Abstract
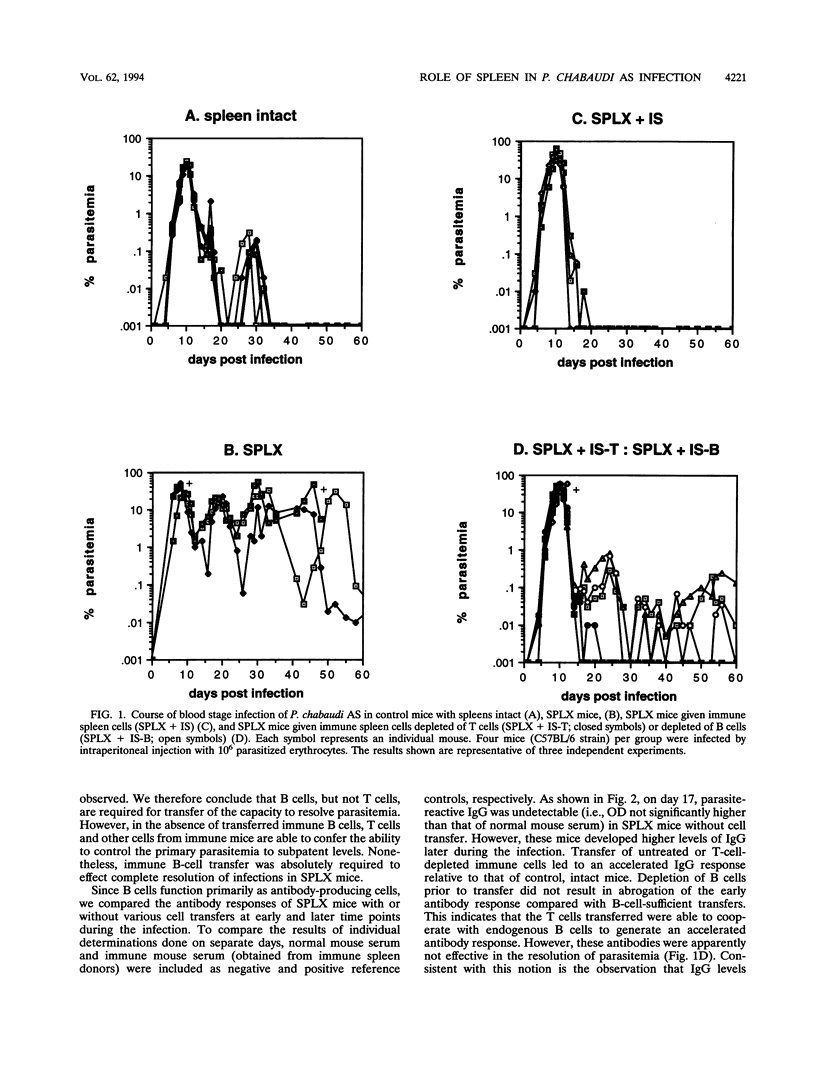
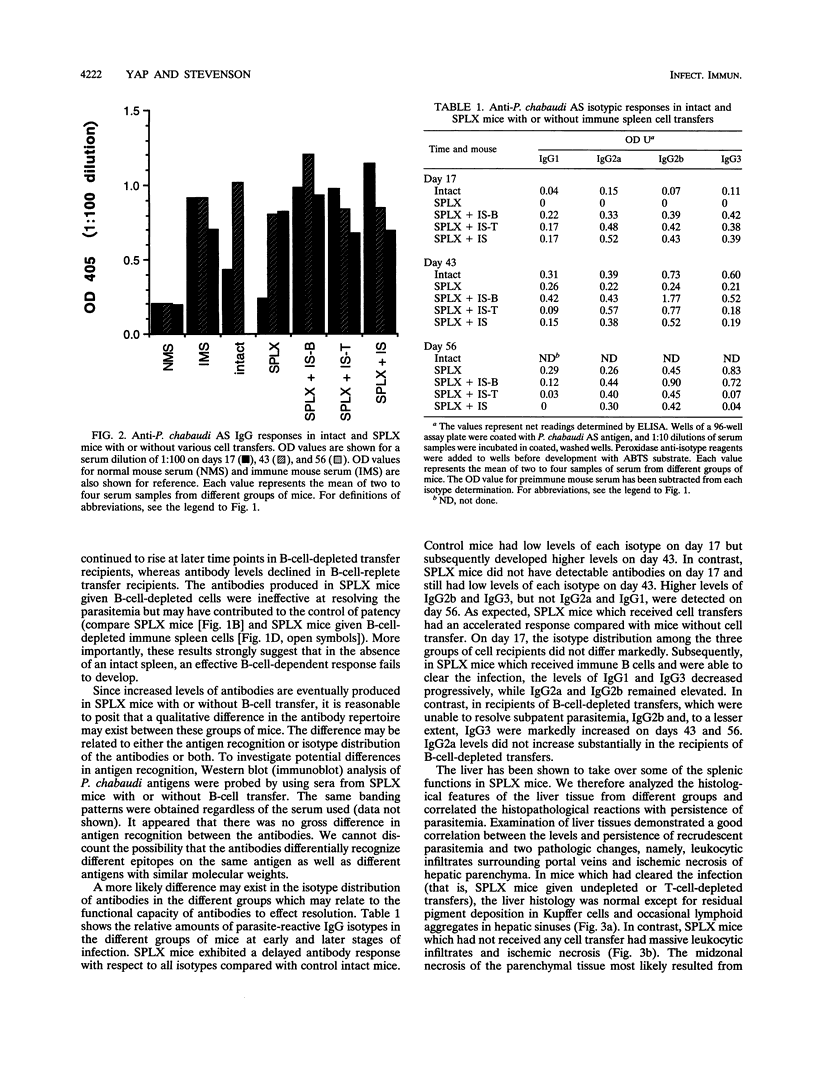
The requirement for an architecturally intact spleen in the afferent and efferent arms of immunity to the murine malaria parasite Plasmodium chabaudi AS was analyzed. C57BL/6 mice with intact spleens develop a single, patent parasitemia and resolve the infection. In contrast, surgically splenectomized mice experience persistent waves of patent parasitemia interrupted briefly by periods of parasitologic crises. Transfer of spleen cells from immune donors, but not transfer of spleen cells from normal mice, into splenectomized mice enabled the recipients to resolve the infection similar to mice with intact spleens. B-cell depletion, but not T-cell depletion, of spleen cells prior to transfer abrogated the ability of splenectomized recipients to resolve the infection. Compared with mice with intact spleens, splenectomized mice exhibited a delayed antibody response whereas all groups of immune cell recipients had an accelerated antibody response. Nevertheless, splenectomized mice and recipients of B-cell-depleted cells failed to resolve infections, despite the development of high-titer antibodies late during the course of infection. Analysis of immunoglobulin G isotype responses showed a lower representation of immunoglobulin G2a in mice which failed to resolve infections. The latter mice had characteristic histopathologic changes in the liver. These observations indicate a unique role of the splenic microenvironment for the induction and development of an effective B-cell-dependent response against malarial parasites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bouharoun-Tayoun H., Druilhe P. Plasmodium falciparum malaria: evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect Immun. 1992 Apr;60(4):1473–1481. doi: 10.1128/iai.60.4.1473-1481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
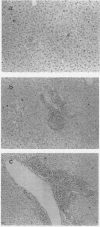
- Dockrell H. M., de Souza J. B., Playfair J. H. The role of the liver in immunity to blood-stage murine malaria. Immunology. 1980 Oct;41(2):421–430. [PMC free article] [PubMed] [Google Scholar]
- Ezekowitz R. A., Bampton M., Gordon S. Macrophage activation selectively enhances expression of Fc receptors for IgG2a. J Exp Med. 1983 Feb 1;157(2):807–812. doi: 10.1084/jem.157.2.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Long C. A., Weidanz W. P. Effects of splenectomy on antibody-independent immunity to Plasmodium chabaudi adami malaria. Infect Immun. 1985 Jun;48(3):853–858. doi: 10.1128/iai.48.3.853-858.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grun J. L., Weidanz W. P. Immunity to Plasmodium chabaudi adami in the B-cell-deficient mouse. Nature. 1981 Mar 12;290(5802):143–145. doi: 10.1038/290143a0. [DOI] [PubMed] [Google Scholar]
- Kumar S., Good M. F., Dontfraid F., Vinetz J. M., Miller L. H. Interdependence of CD4+ T cells and malarial spleen in immunity to Plasmodium vinckei vinckei. Relevance to vaccine development. J Immunol. 1989 Sep 15;143(6):2017–2023. [PubMed] [Google Scholar]
- Langhorne J., Simon-Haarhaus B. Differential T cell responses to Plasmodium chabaudi chabaudi in peripheral blood and spleens of C57BL/6 mice during infection. J Immunol. 1991 Apr 15;146(8):2771–2775. [PubMed] [Google Scholar]
- Langhorne J. The role of CD4+ T-cells in the immune response to Plasmodium chabaudi. Parasitol Today. 1989 Nov;5(11):362–364. doi: 10.1016/0169-4758(89)90113-0. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S., Suntharasamai P., Webster H. K., Ho M. Malaria in splenectomized patients: report of four cases and review. Clin Infect Dis. 1993 Mar;16(3):361–366. doi: 10.1093/clind/16.3.361. [DOI] [PubMed] [Google Scholar]
- Magilavy D. B., Foys K. M., Gajewski T. F. Liver of MRL/lpr mice contain interleukin-4-producing lymphocytes and accessory cells that support the proliferation of Th2 helper T lymphocyte clones. Eur J Immunol. 1992 Sep;22(9):2359–2365. doi: 10.1002/eji.1830220927. [DOI] [PubMed] [Google Scholar]
- Meding S. J., Langhorne J. CD4+ T cells and B cells are necessary for the transfer of protective immunity to Plasmodium chabaudi chabaudi. Eur J Immunol. 1991 Jun;21(6):1433–1438. doi: 10.1002/eji.1830210616. [DOI] [PubMed] [Google Scholar]
- Owens T. A role for adhesion molecules in contact-dependent T help for B cells. Eur J Immunol. 1991 Apr;21(4):979–983. doi: 10.1002/eji.1830210418. [DOI] [PubMed] [Google Scholar]
- Podoba J. E., Stevenson M. M. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991 Jan;59(1):51–58. doi: 10.1128/iai.59.1.51-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond L., Wassom D. L., Hayes C. E. Influence of resistant and susceptible genotype, IL-1, and lymphoid organ on Trichinella spiralis-induced cytokine secretion. J Immunol. 1992 Aug 1;149(3):957–965. [PubMed] [Google Scholar]
- Quinn T. C., Wyler D. J. Resolution of acute malaria (Plasmodium berghei in the rat): reversibility and spleen dependence. Am J Trop Med Hyg. 1980 Jan;29(1):1–4. doi: 10.4269/ajtmh.1980.29.1. [DOI] [PubMed] [Google Scholar]
- Sayles P. C., Cooley A. J., Wassom D. L. A spleen is not necessary to resolve infections with Plasmodium yoelii. Am J Trop Med Hyg. 1991 Jan;44(1):42–48. doi: 10.4269/ajtmh.1991.44.42. [DOI] [PubMed] [Google Scholar]
- Sayles P. C., Yanez D. M., Wassom D. L. Plasmodium yoelii: splenectomy alters the antibody responses of infected mice. Exp Parasitol. 1993 Jun;76(4):377–384. doi: 10.1006/expr.1993.1046. [DOI] [PubMed] [Google Scholar]
- Snapper C. M., Mond J. J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993 Jan;14(1):15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F. Differential induction of helper T cell subsets during blood-stage Plasmodium chabaudi AS infection in resistant and susceptible mice. Clin Exp Immunol. 1993 Apr;92(1):77–83. doi: 10.1111/j.1365-2249.1993.tb05951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Rae D. Dependence on cell-mediated mechanisms for the appearance of crisis forms during Plasmodium chabaudi AS infection in C57BL/6 mice. Microb Pathog. 1990 Nov;9(5):303–314. doi: 10.1016/0882-4010(90)90065-x. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson A. W., Phillips R. S., Severn A., Moncada S., Liew F. Y. The role of TH1 and TH2 cells in a rodent malaria infection. Science. 1993 Jun 25;260(5116):1931–1934. doi: 10.1126/science.8100366. [DOI] [PubMed] [Google Scholar]
- Van den Eertwegh A. J., Boersma W. J., Claassen E. Immunological functions and in vivo cell-cell interactions of T cells in the spleen. Crit Rev Immunol. 1992;11(6):337–380. [PubMed] [Google Scholar]
- Weidanz W. P., Melancon-Kaplan J., Cavacini L. A. Cell-mediated immunity to the asexual blood stages of malarial parasites: animal models. Immunol Lett. 1990 Aug;25(1-3):87–95. doi: 10.1016/0165-2478(90)90097-a. [DOI] [PubMed] [Google Scholar]
- White W. I., Evans C. B., Taylor D. W. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with Plasmodium yoelii. Infect Immun. 1991 Oct;59(10):3547–3554. doi: 10.1128/iai.59.10.3547-3554.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkel K. D., Good M. F. Inability of Plasmodium vinckei-immune spleen cells to transfer protection to recipient mice exposed to vaccine 'vectors' or heterologous species of plasmodium. Parasite Immunol. 1991 Sep;13(5):517–530. doi: 10.1111/j.1365-3024.1991.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Wyler D. J., Quinn T. C., Chen L. T. Relationship of alterations in splenic clearance function and microcirculation to host defense in acute rodent malaria. J Clin Invest. 1981 May;67(5):1400–1404. doi: 10.1172/JCI110168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap G. S., Stevenson M. M. Plasmodium chabaudi AS: erythropoietic responses during infection in resistant and susceptible mice. Exp Parasitol. 1992 Nov;75(3):340–352. doi: 10.1016/0014-4894(92)90219-z. [DOI] [PubMed] [Google Scholar]
- van den Dobbelsteen G. P., Brunekreef K., Kroes H., van Rooijen N., van Rees E. P. Enhanced triggering of mucosal immune responses by reducing splenic phagocytic functions. Eur J Immunol. 1993 Jul;23(7):1488–1493. doi: 10.1002/eji.1830230714. [DOI] [PubMed] [Google Scholar]