Respir Dis. 1987 Jul;136(1):225–244. doi: 10.1164/ajrccm/136.1.225. [DOI] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Basha M. A., Chensue S. W., Lynch J. P., 3rd, Toews G. B., Westwick J., Strieter R. M. Interleukin-8 gene expression by a pulmonary epithelial cell line. A model for cytokine networks in the lung. J Clin Invest. 1990 Dec;86(6):1945–1953. doi: 10.1172/JCI114928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standiford T. J., Kunkel S. L., Rolfe M. W., Evanoff H. L., Allen R. M., Strieter R. M. Regulation of human alveolar macrophage- and blood monocyte-derived interleukin-8 by prostaglandin E2 and dexamethasone. Am J Respir Cell Mol Biol. 1992 Jan;6(1):75–81. doi: 10.1165/ajrcmb/6.1.75. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Shaw J., Hill S. L., Burnett D. Neutrophil chemotaxis in bronchiectasis: a study of peripheral cells and lung secretions. Clin Sci (Lond) 1988 Jun;74(6):645–650. doi: 10.1042/cs0740645. [DOI] [PubMed] [Google Scholar]
- Strieter R. M., Kasahara K., Allen R. M., Standiford T. J., Rolfe M. W., Becker F. S., Chensue S. W., Kunkel S. L. Cytokine-induced neutrophil-derived interleukin-8. Am J Pathol. 1992 Aug;141(2):397–407. [PMC free article] [PubMed] [Google Scholar]
- Strieter R. M., Remick D. G., Lynch J. P., 3rd, Genord M., Raiford C., Spengler R., Kunkel S. L. Differential regulation of tumor necrosis factor-alpha in human alveolar macrophages and peripheral blood monocytes: a cellular and molecular analysis. Am J Respir Cell Mol Biol. 1989 Jul;1(1):57–63. doi: 10.1165/ajrcmb/1.1.57. [DOI] [PubMed] [Google Scholar]
- Tanimoto H. A review of the recent progress in treatment of patients with diffuse panbronchiolitis associated with Pseudomonas aeruginosa infection in Japan. Antibiot Chemother (1971) 1991;44:94–98. doi: 10.1159/000420303. [DOI] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Thornton A. J., Strieter R. M., Lindley I., Baggiolini M., Kunkel S. L. Cytokine-induced gene expression of a neutrophil chemotactic factor/IL-8 in human hepatocytes. J Immunol. 1990 Apr 1;144(7):2609–2613. [PubMed] [Google Scholar]
- Toews G. B., Vial W. C. The role of C5 in polymorphonuclear leukocyte recruitment in response to Streptococcus pneumoniae. Am Rev Respir Dis. 1984 Jan;129(1):82–86. doi: 10.1164/arrd.1984.129.1.82. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Sellers S. E., Swayze S. C., McPhaul K. M., Wittes J. T., Crystal R. G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987 Apr 23;316(17):1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- Wilmott R. W., Kassab J. T., Kilian P. L., Benjamin W. R., Douglas S. D., Wood R. E. Increased levels of interleukin-1 in bronchoalveolar washings from children with bacterial pulmonary infections. Am Rev Respir Dis. 1990 Aug;142(2):365–368. doi: 10.1164/ajrccm/142.2.365. [DOI] [PubMed] [Google Scholar]
- Yamamoto M., Kondo A., Tamura M., Izumi T., Ina Y., Noda M. [Long-term therapeutic effects of erythromycin and newquinolone antibacterial agents on diffuse panbronchiolitis]. Nihon Kyobu Shikkan Gakkai Zasshi. 1990 Oct;28(10):1305–1313. [PubMed] [Google Scholar]
- Yoshimura T., Matsushima K., Tanaka S., Robinson E. A., Appella E., Oppenheim J. J., Leonard E. J. Purification of a human monocyte-derived neutrophil chemotactic factor that has peptide sequence similarity to other host defense cytokines. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9233–9237. doi: 10.1073/pnas.84.24.9233. [DOI] [PMC free article] [PubMed] [Google Scholar]


