Abstract
BACKGROUND AND PURPOSE
Interleukin-15 (IL-15) is important in the activation and proliferation of lymphocytic cell populations and is implicated in inflammatory disease. We report the characterization of a novel monoclonal antibody DISC0280 which is specific for human IL-15.
EXPERIMENTAL APPROACH
DISC0280 was characterized in a direct binding assay of IL-15 with IL-15 receptor α (IL-15Rα) and by its ability to alter IL-15 mediated proliferation of a range of cell lines (cytotoxic T lymphocyte line-2, M-07e, KIT225). A pharmacodynamic model injecting male C57/BL6 mice with IL-15 or IL-15/IL-15Rα, with or without DISC0280, and assessing changes in lymphocytic cell populations and serum cytokines was utilized.
KEY RESULTS
DISC0280 inhibited the binding of IL-15 to IL-15Rα and also potently inhibits IL-15 dependent proliferation of cells expressing IL-15Rα, shared interleukin 2/ interleukin 15 receptor β chain (IL-15Rβ) and common gamma chain (γc). DISC0280 also inhibited the IL-15 dependent proliferation of M-07e cells that only express IL-15Rβ/γc subunits. Human IL-15 injected into mice caused an increase in NK1.1+ and CD3+ cells in the spleen and peripheral blood and these effects were unexpectedly potentiated by giving DISC0280 with human IL-15. This increase in cells caused by DISC0280/IL-15 co-administration was greater than that observed when IL-15 was administered complexed with soluble IL-15Rα.
CONCLUSIONS AND IMPLICATIONS
The ability of DISC0280 to bind to the IL-15Rα-binding site on IL-15 allows trans-presentation of IL-15 by DISC0280 in vivo, similar to the trans-presentation by soluble IL-15Rα. DISC0280 may be therefore suitable as a clinical substitute for IL-15.
Keywords: inflammation, interleukin, antibody, IL-15, trans-presentation, lymphocyte
Introduction
Interleukin-15 (IL-15) and its cognate receptor IL-15 receptor α (IL-15Rα) play an important role in activation and proliferation of natural killer (NK) and CD4+ T cells and are crucial in the proliferation and maintenance of CD8+ T cells involved in memory responses to antigens (Waldmann and Tagaya, 1999; Ma et al., 2006). IL-15−/− or IL-15Rα−/− mice show similar phenotypes with defects in NK, NK-T, intestinal intraepithelial lymphocytes and CD8+ T cells (Kennedy et al., 2000; Lodolce et al., 2002). IL-15 shares the IL-15Rβ and common γ chain (γc) chain with interleukin 2 (IL-2) as the other two components of its trimeric receptor (Giri et al., 1994). IL-15 demonstrates high (100 pM) affinity for IL-15Rα (Giri et al., 1995), and is associated with this receptor at the membrane of cells, particularly monocytic cell types, such as dendritic cells (Ma et al., 2006). Thus basal, soluble IL-15 is usually undetectable in healthy volunteers (Bergamaschi et al., 2008), but has been measured in disease (Fehniger and Caligiuri, 2001; Wuttge et al., 2007) and in supernatants of activated cells, either free or associated with the soluble IL-15 Rα (sIL-15Rα) (Bulanova et al., 2007; Bergamaschi et al., 2008). IL-15 can induce responses in cells expressing both IL-15Rβ and γc and IL-15Rα is dispensable (Meazza et al., 1998). IL-15 can signal either via cis-presentation of IL-15 acting via IL-15Rα and IL-15Rβ/γc subunits in the same cell (Eisenman et al., 2002), or by trans-presentation where the IL-15 bound to IL-15Rα on one cell may present to and activate the signalling IL-15Rβ/γc chains on a different cell (Ma et al., 2006; Mortier et al., 2008). In NK cells the IL-15Rα chain was shown to be dispensable and NK cells are therefore likely to be activated by trans-presentation (Burkett et al., 2004). Reports also suggest that IL-15Rα chaperones IL-15 to the membrane of dendritic cells so that IL-15 may only be expressed in cells co-expressing IL-15Rα (Sandau et al., 2004; Bergamaschi et al., 2008).
Because IL-15 is a key mediator within the innate immune response and especially in the maintenance and persistence of the adaptive immune response, therapeutic approaches explored so far either inhibit IL-15 to treat autoimmune diseases or else use exogenous IL-15 or an IL-15 agonist to stimulate the immune response against tumours, or as an adjuvant to stimulate proliferation and differentiation of specific immune cell lineages (Fehniger and Caligiuri, 2001).
A human anti-IL-15 monoclonal antibody has been shown to be effective in disease models (Villadsen et al., 2003) and in subjects with rheumatoid arthritis (Baslund et al., 2005). Soluble IL-15Rα also acts as an IL-15 antagonist, causing a decrease in NK cell proliferation and antigen-driven T-cell responses, and also inhibiting disease models (Ruchatz et al., 1998; Mortier et al., 2004; Ruckert et al., 2005).
In contrast to this, sIL-15Rα has also been reported to increase the activity of IL-15 both in vivo and in vitro when administered together either as a complex, or as a fusion protein of IL-15 with the extracellular ‘sushi’ domain of the IL-15Rα (Giron-Michel et al., 2005; Mortier et al., 2006; Rubinstein et al., 2006; Stoklasek et al., 2006; Dubois et al., 2008; Huntington et al., 2009). In vivo, exogenous complexes of sIL-15Rα and IL-15 induce a strong increase in CD8+ T cells and NK cells plus enlargement of the spleen and have been shown to inhibit tumour growth in mouse models (Dubois et al., 2008).
The disparity in effects of sIL-15Rα is echoed in vitro where it inhibits responses in cells attributable to human IL-15 (Eisenman et al., 2002), but also has been reported to increase responses to mouse IL-15 in cytotoxic T lymphocyte line (CTLL) cells (Bulanova et al., 2007). Significant steps have been made to elucidate the role of sIL-15Rα and its effects on IL-15 in vitro cell systems (Bouchaud et al., 2008).
In this paper we report the characterization of a novel antibody to hIL-15 (DISC0280). Our aim was to characterize its in vitro and in vivo activities for its potential use as a therapeutic antibody. We demonstrate that in vitro DISC0280 inhibits the activity of soluble hIL-15 and prevents binding of hIL-15 to sIL-15Rα. However, in an in vivo model of hIL-15 activity, we also show an opposing action for DISC0280, highlighting the complexity of pursuing IL-15 as a therapeutic target. These observations raise the possibility that DISC0280 or equivalent antibodies could be used to substitute clinically for IL-15 where a specific immunostimulation is desirable.
Methods
Isolation of antibody DISC0280
Phage display technology was used to isolate a panel of novel human monoclonal single chain antibody fragments (scFv), specific for hIL-15 by performing selections to enrich for scFv that bind to biotinylated hIL-15 (Vaughan et al., 1996). DISC0100, identified in this process, was shown to inhibit soluble hIL-15 in vitro biological activity assays, such as hIL-15 dependent survival of the mouse T cell line CTLL-2. This antibody fragment was then optimized using phage display (Thompson et al., 1996) to improve the overall potency by several orders of magnitude, and a variant of DISC0100 was identified as a potent inhibitor of hIL-15 activity in vitro. The cDNA encoding this scFv was recloned, expressed and purified as a fully human IgG1 antibody and named DISC0280.
Generation of hIL-15Rα
A cDNA encoding the extracellular portion of the hIL-15Rα (residues 1-205) was cloned into two mammalian expression vectors based on pDEST12.2. These vectors either incorporated a C-terminal FLAG-polyhistidine [FLAG-(HIS)10] tag on the end of the IL-15Rα sequence or a C-terminal human IgG1 Fc fusion respectively. The IL-15Rα was expressed in HEK-EBNA cells transfected using PEI (polyethylene imine; Sigma-Aldrich, Dorset, UK) as transfection reagent. The IL-15Rα-Fc fusion protein was purified from the conditioned media using protein G affinity chromatography (GE Healthcare LifeSciences, Amersham, UK) and the IL-15Rα-FLAG-(HIS)10 protein was purified from the conditioned media using Ni-affinity chromatography and both proteins were further purified using size exclusion chromatography. The activity of these proteins was confirmed by comparison to recombinant IL-15Rα from R&D Systems (Abingdon, UK) in a CTLL-2 proliferation assay (data not shown).
Cell culture
Cytotoxic T lymphocyte line-2 and M-07e cells were purchased from ECACC (Salisbury, UK) and DSMZ (Braunschweig, Germany) respectively, and were cultured under conditions recommended by the suppliers. KIT225 cells (Hori et al., 1987) were a kind gift from Doreen Cantrell at ICRF, Lincoln's Inn Fields, London, UK. Cells were incubated at 37°C with 5% CO2, 95% humidity.
IL-15-dependent assays using CTLL-2, M-07e and KIT225 cells
CTLL-2 cells were grown as recommended by the supplier, in RPMI-1640 with Glutamax I containing 10% fetal bovine serum (FBS) and 100 U mL−1 penicillin/streptomycin, and IL-2 (5 ng mL−1) as growth supplement. During subculture, the CTLL-2 cells were not allowed to achieve a density greater than 1 × 105 cells mL−1 in order to maintain IL-15 responsiveness. Cells were removed from IL-2-containing media and immediately plated into microtitre plates containing hIL-15 ± antibodies. The assay media were RPMI-1640 with Glutamax I containing 10% FBS and 100 U mL−1 penicillin/streptomycin. Test dilutions of anti-IL-15 antibodies and CAT-002 IgG1 as negative isotype control were plated in 96-well format in duplicate. Recombinant hIL-15 (R&D Systems) at an EC80 concentration was incubated with the IL-15 inhibitors for 30 min at room temperature before cells were added at 50 000 cells per well. A titration of hIL-15 alone was also performed in each experiment to confirm IL-15 responsiveness. After 24 h incubation at 37°C in 5% CO2/ 95% humidity 100 µL well of Cell-Titer Glo was added and luminescence read using a Wallac Victor absorbance/luminescence reader.
KIT225 and M-07e assays were undertaken in a similar fashion. Both cell types were grown in the presence of IL-2 in identical media to CTLL-2 and subcultured according to suppliers instructions; however, a period of IL-2 starvation improved their responsiveness to IL-15, and so IL-2 but not serum was removed from the growth media 72 h before the assay and cells seeded in this media in flasks at a density of 2.5 × 105 cells·mL−1. The assay was set up essentially as for CTLL-2 cells, with the exception that these two cell types were plated at a density of 100 000 cells per well. The cells were incubated for 48 h ± IL-15 and inhibitors before cell viability measured using Cell Titer Glo as for CTLL-2 cells. Although greater cell numbers were measured in the presence of hIL-15 compared with assay media without IL-2, the cells were not actively proliferating: they did not incorporate [3H]-thymidine to any great extent for the duration of the assay when this end point was substituted for Cell Titer Glo (data not shown). More properly these assays should be described as assays of IL-15 induced rescue from cytokine withdrawal-induced apoptosis which also was demonstrated using Annexin V staining (data not shown).
Inhibition of biotinylated huIL-15 binding to immobilized IL-15RαFc
Interleukin-15Rα-Fc in phosphate-buffered saline (PBS) at a concentration of 600 pM was coated onto Nunc MaxiSorp™ 96-well plates (Nunc, ThermoFisher Scientific, Loughborough, UK) by incubation at 4°C for 16 h. The wells were washed with PBS and blocked with PBS containing 3% (w·v−1) bovine serum albumin (BSA) for 2 h and washed again with PBS. Inhibitors were diluted in PBS with 0.1% (w·v−1) BSA and added to the IL-15Rα coated assay wells. Biotinylated hIL-15 at a final concentration of 100 pM was added and the assay plates incubated for 1 h. The plates were then washed three times with PBS containing 0.1% (v·v−1) Tween20 followed by addition of diluted Streptavidin-Eu in DELFIA® assay buffer. After 30 min incubation the plates were washed seven times with DELFIA® wash buffer. Finally DELFIA® Enhancement Solution was added, and after 10 min time resolved fluorescence was measured at 620 nm emission wavelength using an Envision 2101 multi-label plate reader (PerkinElmer, Buckinghamshire, UK).
Biotinylated B-E29 epitope competition assay
A homogeneous time-resolved fluorescence (HTRF®) assay was used to measure inhibition of biotinylated B-E29 binding to europium chelate-labelled hIL-15 by anti-IL-15 antibodies. Biotinylated B-E29 was allowed to bind to streptavidin XLent! (CIS Bio International, Bagnols/Cèze, France) by pre-incubating in the dark for 30 min at room temperature. After pre-incubation, biotinylated B-E29/streptavidin mix was added to a 384-well black Optiplate (PerkinElmer). This was followed by the addition of diluted antibody and the diluted europium chelate-labelled IL-15. Unlabelled B-E29 antibody was used as a positive inhibitor control. After 1 h incubation in the dark, time resolved fluorescence at 620 nm and 665 nm was read using an Envision 2101 reader.
Selectivity and species cross reactivity assays
Purified DISC0280 was adsorbed onto 96-well MaxiSorp™ microtitre plates (4 nM in PBS incubated overnight at RT). The wells were washed with PBS-Tween (0.1% v·v−1) and blocked with PBS-Marvel (3% w·v−1) for 1 h. A dilution series of each of the test proteins was prepared in PBS. Non-biotinylated hIL-15 was used as a positive control. To this series, an equal volume of biotinylated recombinant hIL-15 at a concentration of approximately twofold the apparent KD was added (resulting in a series starting at a ratio of competitor antigen : biotinylated hIL-15 of approximately 100:1; KD previously estimated by saturation analysis of bio-IL-15 binding to DISC0280 by essentially the same method). These mixtures were then transferred onto the blocked IgG and allowed to equilibrate for 1.5 h at room temperature. Unbound antigen was removed by washing with PBS-Tween (0.1% v·v−1), while the remaining biotinylated human IL-15 was detected by Streptavidin-Eu conjugate (DELFIA® reagents, PerkinElmer as before). Time-resolved fluorescence was measured at 620 nm on an Envision plate reader (PerkinElmer).
IL-15 and sIL-15Rα administration in vivo
All animal care and experimental procedures involved in these studies complied with the Animals (Scientific Procedures) Act in the UK and were approved by the AstraZeneca R&D UK Ethical Review Committee. Mice (approximately 20 g adult males C57BL/6 strain; 4–5 mice per experimental group) were individually housed in a temperature regulated room on a 12 h/12 h day/night cycle and were given food and water ad libitum. Mice were dosed once per day for three consecutive days with recombinant proteins [human IL-15; 1 µg per mouse (R&D Systems), human IL-15Rα-FLAG-HIS10; 5 µg per mouse] and PBS vehicle or inhibitors via the intra-peritoneal, or subcutaneous route (B-E29, 100 µg per mouse; CAT002, 100 µg per mouse and DISC0280, 30 or 100 µg per mouse). The mice were killed 24 h after the last dose with a high concentration of CO2. The spleens were dissected and temporarily stored in 2 mL of complete RPMI (RPMI 1640 containing 10% FBS, 2 mM glutamine and 100 U·mL−1 penicillin/streptomycin). Half of each spleen was gently pushed through a 70 µm BD Falcon cell strainer (BD Biosciences, Oxford, UK) using a syringe plunger into complete RPMI (Invitrogen, Paisley, UK) in order to generate a single cell suspension. Cells were then pushed through a second cell strainer and counted. The cells were centrifuged and re-suspended in complete RPMI. Cells (1 × 106 per staining condition) were washed with buffer (staining buffer: PBS/0.5%BSA) and re-suspended in 100 µL buffer containing 0.5 µg Fc block [Clone 2.4G2, BD Pharmingen (Oxford, UK) ] and incubated for 10 min at room temperature. After washing the cells, 1 µL of the conjugated antibodies [anti-mouse NK1.1- PE; anti-mouse CD19-FITC or -PE; anti-mouse CD3ε -Cy5; anti-mouse CD8α-PE or isotype control mouse or rat IgG2a-FITC, -PE or -Cy5 (all BD Pharmingen except Mouse IgG2a -PE (Dako, Stockport, UK) ] were then added in the same buffer and incubated at 4°C for 30 min. They were washed once with buffer, after which 500 µL VersaLyse (Beckman Coulter, High Wycombe, UK) was added and incubated for 10 min before a final addition of 500 µL buffer. The samples were analysed on a Beckman Coulter FC500 Flow Cytometer.
In addition, terminal blood samples were taken in EDTA tubes and the presence of NK1.1+ positive cells in whole blood was determined by flow cytometry using an anti-NK1.1- PE labelled antibody (BD Pharmingen). Plasma was used for cytokine analysis. The concentration of six inflammatory cytokines [IL-1β, IL-5, IL-6, TNF-α, RANTES (CCL5) and KC] was measured using the MCYTO_70 K multiplex kit (Millipore, Watford, UK) in accordance with manufacturer's instructions.
Data analysis and statistics
All assay data were analysed using GraphPad Prism software and curve fitting using a 4-parameter logistic equation to generate IC50 values in each of the assays. Activities were typically expressed as the geometric mean IC50 with 95% confidence intervals in brackets. Where HTRF methods were used, ΔF was calculated using equations as advised by CIS Bio International.
Statistical analysis of in vivo data was performed using anova to analyse the entire data set, then using the paired t-test to confirm and run statistical comparisons between individual groups (using GraphPad InStat software).
Materials
Heat-inactivated FBS was obtained from Sigma-Aldrich (UK). Streptomycin, penicillin, geneticin, hygromycin B, Glutamax I, sodium pyruvate, Dulbecco's Modified Eagle's Medium (DMEM) and RPMI 1640 were purchased from Invitrogen (Paisley, UK). Cell-Titer Glo was obtained from Promega (Southampton, UK); cell culture flasks and 96-well tissue culture plates were obtained from Fisher Scientific (Loughborough, UK); oligonucleotides were obtained from Eurogentec (Southampton, UK). All other chemicals were purchased from Sigma-Aldrich (UK) and were of the highest purity available.
Plasmid pDEST12.2 was used for cloning (Life Technologies, Invitrogen, Paisley,UK). DISC0280 was isolated at MedImmune as described below, and CAT002 is a human IgG1κ isotype control (MedImmune, Cambridge, UK); B-E29 was obtained from Diaclone (IDS Ltd, Boldon, Tyne and Wear, UK); MAB647, MAB247, recombinant hIL-2 and hIL-15 were obtained from R&D Systems (Abingdon, UK). Rat and Mouse IL-15 were sourced from Peprotech EC Ltd (London, UK). hIL-21 was obtained from Biosource (Invitrogen, Paisley, UK). Biotinylation of hIL-15 was performed using EZ link NHS-LC-Biotin (Pierce Protein Research products, ThermoFisher, Northumberland, UK) and biotinylation of B-E29 using EZ link Sulfo-NHS-LC-Biotin (Pierce Protein Research products, ThermoFisher, Northumberland, UK). Europium chelate labelling of IL-15 was performed using the LANCE® Eu-W1024-ITC chelate kit (PerkinElmer LAS UK Ltd. Beaconsfield, UK) according to manufacturer's instructions. Europium-labelled streptavidin (Streptavidin-Eu) and all DELFIA buffers also were purchased from PerkinElmer LAS UK. Ltd.
Results
Characterization of DISC0280 in IL-15 dependent cell assays
To characterize the properties of DISC0280, we evaluated its activity in a panel of hIL-15-dependent cell assays. IL-15 has been shown to cause the survival and proliferation of the mouse CTLL-2 and human KIT225 T cell lines (Eisenman et al., 2002; Mortier et al., 2004). The EC50 of hIL-15 was ∼20 pM in both assays. The activity of DISC0280 in these assays was compared with previously characterized (Bernard et al., 2004) anti-hIL-15 antibodies: B-E29, MAB647 and MAB247 (Figure 1A and B). In the CTLL-2 and KIT225 assays DISC0280 was shown to dose-dependently inhibit the activity of hIL-15 with an IC50 3.4 pM (1.2 to 9.2 pM)) and 66.5 pM (58.7 to 75.3 pM) respectively. This activity was greater than or equivalent to the potency of B-E29, MAB247 or MAB647. Thus DISC0280 antagonizes the actions of hIL-15 at either human or mouse IL-15 receptors in these assays. As CTLL-2 and KIT225 cells express IL-15Rα, β and γc we next investigated whether DISC0280 could inhibit IL-15 signalling through IL-15β and γc alone.
Figure 1.
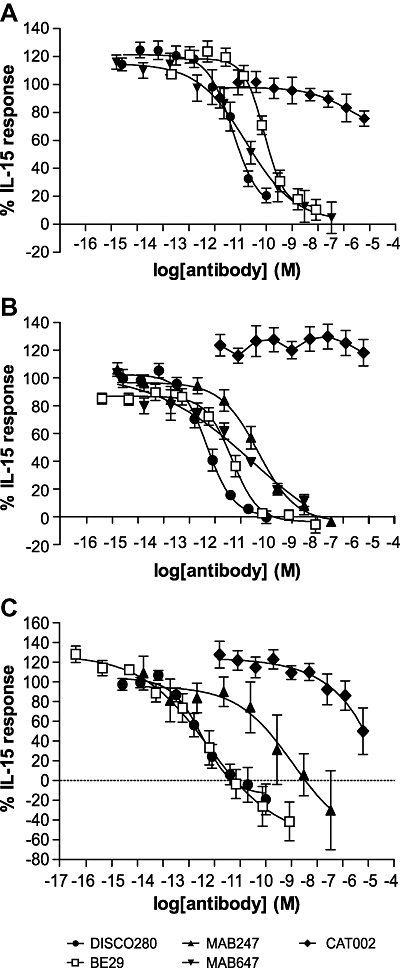
Inhibition of hIL-15-dependent cell effects in CTLL-2, KIT225 and M-07e cells by anti-IL-15 mAbs or CAT002, a human IgG1 isotype control (A) IL-15 dependent KIT225 cell survival assay, (B) IL-15 dependent CTLL-2 cell survival assay, (C) IL-15 dependent M-07e cell survival assay. All data is the mean of three experiments and error bars represent standard deviation. hIL-15, human IL-15; hIL-15/sIL-15Rα, pre-associated complex of human IL-15 and soluble IL-15 receptor α; IL-15, interleukin-15.
M-07e, a cell line expressing only the IL-15Rβ and γc subunits, has been described as being IL-15 dependent (Meazza et al., 1998). The absence of IL-15Rα in this cell line was additionally suggested by RT-PCR (normalizing to an IL-15 non-responsive cell line) and flow cytometry in cells which had been grown in either the presence or absence of IL-2 (Figures S1 and S2, Table S1). In this assay, the EC50 of hIL-15 for the survival of M-07e cells was ∼30 pM, equivalent to the observation in KIT225 cells. DISC0280, B-E29 and MAB647 were able to completely inhibit the activity of hIL-15 in this M-07e assay, with IC50s of 8.5 pM (0.7 pM–99.7 pM), 5.7 pM (0.5 to 65 pM) and 3 nM respectively (Figure 1C). MAB247 was not directly compared with DISC0280 in this assay.
Characterization of DISC0280 epitope
To understand the epitope recognized by DISC0280, it was tested in a time-resolved fluorescence assay that measured the binding of biotinylated hIL-15 to immobilized recombinant sIL-15Rα. A decrease of signal in this assay on incubation with antibodies demonstrates a disruption of the interaction between IL-15 and IL-15Rα either by direct competition or by steric hindrance of the interaction. DISC0280 completely inhibited the binding of biotinylated hIL-15 to IL-15Rα with an IC50 of 47 pM (31 to 71 pM) (Figure 2). In contrast, neither B-E29 nor MAB647 nor MAB247 gave full inhibition in this assay, although B-E29 and MAB247 partially inhibited hIL-15 and sIL-15Rα binding at concentrations of 30 nM and above.
Figure 2.
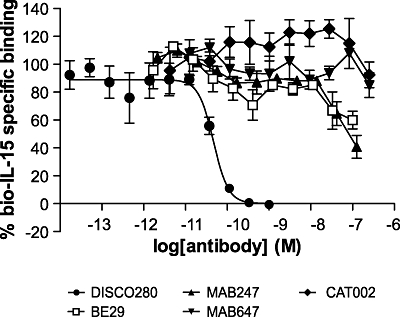
Inhibitory effects of DISC0280 on human interleukin-15 (hIL-15) binding to interleukin 15 receptor α (IL-15Rα). Labelled hIL-15 at a single concentration was incubated with immobilized IL-15Rα-Fc in the presence of a titration of inhibitor, as described. All data is the mean of three experiments and error bars represent standard deviation.
As the epitope of B-E29 has been previously described (Bernard et al., 2004), we used this antibody in an epitope competition assay with DISC0280. In this assay, the binding of biotinylated B-E29 to europium chelate-labelled hIL-15 is detected in a homogeneous format. A decrease in the signal in this assay indicates the epitope for the antibody tested and B-E29 are at least overlapping, or that binding of the test antibody to IL-15 sterically inhibits binding of IL-15 to B-E29. DISC0280 completely inhibited the binding of labelled B-E29 (IC50s of 0.82 nM and 0.53 nM in two independent experiments) to hIL-15 (Figure 3). In contrast, MAB647 and MAB247 did not compete with B-E29 binding in this assay with no inhibition detected up to a concentration of 3 µM (data not shown).
Figure 3.
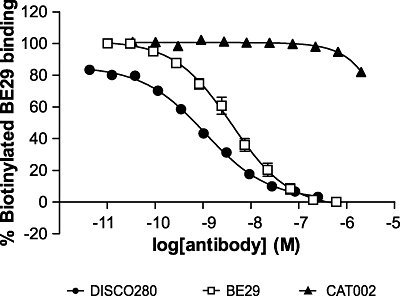
DISC0280 shares an overlapping epitope with B-E29. The inhibition of labelled B-E29 binding to human interleukin-15 by mAbs was measured by a homogeneous time resolved fluorescence method, as described. The experiment was repeated twice and representative data from one experiment are presented.
Characterization of DISC0280 with IL-15-dependent effects in vivo
Prior to evaluating the antibody in vivo, we confirmed the selectivity and specificity of the antibody by competition elisa. DISC0280 was specific for hIL-15 with an IC50 in this assay of 0.7 nM. It did not bind mouse or rat IL-15, nor hIL-2 or IL-21, the proteins most closely related by amino acid sequence, at concentrations of each up to 10 µM. In order to test the effects of DISC0280 on IL-15-mediated effects in vivo a mouse model was set up which measured the increase in NK1.1+ and CD3+ cells as a result of once daily dosing of hIL-15 over 3 days. Consistent with previous observations (Rubinstein et al., 2002; 2006; Stoklasek et al., 2006; Dubois et al., 2008) intraperitoneal administration of IL-15 over 3 days induced a significant expansion of NK1.1+ cells (P < 0.001) in the spleens of treated mice (Figure 4A), an effect which is increased further by the co-administration of sIL-15Rα (without an IgG1 Fc domain) as a complex with hIL-15 (Figure 4A column 4, P < 0.001). In addition, when hIL-15 and IL-15Rα were administered separately at different sites 1 h apart, the same effect on NK1.1+ cells was seen (Figure 4A column 5, P < 0.01). The administration of pre-associated IL-15/IL-15Rα complex also increased progenitor/NK1.1+ cells in the peripheral blood and induced myeloid hyperplasia coincident with expansion of the NK1.1+ population (data not shown). Also consistent with previous observations, co-administration of IL-15/IL15Rα additionally produced a significant increase in splenic CD3+ cells, only a proportion of which can be attributed to an expansion of CD8+ cells (Figure 4B), and also increases in CD19+ cells were observed (P < 0.001, data not shown).
Figure 4.
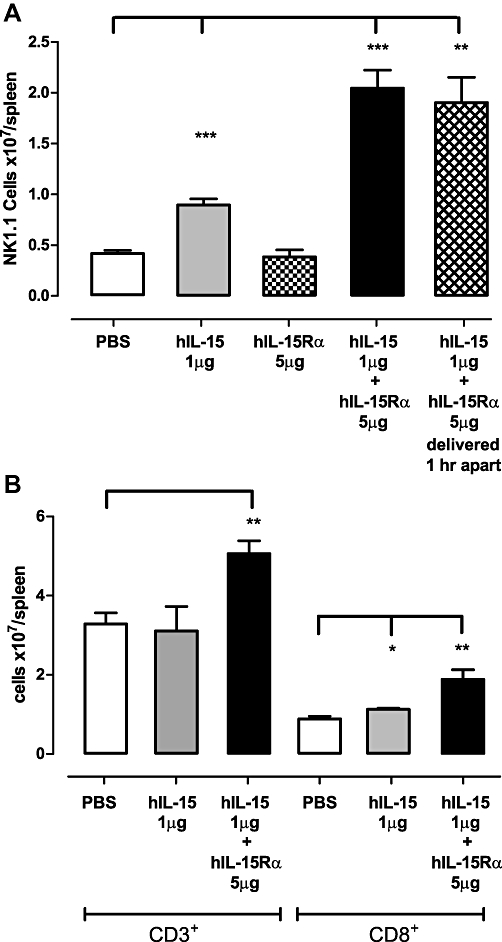
Effect of hIL-15 and sIL-15Rα administration on total numbers of (A) NK1.1+ cells, (B) CD3+/CD8+ cells in the spleens of treated mice. C57BL/6/J male mice (n = 4 per group) were dosed once per day for three consecutive days with recombinant proteins as indicated. 24 h post treatment spleens were extracted and the total number of NK1.1+ CD3+ and CD8+ cells measured according to Materials and Methods. hIL-15 alone and pre-associated IL-15/sIL-15Rα complex significantly increased numbers of NK1.1+ cells in the spleen compared with PBS-treated animals. Administration of hIL-15 followed by sIL-15Rα 1 h apart at separate sites caused a significant increase in numbers of NK1.1+ cells compared to PBS-treated animals. *P < 0.05, **P < 0.01, ***P < 0.001. hIL-15, human interleukin-15; hIL-15/sIL-15Rα, pre-associated complex of human IL-15 and soluble IL-15 receptor α; IL-15, interleukin-15; PBS, phosphate-buffered saline.
The increase in NK1.1+ cells in the spleen caused by hIL-15 alone was shown to be IL-15 specific as it could be dose-proportionally inhibited by the anti-hIL-15 antibody B-E29 (Figure 5A); however, dosing with an irrelevant IgG1 control had no effect. In addition to this, B-E29 was also able to inhibit the enhanced NK1.1+ cell production induced by administration of the hIL-15/sIL-15Rα complex (Figure 5B).
Figure 5.
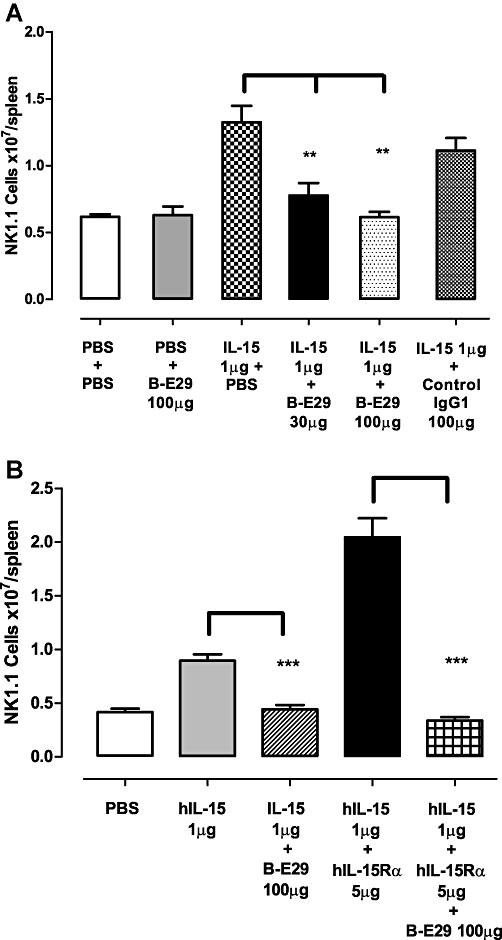
(A) Treatment of mice with B-E29 causes a dose dependent decrease in the effect of hIL-15 on NK1.1+ cells. An irrelevant IgG1 control has no effect on the response to IL-15. (B) Treatment of mice with B-E29 significantly inhibited the effects of hIL-15 and pre-associated hIL-15/IL-15Rα complex. Error bars indicate SEM values. *P < 0.05, **P < 0.01, ***P < 0.001. hIL-15, human interleukin-15; hIL-15/sIL-15Rα, pre-associated complex of human IL-15 and soluble IL-15 receptor α; IL-15, interleukin-15; PBS, phosphate-buffered saline.
However, while the administration of DISC0280 alone had no significant effect compared with vehicle-treated animals, when 30 or 100 µg of DISC0280 was administered in the presence of hIL-15, there was a 6.9-fold to eightfold increase in NK1.1+ cells in the spleen compared with vehicle-treated animals (P < 0.001) and animals treated with hIL-15 only (P < 0.001) (Figure 6A). CD3+ T cell numbers in the spleen also increased in response to DISC0280 treatment in line with NK1.1 cells (CD8+, CD19+ cells were not measured on this occasion) (Figure 6B). When DISC0280 was dosed into animals treated with pre-associated IL-15/IL-15Rα complex there were significantly fewer spleen NK1.1+ cells compared with animals dosed with DISC0280 and IL-15 alone (P < 0.01). This observation was also repeated for CD3 + cell numbers.
Figure 6.
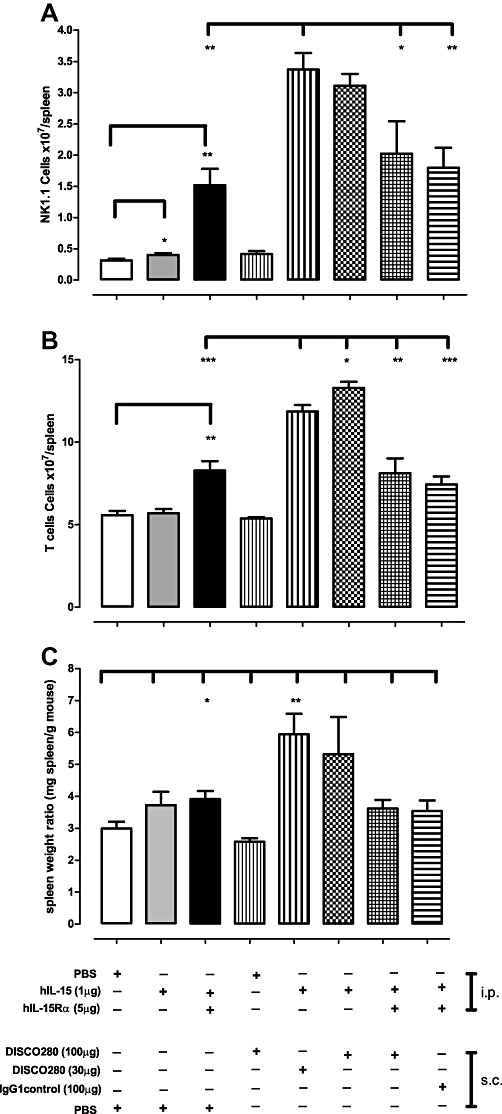
Effect of DISC0280 on hIL-15- and sIL-15Rα-induced increase in splenic NK1.1+ cells, CD3+ cells and spleen weight. C57BL/6/J male mice (n = 5 per group) were dosed once per day for 3 consecutive days with recombinant proteins as indicated. 24 h post treatment the spleens were weighed and cells extracted and (A) the number of NK1.1+ cells (B) CD3+ cells measured according to materials and methods and (C) weight of spleens reported as a ratio to body weight of animal. Error bars indicate SEM values. *P < 0.05, **P < 0.01, ***P < 0.001. hIL-15, human interleukin-15; hIL-15Rα, human interleukin 15 receptor α; PBS, phosphate-buffered saline; sIL-15Rα, soluble interleukin 15 receptor α.
Co-administration of DISC0280 and IL-15 generated a marked splenomegaly in treated animals (Figure 6C). Increases in NK1.1+ cells were also observed in peripheral blood. Despite the increase in spleen size and expansion of NK1.1+ and CD3+ cells, there were no obvious adverse effects seen in the mice over the course of the experiment.
In order to explore the possibility that effects were caused by a non-specific proinflammatory mechanism of DISC0280 or by contributing putative contaminants, the terminal blood samples of all treated mice were tested for six murine proinflammatory cytokines (IL-1β, IL-6, KC, TNF-α, IL-5 and CCL5). Animals treated with hIL-15 and 30 or 100 µg of DISC0280 respectively, showed significant increases in IL-5 concentrations when compared with control treatment groups (P < 0.05 and <0.001 respectively) (Figure 7). Plasma levels of TNF-α, IL-1β and KC remained unchanged between groups (Table S2). Variable responses with evidence of increased IL-6 and CCL5 in some animals were observed within groups. In contrast, when IL-15 or IL-15 plus DISC0280 (IgG or FAb) were incubated with isolated mouse splenocytes in vitro for 24 h or 48 h, no detectable level of IL-5, or other cytokines from a panel of TH1/TH2 cytokines [interferon (IFN)γ, IL-1β, IL-10, IL-12 total, IL-2, IL-4, IL-5, KC, TNF-α] was measured above background levels in the tissue culture supernatant (data not shown).
Figure 7.
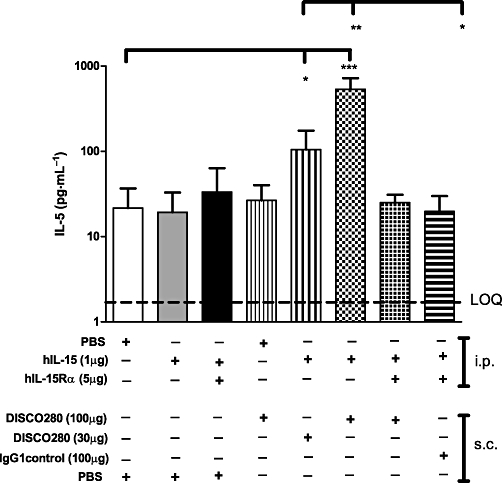
Administration of DISC0280 and hIL-15 increases IL-5 levels. The plasma concentrations of IL-5 in the terminal bleeds of all the mice treated as in Figure 6, were measured. Limit of Quantification (LOQ) is shown at 3 pg·mL−1. Error bars show standard deviation from the mean. *P < 0.05, **P < 0.01, ***P < 0.001. hIL-15, human interleukin-15; hIL-15Rα, human interleukin 15 receptor α; IL-5, interleukin-5.
Discussion
DISC0280 was originally isolated as an anti-hIL-15 antibody as a potential treatment of inflammatory diseases. Our epitope mapping experiments demonstrated that DISC0280 disrupted the interaction between IL-15 and its cognate receptor IL-15Rα.
Additionally, the co-crystal structure of DISC0280 FAb complexed with IL15 has recently been generated (Dr D. Lowe et al., pers. comm.). In this study it was demonstrated that DISC0280 and IL-15Rα have overlapping epitopes on IL-15 supporting our observations in the current study. The epitope of B-E29 was previously mapped (Bernard et al., 2004) using peptide scanning technology to residues 61–72, which partially overlaps with the IL-15/IL-15Rα interface and the epitope of DISC0280.
Moreover, we have demonstrated that DISC0280 is a potent inhibitor of IL-15-dependent CTLL-2 and KIT225 cell survival. This is supported by recent observations (Bouchaud et al., 2008), which showed that IL-15-dependent survival of these cells is also competitively inhibited by sIL-15Rα. These cell lines endogenously express IL-15Rα, IL-15Rβ and γc proteins, but interestingly DISC0280 also inhibits the IL-15-dependent responses of M-07e cells, which do not express IL-15Rα at their surface (Meazza et al., 1998, and our data).
Thus, based on these observations that DISC0280 not only inhibits IL-15 binding to IL-15Rα but also inhibits its interaction with the βγ chain, we decided to investigate this further by comparing the effects of DISC0280 with those of B-E29. This antibody partially inhibits binding of hIL-15 to IL-15Rα (Bernard et al., 2004) and its mapped epitope corresponds to a peptide sequence in IL-15 which forms part of both the IL-15Rβ binding site and the IL-15Rα binding site (Olsen et al., 2007). In our studies DISC0280 competes with B-E29 for binding to IL-15 suggesting these antibodies have overlapping epitopes, however they are sufficiently different that, despite being of similar potency in IL-15-dependent cell assays, DISC0280 completely inhibits IL-15 binding to IL-15Rα whereas B-E29 only gives partial inhibition.
Previous investigators have shown that sIL-15Rα plus IL-15, or the extracellular ‘sushi’ domain of IL-15Rα fused to IL-15, can act as ‘hyperagonists’ (Mortier et al., 2006; Rubinstein et al., 2006; Stoklasek et al., 2006). This is in contrast to IL-2 which is inhibited by addition of sIL-2Rα (Rubinstein et al., 2006). The in vivo effects of IL-15 administration in mice with combinations of sIL-15Rα-Fc have been well documented (Rubinstein et al., 2006; Stoklasek et al., 2006; Dubois et al., 2008). Therefore, we generated a mouse model to confirm these findings, and to investigate the effects of DISC0280 in vivo in the presence of hIL-15 or with the complex of hIL-15/sIL-15Rα.
Consistent with previous reports, we showed that dosing either hIL-15 or pre-associated hIL-15/sIL-15Rα complex gave a significant increase in NK1.1+ cells, CD3+ T cells, CD8+ cells and CD19+ cells in the spleen and systemic circulation. Similar effects were seen with exogenous murine IL-15 (data not shown). We also showed that separate injections of hIL-15 and sIL-15Rα at different sites of administration, spaced 1 h apart was still sufficient to generate an increase in NK1.1+ cells over and above IL-15 alone. In order to best mimic endogenous sIL-15Rα which is expected to be present in vivo (Budagian et al., 2004; Mortier et al., 2004), we used the soluble extracellular domain of IL-15Rα and not an Fc fusion construct (Rubinstein et al., 2006; Stoklasek et al., 2006).
Using this model, B-E29 completely inhibited the NK1.1+ proliferation in both hIL-15 or hIL-15/IL-15Rα complex treated mice, consistent with it being an antagonist of hIL-15 activity. By contrast in the same model DISC0280 in hIL-15-treated mice caused an increase of NK1.1+ and CD3+ T cells plus spleen enlargement over and above that seen for hIL-15 alone or hIL-15/sIL-15Rα complex. In addition, the administration of DISC0280 did not inhibit the effect of hIL-15/sIL-15Rα complex. There were no general increases in terminal plasma TNF-α, KC or IL-1β with any particular treatment, demonstrating that the effects seen were not via a non-specific proinflammatory mechanism. Detectable levels of CCL5 or IL-6 that were observed in some animals treated with DISC0280 alone, were not likely to be the cause of the enhanced NK1.1+ or CD3+ effects, as IL-6 and CCL5 levels did not correlate with the proliferation of NK1.1+ /CD3+ cells observed.
We showed in vitro that DISC0280 binds specifically to hIL-15 and not mIL-15 or related proteins. In association with this, DISC0280 treatment alone in vivo gave no significant increase in NK1.1+ or CD3+ cells or increase in spleen weight so the effects seen with DISC0280 in vivo appear to be specific to the co-administration of the human form of IL-15, consistent with specificity of binding to hIL-15. To date, the precise mechanism for the observed increases in plasma IL-5 in DISC0280 plus IL-15-treated mice remain unclear; however, IL-5 production has been noted at intermediate stages of differentiation of NK cells from progenitor cells stimulated by IL-15 and IL-2 (Loza et al., 2002). Furthermore, IL-15 has also been noted as a key promoter of IL-5 production from T-helper cells (Mori et al., 1996), consistent with our observation of increased NK and T cell numbers in response to DISC0280 in this model. This observation could not be replicated in vitro by incubating hIL-15 ± DISC0280 with isolated mouse splenocytes (data not shown), suggesting some contextual activation of this response irrespective of extension of half-life of IL-15 in the circulation of these animals by DISC0280, because availability of IL-15 and DISC0280 to the cells in vitro would not be a limiting factor. Additionally, IL-15 has been reported to increase cytokine production in a cell-contact dependent manner, for example TNF-α production from macrophages was increased when co-cultured with peripheral blood monocytes isolated from rheumatoid arthritis patients in vitro via an IL-15 and cell contact dependent mechanism (McInnes et al., 1997). In our study an increase in serum TNF-α was not observed with IL-15, IL-15Rα nor DISC0280 despite the increase in T cell and NK numbers and despite the presumed increase in half-life of IL15 which occurs when bound to DISC0280. This lack of TNF-α production but increase in IL-5 could be of mechanistic significance to DISC0280, and further investigation of a wider panel of TH1/TH2 cytokines is required to elucidate this mechanism.
In this study, we have shown that DISC0280 and sIL-15Rα have a competing epitope on hIL-15. Soluble IL-15Rα can act as an inhibitor of IL-15 dependent assays as well as acting as a hyperagonist of IL-15 in vivo (Dubois et al., 2008). Based on these observations we hypothesize that DISC0280 enhanced the activity of hIL-15 in vivo by providing a platform for trans-presentation of hIL-15 in a manner analogous to that previously described for sIL-15Rα. It is unlikely that the effects of DISC0280 on NK1.1+ cells, T cells or IL-5 can be explained simply by an increase in the half-life of hIL-15 imparted by the antibody as the ability to extend the half-life in plasma of IL-15 from hours to days would be expected to be equal for B-E29 and DISC0280. However, no formal analysis of the pharmacokinetics of the antibody-IL-15 complexes in the circulation of the animals has been performed. It is also unlikely that DISC0280 may enhance the hIL-15 activity by increasing the affinity of hIL-15 for IL-15Rβ/γc, as if this were the mechanism, the same effect would be expected in vitro and was not seen in M0-7e assays. Human IgG1 mAbs have been shown to be capable of binding mouse FcγR, which is expressed on the surface of a number of cells including macrophages and dendritic cells. It is therefore possible that DISC0280 complexed with hIL-15 binds to FcγR on the surface of mouse cells, which in turn present the hIL-15 to circulating NK cells. In this way, DISC0280 may more effectively trans-present IL-15 than the sIL-15Rα used in this study. In a recent study, Huntington et al., 2009 have used a Rag2-/-γc-/- mouse to reconstitute and study human NK cell development in mice and suggested that IL-15 agonists did not require an Fc domain to increase NK cell number. However, by comparing a covalently linked sushi domain-IL-15 molecule without Fc-binding domain and a non-covalently-linked complex with Fc domain, it is possible that the relative importance of covalent linkage versus Fc domain interactions under physiological conditions may not have been fully assessed. Whether mechanistically required or not, the Fc domain confers favourable extension of half-life via FcRN binding for more attractive therapeutic dosing regimens. In considering whether an anti-IL-15 agonistic antibody or IL-15Rα-Fc would make better clinical substitutes for IL-15, it is worth considering that the manufacture of IgG1 for therapeutic use is well established and may offer a greater chance of technical and regulatory success to the clinic.
While it is unknown whether identical mechanisms exist for DISC0280 and IL-15, it is interesting to note that a neutralizing anti-IL-2 antibody (S4B6) in vitro, when co-administered with IL-2 in vivo caused a >100-fold expansion of CD8+ cells (Boyman et al., 2006). S4B6 has an epitope on IL-2 that apparently overlaps the binding site of IL-2Rα, analogous with our observations with DISC0280 and IL-15.
If DISC0280 were to be considered as a therapeutic alternative to IL-15 or IL-15Rα it would be important to elucidate further its effects on T cell subsets. While we have demonstrated that CD3+ cells do increase in response to DISC0280, the precise effects of DISC0280 on T cells such as CD8+/CD4+ cells, γ/δ T cells and TReg cells has not been tested. Also, the theoretical effect of engagement of trans-presented IL-15 on activated myeloid cells should be investigated. Although we would anticipate that DISC0280 would not be able to bind to IL-15 presented by IL-15Rα at the surface of cells due to the competitive nature of the binding sites, it is theoretically possible that DISC0280 could bind IL-15 presented in different ways by myeloid cells.
With regard to the therapeutic utility of IL-15 antibodies, what is clear is that the epitope is crucial to whether the antibody behaves as an antagonist or agonist in vivo. Despite binding to at least some common regions on IL-15, B-E29 and DISC0280 antibodies have opposing effects in vivo, while retaining similar in vitro activity. This highlights the complexity of identifying anti-IL-15 antibodies as therapies for diseases where IL-15 has been implicated, such as rheumatoid arthritis and psoriasis. It also indicates that much more is yet to be elucidated regarding the way in which IL-15 communicates between cell subtypes under physiological and pathological situations. Nevertheless, these data also demonstrate that an anti-IL-15 antibody in a pharmaceutically feasible IgG1 format, may be able to substitute therapeutically for IL-15.
Acknowledgments
The authors would like to acknowledge the expert technical help of David Rider and Dorothy Sims for M-07e/KIT225 flow cytometry and Joanne Woods for M-07e/KIT225 Taqman analysis.
Glossary
Abbreviations
- γc
common γ chain
- hIL-15
human IL-15
- hIL-15/sIL-15Rα
pre-associated complex of human IL-15 and soluble IL-15Rα
- IL-2
interleukin 2
- (s)IL-15Rα
(soluble)interleukin 15 receptor α
- IL-15Rβ
shared interleukin 2/ interleukin 15 receptor β chain
Conflicts of interest
All work was funded by AstraZeneca R&D or MedImmune, and all authors are or were employees of these organizations.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Teaching Materials; Figs 1–7 as PowerPoint slide.
Figure S1 Relative expression levels of mRNA of (A) IL-15Rα (B) IL-2Rα (C) IL-2Rβ and (D) IL-2Rγ in a panel of cell lines. HEK293 cells are used as the comparator cell line as these do not respond to IL-15 or IL-2. These cells were not growth factor starved (Mean ± SEM for three samples; representative of two experiments).
Figure S2 IL-15Rα surface expression was determined by flow cytometry using monoclonal antibodies directly labelled with fluorophores as described in Supplementary Methods. Representative histograms of one of three experiments are shown. (A) M0-7e and (B) KIT225 cells were stained after growth-factor starvation for 72 h. Staining was also performed before growth factor starvation with very similar results (data not shown). Dead cells were excluded by gating for 7AAD/Annexin V positive staining. Responsiveness to IL-15 was confirmed by proliferation in a parallel experiment with the same cells (data not shown) and IL-2Rβ and γc receptor expression confirmed by flow cytometry with analogous methods (data not shown). Staining with the anti-IL-15Rα-FITC antibody is shown shaded with broken line; isotype control-FITC staining is unshaded with solid line.
Table S1 Primers and probes designed to the target IL-15 and IL-2 receptor genes or 18S ribosomal RNA housekeeping control
Table S2 Cytokine levels in the terminal plasma samples of mice treated with recombinant proteins and mAbs
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Baslund B, Tvede N, Danneskiold-Samsoe B, Larsson P, Panayi G, Petersen J, et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005;52:2686–2692. doi: 10.1002/art.21249. [DOI] [PubMed] [Google Scholar]
- Bergamaschi C, Rosati M, Jalah R, Valentin A, Kulkarni V, Alicea C, et al. Intracellular interaction of interleukin-15 with its receptor alpha during production leads to a mutual stabilisation and increased bioactivity. J Biol Chem. 2008;283:4189–4199. doi: 10.1074/jbc.M705725200. [DOI] [PubMed] [Google Scholar]
- Bernard J, Harb C, Mortier E, Quemener A, Meloen RH, Vermot-Desroches C, et al. Identification of an interleukin-15alpha receptor-binding site on human interleukin-15. J Biol Chem. 2004;279:24313–24322. doi: 10.1074/jbc.M312458200. [DOI] [PubMed] [Google Scholar]
- Bouchaud G, Garrigue-Antar L, Solé V, Quéméner A, Boublik Y, Mortier E, et al. The exon-3-encoded domain of IL-15Rα contributes to IL-15 high-affinity binding and is crucial for the IL-15 antagonistic effect of soluble IL-15Rα. J Mol Biol. 2008;382:1–12. doi: 10.1016/j.jmb.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- Budagian V, Bulanova E, Orinska Z, Pohl T, Borden EC, Silverman R, et al. Reverse signaling through membrane-bound interleukin-15. J Biol Chem. 2004;279:42192–42201. doi: 10.1074/jbc.M403182200. [DOI] [PubMed] [Google Scholar]
- Bulanova E, Budagian V, Duitman E, Orinska Z, Krause H, Ruckert R, et al. Soluble Interleukin IL-15Ralpha is generated by alternative splicing or proteolytic cleavage and forms functional complexes with IL-15. J Biol Chem. 2007;282:13167–13179. doi: 10.1074/jbc.M610036200. [DOI] [PubMed] [Google Scholar]
- Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200:825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15Rα-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T Cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- Eisenman J, Ahdieh M, Beers C, Brasel K, Kennedy MK, Le T, et al. Interleukin-15 interactions with interleukin-15 receptor complexes: characterization and species specificity. Cytokine. 2002;20:121–129. doi: 10.1006/cyto.2002.1989. [DOI] [PubMed] [Google Scholar]
- Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- Giri JG, Ahdieh M, Eisenman J, Shanebeck K, Grabstein K, Kumaki S, et al. Utilization of the beta and gamma chains of the IL-2 receptor by the novel cytokine IL-15. EMBO J. 1994;13:2822–2830. doi: 10.1002/j.1460-2075.1994.tb06576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri JG, Kumaki S, Ahdieh M, Friend DJ, Loomis A, Shanebeck K, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995;14:3654–3663. doi: 10.1002/j.1460-2075.1995.tb00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron-Michel J, Giuliani M, Fogli M, Brouty-Boye D, Ferrini S, Baychelier F, et al. Membrane-bound and soluble IL-15/IL-15Ralpha complexes display differential signaling and functions on human hematopoietic progenitors. Blood. 2005;106:2302–2310. doi: 10.1182/blood-2005-01-0064. [DOI] [PubMed] [Google Scholar]
- Hori T, Uchiyama T, Tsudo M, Umadome H, Ohno H, Fukuhara S, et al. Establishment of an interleukin 2-dependent human T cell line from a patient with T cell chronic lymphocytic leukemia who is not infected with human T cell leukemia/lymphoma virus. Blood. 1987;70:1069–1072. [PubMed] [Google Scholar]
- Huntington ND, Legrand N, Alves NL, Jaron B, Weijer K, Plet A, et al. IL-15 trans-presentation promotes human NK cell development and differentiation in vivo. J Exp Med. 2009;206:25–34. doi: 10.1084/jem.20082013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev. 2002;13:429–439. doi: 10.1016/s1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Loza M, Zamai L, Azzoni L, Rosati E, Perussia B. Expression of type 1 (interferon gamma) and type 2 (interleukin-13, interleukin-5) cytokines at distinct stages of natural killer cell differentiation from progenitor cells. Blood. 2002;99:1273–1281. doi: 10.1182/blood.v99.4.1273. [DOI] [PubMed] [Google Scholar]
- Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-α production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- Meazza R, Basso S, Gaggero A, Detotero D, Trentin L, Pereno R, et al. Interleukin (IL)-15 induces survival and proliferation of the growth factor-dependent acute myeloid leukemia M-07e through the IL-2 receptor beta/gamma. Int J Cancer. 1998;78:189–195. doi: 10.1002/(sici)1097-0215(19981005)78:2<189::aid-ijc12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Mori A, Suko M, Kaminuma O, Inoue S, Ohmura T, Nishizaki Y, et al. IL-15 promotes cytokine production of human T helper cells. J Immunol. 1996;156:2400–2405. [PubMed] [Google Scholar]
- Mortier E, Bernard J, Plet A, Jacques Y. Natural, proteolytic release of a soluble form of human IL-15 receptor alpha-chain that behaves as a specific, high affinity IL-15 antagonist. J Immunol. 2004;173:1681–1688. doi: 10.4049/jimmunol.173.3.1681. [DOI] [PubMed] [Google Scholar]
- Mortier E, Quemener A, Vusio P, Lorenzen I, Boublik Y, Grotzinger J, et al. Soluble interleukin-15 receptor alpha (IL-15Rα)-sushi as a selective and potent agonist of IL-15 action through IL-15Rbeta/gamma: hyperagonist IL-15- IL-15Rα fusion proteins. J Biol Chem. 2006;281:1612–1619. doi: 10.1074/jbc.M508624200. [DOI] [PubMed] [Google Scholar]
- Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15Rα chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen SK, Ota N, Kishishita S, Kukimoto-Niino M, Murayama K, Uchiyama H, et al. Crystal structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation. J Biol Chem. 2007;282:37191–37204. doi: 10.1074/jbc.M706150200. [DOI] [PubMed] [Google Scholar]
- Rubinstein MP, Kadima AN, Salem ML, Nguyen CL, Gillanders WE, Cole DJ. Systemic administration of IL-15 augments the antigen-specific primary CD8+ T cell response following vaccination with peptide-pulsed dendritic cells. J Immunol. 2002;169:4928–4935. doi: 10.4049/jimmunol.169.9.4928. [DOI] [PubMed] [Google Scholar]
- Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, et al. Converting IL-15 to a superagonist by binding to soluble IL-15Rα. Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchatz H, Leung BP, Wei XQ, McInnes IB, Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998;160:5654–5660. [PubMed] [Google Scholar]
- Ruckert R, Brandt K, Braun A, Hoymann HG, Herz U, Budagian V, et al. Blocking IL-15 prevents the induction of allergen-specific T cells and allergic inflammation in vivo. J Immunol. 2005;174:5507–5515. doi: 10.4049/jimmunol.174.9.5507. [DOI] [PubMed] [Google Scholar]
- Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173:6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J, Pope T, Tung J-S, Chan C, Hollis G, Mark G, et al. Affinity maturation of a high-affinity human monoclonal antibody against the third hypervariable loop of human immunodeficiency virus: use of phage display to improve affinity and broaden strain reactivity. J Mol Biol. 1996;256:77–88. doi: 10.1006/jmbi.1996.0069. [DOI] [PubMed] [Google Scholar]
- Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, et al. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. 1996;14:309–314. doi: 10.1038/nbt0396-309. [DOI] [PubMed] [Google Scholar]
- Villadsen LS, Schuurman J, Beurskens F, Dam TN, Dagnaes-Hansen F, Skov L, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. J Clin Invest. 2003;112:1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann TA, Tagaya Y. The multifaceted regulation of Interleukin-15 expresssion and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- Wuttge DM, Wildt M, Geborek P, Wollheim FA, Scheja A, Akesson A. Serum IL-15 in patients with early systemic sclerosis: a potential novel marker of lung disease. Arthritis Res Ther. 2007;9:R85. doi: 10.1186/ar2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


