Abstract
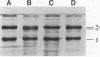
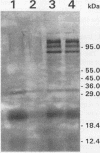
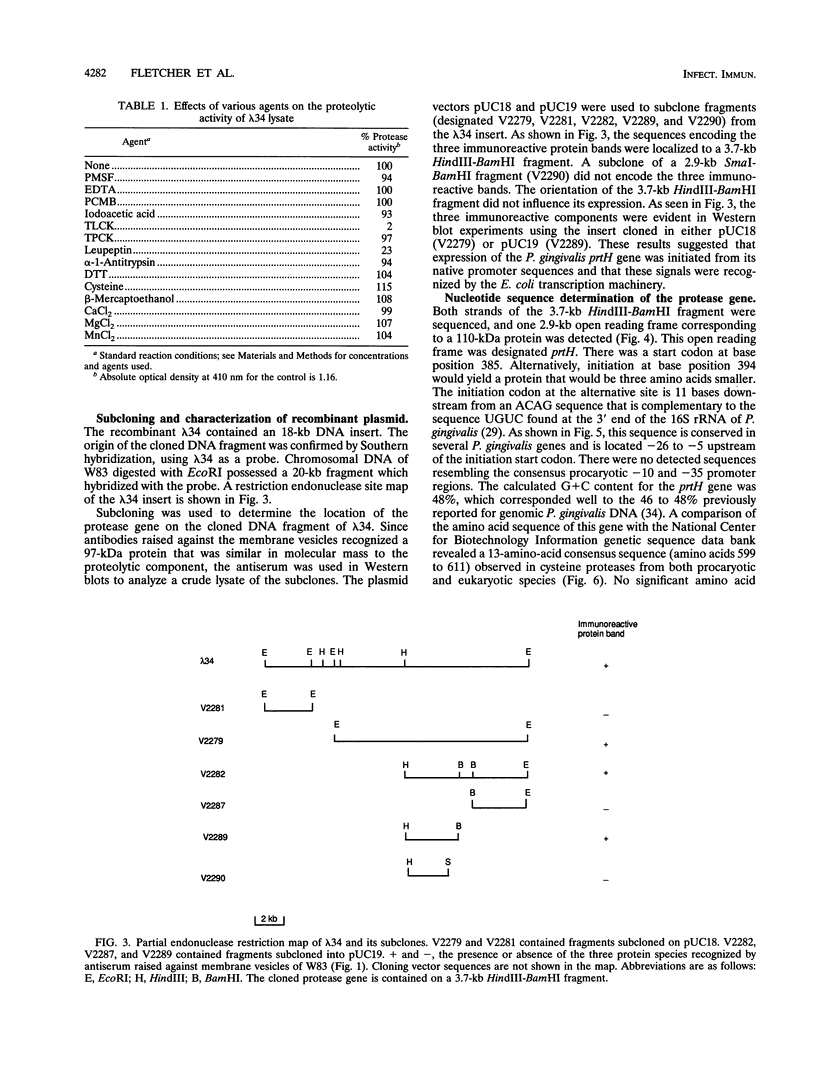
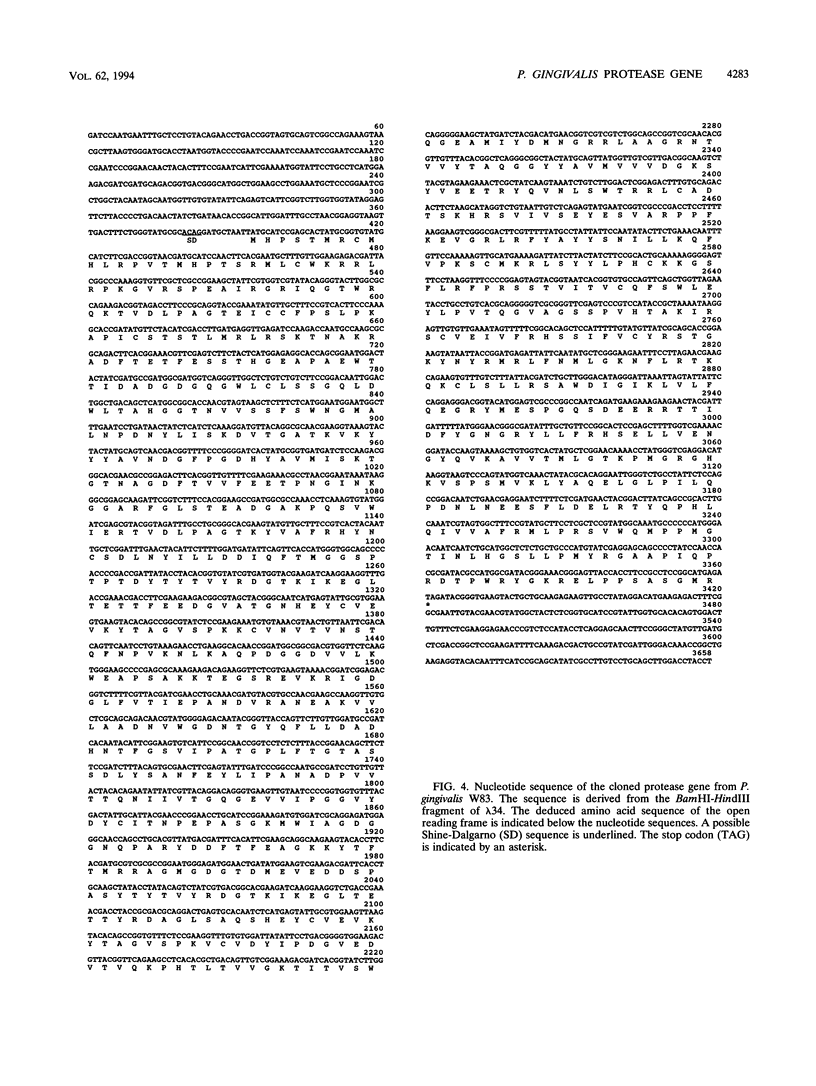
Porphyromonas gingivalis has been implicated as a contributing etiological agent of adult periodontitis and generalized forms of early-onset periodontitis. Proteases of P. gingivalis may contribute to its pathogenicity by destroying connective tissue as well as inactivating key plasma proteins that might mediate protective host functions. In order to explore this problem, antiserum raised against membrane vesicles of P. gingivalis W83 was used to screen a genomic library of strain W83 constructed by using the lambda DASH vector system. A recombinant phage (lambda 34) expressing a P. gingivalis protease from the library was identified and characterized. Casein substrate zymography of lambda 34 lysates revealed a protease with an apparent molecular mass of 97 kDa. The gene encoding this protease was designated prtH. It was localized to a 3.7-kb HindIII-BamHI fragment and specified an enzyme which hydrolyzed the human C3 complement protein under defined conditions. The nucleotide sequence of this 3.7-kb fragment was determined, and one 2.9-kb open reading frame (992 amino acids) corresponding to a 110-kDa protein was detected, suggesting it might be a precursor of the 97-kDa active protease. prtH is not similar to any previously cloned protease gene from P. gingivalis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott M. A., Rigg G., Shah H., Williams D., Wallace A., Roberts I. S. Cloning and expression of a Porphyromonas (Bacteroides) gingivalis protease gene in Escherichia coli. Arch Oral Biol. 1990;35 (Suppl):97S–99S. doi: 10.1016/0003-9969(90)90137-y. [DOI] [PubMed] [Google Scholar]
- Banas J. A., Ferretti J. J., Progulske-Fox A. Identification and sequence analysis of a methylase gene in Porphyromonas gingivalis. Nucleic Acids Res. 1991 Aug 11;19(15):4189–4192. doi: 10.1093/nar/19.15.4189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonham-Smith P. C., Bourque D. P. Translation of chloroplast-encoded mRNA: potential initiation and termination signals. Nucleic Acids Res. 1989 Mar 11;17(5):2057–2080. doi: 10.1093/nar/17.5.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeau G., Lapointe H., Péloquin P., Mayrand D. Cloning, expression, and sequencing of a protease gene (tpr) from Porphyromonas gingivalis W83 in Escherichia coli. Infect Immun. 1992 Aug;60(8):3186–3192. doi: 10.1128/iai.60.8.3186-3192.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. I., Takahashi N., Kato T., Kuramitsu H. K. Isolation, expression, and nucleotide sequence of the sod gene from Porphyromonas gingivalis. Infect Immun. 1991 Apr;59(4):1564–1566. doi: 10.1128/iai.59.4.1564-1566.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Kubiniec M. A., Yoshimura F., Genco R. J. Molecular cloning and sequencing of the gene encoding the fimbrial subunit protein of Bacteroides gingivalis. J Bacteriol. 1988 Apr;170(4):1658–1665. doi: 10.1128/jb.170.4.1658-1665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genco R. J. Host responses in periodontal diseases: current concepts. J Periodontol. 1992 Apr;63(4 Suppl):338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- Gregory R. L., Kim D. E., Kindle J. C., Hobbs L. C., Lloyd D. R. Immunoglobulin-degrading enzymes in localized juvenile periodontitis. J Periodontal Res. 1992 May;27(3):176–183. doi: 10.1111/j.1600-0765.1992.tb01666.x. [DOI] [PubMed] [Google Scholar]
- Grenier D., Chao G., McBride B. C. Characterization of sodium dodecyl sulfate-stable Bacteroides gingivalis proteases by polyacrylamide gel electrophoresis. Infect Immun. 1989 Jan;57(1):95–99. doi: 10.1128/iai.57.1.95-99.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D. Inactivation of human serum bactericidal activity by a trypsinlike protease isolated from Porphyromonas gingivalis. Infect Immun. 1992 May;60(5):1854–1857. doi: 10.1128/iai.60.5.1854-1857.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect Immun. 1987 Jan;55(1):111–117. doi: 10.1128/iai.55.1.111-117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenier D., Mayrand D., McBride B. C. Further studies on the degradation of immunoglobulins by black-pigmented Bacteroides. Oral Microbiol Immunol. 1989 Mar;4(1):12–18. doi: 10.1111/j.1399-302x.1989.tb00400.x. [DOI] [PubMed] [Google Scholar]
- Kato T., Takahashi N., Kuramitsu H. K. Sequence analysis and characterization of the Porphyromonas gingivalis prtC gene, which expresses a novel collagenase activity. J Bacteriol. 1992 Jun;174(12):3889–3895. doi: 10.1128/jb.174.12.3889-3895.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher P. J., Juliano R. L. Detection of proteases in polyacrylamide gels containing covalently bound substrates. Anal Biochem. 1984 Feb;136(2):470–475. doi: 10.1016/0003-2697(84)90246-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawson D. A., Meyer T. F. Biochemical characterization of Porphyromonas (Bacteroides) gingivalis collagenase. Infect Immun. 1992 Apr;60(4):1524–1529. doi: 10.1128/iai.60.4.1524-1529.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrina F. L., Mays T. K., Smith C. J., Welch R. A. Non-plasmid associated transfer of antibiotic resistance in Bacteroides. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):77–86. doi: 10.1093/jac/8.suppl_d.77. [DOI] [PubMed] [Google Scholar]
- Mayrand D., Holt S. C. Biology of asaccharolytic black-pigmented Bacteroides species. Microbiol Rev. 1988 Mar;52(1):134–152. doi: 10.1128/mr.52.1.134-152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee R. A., Adams S., Matthews J. A., Smith C. J., Smith H. Molecular cloning of two cysteine proteinases from paw-paw (Carica papaya). Biochem J. 1986 Jul 1;237(1):105–110. doi: 10.1042/bj2370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K. The superoxide dismutase-encoding gene of the obligately anaerobic bacterium Bacteroides gingivalis. Gene. 1990 Nov 30;96(1):149–150. doi: 10.1016/0378-1119(90)90357-w. [DOI] [PubMed] [Google Scholar]
- Otogoto J., Kuramitsu H. K. Isolation and characterization of the Porphyromonas gingivalis prtT gene, coding for protease activity. Infect Immun. 1993 Jan;61(1):117–123. doi: 10.1128/iai.61.1.117-123.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y., McBride B. C. Cloning of a Porphyromonas (Bacteroides) gingivalis protease gene and characterization of its product. FEMS Microbiol Lett. 1992 May 1;71(3):273–278. doi: 10.1016/0378-1097(92)90721-y. [DOI] [PubMed] [Google Scholar]
- Paster B. J., Dewhirst F. E., Olsen I., Fraser G. J. Phylogeny of Bacteroides, Prevotella, and Porphyromonas spp. and related bacteria. J Bacteriol. 1994 Feb;176(3):725–732. doi: 10.1128/jb.176.3.725-732.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkein H. A. The effect of periodontal proteolytic Bacteroides species on proteins of the human complement system. J Periodontal Res. 1988 May;23(3):187–192. doi: 10.1111/j.1600-0765.1988.tb01356.x. [DOI] [PubMed] [Google Scholar]
- Schenkein H. A. The role of complement in periodontal diseases. Crit Rev Oral Biol Med. 1991;2(1):65–81. doi: 10.1177/10454411910020010501. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Kato T., Kuramitsu H. K. Isolation and preliminary characterization of the Porphyromonas gingivalis prtC gene expressing collagenase activity. FEMS Microbiol Lett. 1991 Nov 15;68(2):135–138. doi: 10.1016/0378-1097(91)90116-r. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingrove J. A., DiScipio R. G., Chen Z., Potempa J., Travis J., Hugli T. E. Activation of complement components C3 and C5 by a cysteine proteinase (gingipain-1) from Porphyromonas (Bacteroides) gingivalis. J Biol Chem. 1992 Sep 15;267(26):18902–18907. [PubMed] [Google Scholar]