Abstract
Mutations in transcription factor RUNX1 are associated with familial platelet disorder, thrombocytopenia, and predisposition to leukemia. We have described a patient with thrombocytopenia and impaired agonist-induced platelet aggregation, secretion, and glycoprotein (GP) IIb-IIIa activation, associated with a RUNX1 mutation. Platelet myosin light chain (MLC) phosphorylation and transcript levels of its gene MYL9 were decreased. Myosin IIA and MLC phosphorylation are important in platelet responses to activation and regulate thrombopoiesis by a negative regulatory effect on premature proplatelet formation. We addressed the hypothesis that MYL9 is a transcriptional target of RUNX1. Chromatin immunoprecipitation (ChIP) using megakaryocytic cells revealed RUNX1 binding to MYL9 promoter region −729/−542 basepairs (bp), which contains 4 RUNX1 sites. Electrophoretic mobility shift assay showed RUNX1 binding to each site. In transient ChIP assay, mutation of these sites abolished binding of RUNX1 to MYL9 promoter construct. In reporter gene assays, deletion of each RUNX1 site reduced activity. MYL9 expression was inhibited by RUNX1 short interfering RNA (siRNA) and enhanced by RUNX1 overexpression. RUNX1 siRNA decreased cell spreading on collagen and fibrinogen. Our results constitute the first evidence that the MYL9 gene is a direct target of RUNX1 and provide a mechanism for decreased platelet MYL9 expression, MLC phosphorylation, thrombocytopenia, and platelet dysfunction associated with RUNX1 mutations.
Introduction
Transcription factor RUNX1 (also called core binding factor A2 [CBFA2] and acute myeloid leukemia-1 [AML-1]) plays an important role in hematopoiesis.1–3 RUNX1 is critical for embryonic definitive hematopoiesis and maintenance of adult hematopoiesis.4,5 It is required for megakaryocytic maturation and in lymphocyte differentiation.6 RUNX1 protein binds to consensus sequence TGt/cGGT or ACCa/gCA to regulate various genes related to hematopoiesis.7,8 In humans, germline heterozygous mutations in RUNX1 are associated with a familial platelet disorder, moderate thrombocytopenia, and propensity to develop myeloid malignancy.9–11 RUNX1 mutations have been identified in several pedigrees, including missense, frameshift, and nonsense mutations, and a large intragenic deletion.9,10,12–15 Most of the mutations have occurred in the conserved RUNT homology domain leading to the loss of DNA binding.10,16
We have previously reported studies in a patient with inherited thrombocytopenia, impaired platelet aggregation, and secretion responses associated with diminished agonist induced myosin light chain (MLC) phosphorylation.17 This patient has a heterozygous mutation in RUNX1 characterized by a single nucleotide mutation between intron 3 and exon 4, at the splice acceptor site for exon 4, that generates a frameshift and premature termination in the RUNT domain.12,17 Subsequent studies using platelet expression profiling on this patient showed a striking down-regulation of MLC gene MYL9 by approximately 77-fold, but not of other MLC genes (MRCL3, MRCL2, and MYL5) or in MLC kinase.18 Additional platelet abnormalities reported in our patient were diminished GPIIb-IIIa activation, impaired pleckstrin phosphorylation, and decreased platelet protein kinase C-θ and platelet factor 4.12,17,19
Myosins are a diverse superfamily of actin-dependent molecular motors consisting of distinct structural and functional classes and are implicated in cell shape, migration, adhesion, intracellular transport of organelles, signal transduction, and tumor suppression.20,21 Megakaryocytes (MKs) contain abundant amounts of myosin IIA (gene MYH9), the only member of myosin-II family in MKs.22 Conventional myosin II-complexes are hexamers consisting of 2 heavy chains, 2 regulatory MLCs, and 2 essential light chains.20 The activity of myosin IIA is regulated by reversible phosphorylation of specific amino acids in the regulatory MLC.20 Phosphorylation of MLC is a critical step in its role in platelet responses to activation, and the best-characterized signaling pathways for MLC phosphorylation are through the Ca2+/calmodulin–dependent MLC kinase23 and the Ca2+-independent Rho/Rho–associated coiled-coil-forming kinase (ROCK)24–26 and myosin phosphatase.27 Myosin light chain phosphorylation plays an important role in platelet function, including in regulating shape change and secretion upon activation. Platelet shape change results from a rapid reorganization of the cytoskeleton, including formation of new actin filaments, actomyosin polymerization through MLC phosphorylation, and centralization of granules.28–31 Inhibition of MLC phosphorylation results in decreased secretion.32–34
Megakaryocytes release platelets by using cytoplasmic extensions called proplatelets; this process requires extensive reorganization in the microtubules and actin filaments. Microtubular protein myosin IIA is a major player in proplatelet extension and platelet release.35–37 Proplatelet formation (PPF) is paradoxically enhanced in MKs derived from embryonic stem cells deficient in myosin heavy chain (MYH9−/−), causing premature release of platelets.35 This has been proposed as a mechanism for the thrombocytopenia in May-Hegglin syndrome and related disorders associated with mutations in MYH9.20,35,36 Moreover, inhibition of Rho/ROCK in MKs leads to a decrease in MLC phosphorylation and myosin contractility, and increases proplatelet formation, indicating that ROCK influences PPF through regulation of MLC.36
Despite the important role of MLC in platelet function and production, very little is known regarding its gene transcriptional regulation. Based on the decreased platelet expression of MLC gene MYL9 and the impaired MLC phosphorylation in our patient with a RUNX1 mutation, we hypothesized that MYL9 is a direct transcriptional target of RUNX1. The present studies provide evidence for this. These findings not only advance a cogent mechanism for the impaired MLC-phosphorylation in our patient, but also provide a mechanism for the thrombocytopenia and platelet dysfunction associated with RUNX1 mutations.
Methods
Patient information
We have previously described12,17 the clinical presentation and detailed studies in this 24-year-old white male, documenting decreased platelet aggregation, secretion, activation of GPIIb-IIIa, pleckstrin and MLC phosphorylation, and PKC-θ level. The patient has a single point mutation in intron 3 at the splice acceptor site for exon 4 leading to a frameshift with premature termination in the conserved Runt homology domain of RUNX1.12 Platelet expression profiling studies in this patient showed18 decreased expression of MYL9 (by 77-fold compared with normal platelets) and other genes. This study was approved by the Temple University Institutional Review Board.
Cell culture
Human erythroleukemia (HEL) cells were grown in RPMI-1640 medium (Mediatech) supplemented with 10% fetal bovine serum (Biomeda) and 1% of antibiotics (penicillin and streptomycin). To induce megakaryocytic transformation, HEL cells were grown in the presence of 10nM phorbol 12-myristate 13-acetate (PMA) for 24 hours, unless indicated otherwise.38
ChIP assay
Chromatin immunoprecipitation (ChIP) assays were performed on HEL cells (1 × 108) induced with PMA for 24 hours and then crosslinked by adding formaldehyde (final concentration 1%). After 10 minutes, crosslinks were quenched with glycine at a final concentration of 0.125 M and washed twice with phosphate-buffered saline (PBS). Lysis and shearing of chromatin to 150-500 bp fragments was performed using ChIP-It kit (Active Motif). Chromatin samples were immunoprecipitated with anti-RUNX1 antibody (sc-8564x, Santa Cruz Biotechnology Inc). Immunoprecipitated samples were analyzed by polymerase chain reaction (PCR) using primers for the MYL9 promoter region −729/−542 (forward primer, −729/−710 5′-CCGATAAGCAGTGGGGAGTG-3′ and reverse primer, −542/−561 5′-TCCTGCATTCCTGCCGTTCC-3′), and for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Amplification was performed using Go Taq Green Master Mix (Promega) with one cycle at 94°C for 1 minute followed by 35 cycles of 94°C for 1 minute, 62°C for 1 minute, and 72°C for 1 minute. Amplified products were analyzed by agarose gel electrophoresis.
Construction of luciferase reporter plasmids
The human MYL9 promoter region (−691/+4) was generated by standard PCR methods using Go Taq green master mix (Promega), with human genomic DNA as a template. It was subcloned between the Xho1 and HindIII recognition sites of pGL3-Basic (Promega). The primer sequences of wild-type (Wt) construct were: forward at −691/−672, 5′-aaactcgag CAGGATCAGTCTGTGACCAC-3′, and reverse at +4/−15 5′-cagaagctt ACATCTTGGCTTCTGGTGGG-3′ (lower cases indicate flanking sequences). Mutant constructs were generated by deleting nucleotides (underlined) from each RUNX1 site (in bold) in the forward primer, but the reverse primer was identical to all constructs. The primer sequences were –691/–662, 5′-aaactcgagCAGGATCAGTCTGTGACCACTCACCACT-GG-3′ (Mut-1), –691/–657 5′-aaactcgagCAGGATCAGTCTGTGACCACTC- ACCACTGGGGGGC-3′ (Mut-2), –691/–632 5′-aaactcgagCAGGATCAGTCTGTGACCACTCACCACTGGGGGGCTGGTCAAGCTCCCTGAGGTGG- ACAG 3′ (Mut-3), and –691/–593 5′-aaactcgagCAGGATCAGTCTGTGACCACTCACCACTGGGGGGCTGGTCAAGCTCCCTGAGGTGGACAGG- CGGGGGTTTGACTGCCCAGAGCCTCCTAGACCTCAGGC-3′ (Mut-4). A truncated construct with no RUNX1 sites was generated using forward primer −591/−573, 5′-aaactcgagGGGCAGCAGAGGGATGGAG-3′ and a common reverse primer described above. PCR products were confirmed by DNA sequencing on the ABI Prism 377 (Applied Biosystems).
Transient ChIP assay
Transient ChIP Assay was performed as described by Lavrrar et al39 with slight modifications. HEL cells were transiently transfected with luciferase-reporter constructs containing either Wt MYL9 promoter region −691/+4 or the same promoter region with mutations in the individual RUNX1 binding sites. This was followed by ChIP analysis using anti-RUNX1 antibodies. HEL cells (107) were transfected with 3 μg of the promoter-reporter construct using a Genfect transfection agent (KD Medical, Inc). After 5 hours, cells were transferred to medium containing PMA (concentration of 10nM). After 24 hours, cells were cross-linked with formaldehyde, then analyzed with the ChIP-It kit. The resulting chromatin samples were immunoprecipitated with anti-RUNX1 antibody. Immunoprecipitated samples were analyzed by PCR with a forward primer in a downstream portion of the MYL9 promoter at −140/−107 (5′-ATCCGTGGGCAGCCTTGAATG-3′) and a reverse primer in luciferase vector backbone (GL primer 2 from Promega). In addition, as a control, samples were analyzed by PCR using both forward and reverse primers specific for the endogenous MYL9 promoter region −729/−542 and for GAPDH, as described in the previous paragraph.
Electrophoretic mobility shift assay
Nuclear proteins from PMA-induced HEL cells were prepared by the method of Dignam et al,40 and protein was estimated using the Bio-Rad protein assay reagent. MYL9 promoter region −729/−542 has 4 RUNX1 consensus sites. Double-stranded oligonucleotide probes encompassing these sites were labeled with T4 polynucleotide kinase (Promega) and [γ32] ATP (PerkinElmer). For the competition experiments, the 200-fold excessive cold double-strand oligos were added with the labeled probe. The sequences of oligonucleotide probes with RUNX1 sites (in bold) and their mutants with deletions (underlined) were as follows: Wt probe (–680/–662) containing RUNX1 site I and site II separated by a single nucleotide (5′-TGTGACCACTCACCACTGGGG-3′) and its deletion mutants with one site mutated (5′-TGTGACCACTCACCACTGGGG-3′) and (5′- TGTGACCACTCACCACTGGGG-3′); Wt probe (–652/–632 containing RUNX1 site III (5′-CAAGCTCCCTGAGGTGGACAG-3′) and its mutant (5′-CAAGCTCCC TCGAGGTGGACAG-5′); and Wt probe (–609/–589) containing RUNX1 site IV (5′ -CTCCTAGAACCTCAGGCGGG-3′) and its mutant (5′-CTCCTAGAACCTCAGGCGGG-3′). Five micrograms of the nuclear extract were incubated on ice for 30 minutes with labeled probes. For the supershift experiments, anti-RUNX1 antibody (sc-8563x, Santa Cruz Biotechnology) was preincubated with nuclear proteins on ice for 30 minutes before the addition of the probe. The reaction mixture was loaded onto 8% polyacrylamide gel in 0.5× Tris-borate-EDTA buffer and resolved by electrophoresis. Gels were dried and visualized by autoradiography.
Binding studies were also performed using recombinant RUNX1 protein (200 ng) (TP323854, OriGene Technologies Inc) and MYL9 Wt probes labeled with infrared dye 700 (LI-COR Biosciences). Binding reaction was performed on ice for 1 hour in a low-ionic-strength buffer containing 0.6mM HEPES (pH 8.0), 1mM dithiothreitol (DTT), 0.01% triton X-100, and 2% glycerol, along with 5 μg/μL of bovine serum albumin and 100mM NaCl. Unlabeled identical probes were used in competition studies. For supershift studies, anti-RUNX1 antibodies (sc-8564x; Santa Cruz Biotechnology Inc) or nonspecific IgG (Santa Cruz Biotechnology Inc) was preincubated for 30 minutes on ice with purified RUNX1 protein before the addition of a labeled probe. The reaction mixture was loaded onto 5% TBE readymade gels (Bio-Rad) and resolved by electrophoresis. Gels were visualized on Odyssey Infrared Imaging System (LI-COR Biosciences).
Luciferase reporter assays
HEL cells (2 × 106 cells) were cotransfected with MYL9-reporter construct (5 μg) and pRL-TK (0.1 μg), containing Renilla luciferase gene at a ratio of 50:1 using Genfect transfection reagent. An empty vector, pGL3-Basic, was transfected as a control vector. After incubation for 3 to 4 hours at 37°C in 5% CO2, cells were transferred to a medium containing phorbol 12-myristate 13-acetate (PMA; concentration of 10nM). After 24 hours, cells were lysed and luciferase activity was measured with a Dual Luciferase Assay System (Promega). Promoter activity was expressed as firefly luciferase activity/Renilla luciferase activity relative to that of the empty vector. All transfection experiments were performed 3 to 4 times in triplicate.
Effect of overexpression of RUNX1 on MYL9
To study the effect of overexpression of RUNX1 on MYL9 gene, HEL cells (1 × 106) were cotransfected with equal amounts (1 μg each) of Wt or mutant MYL9 reporter construct along with RUNX1expression plasmid, RUNX1-pCMV6–XL4 (SC106348, OriGene Technologies Inc), or empty expression vector (pCMV6-XL4), using transfection agent Turbofectin 8.0 (OriGene Technologies Inc). Renilla luciferase reporter plasmid was used as an internal standard and transfections were performed as described above. Cells were lysed after 48 hours and assayed for luciferase activities. To see the effect on endogenous RUNX1 and MLC in overexpression studies, HEL cell lysates were subjected to immunoblotting using antibodies against RUNX1 (sc-8563; Santa Cruz Biotechnology Inc), actin (sc-1616R; Santa Cruz Biotechnology Inc), and MLC (3672; Cell Signaling Technology).
Effect of siRNA-mediated knockdown of RUNX1
Ribonucleic acids siRNA against RUNX1 and unrelated mock siRNA were purchased from Santa Cruz Biotechnology Inc. The RUNX1 siRNA used was a pool of 3 target-specific 19-25 nucleotide siRNAs designed to knock down gene expression. For transfection, 400nM siRNA were dissolved in 100 μL of Optimem media (Invitrogen). In another tube, transfection media was dissolved in 100 μL of Optimem media. The 2 mixtures were then combined and incubated for 45 minutes at room temperature. The mixture was added to 5 × 105 HEL cells resuspended in 400 μL of serum-free media, plated in a 24-well plate and incubated for 5 hours at 37°C. After transfection, 2× fetal bovine serum medium containing 50nM PMA was added to the wells. On the following day, the 2× medium was replaced with regular medium containing 50nM PMA. Cells were harvested at 48 hours. The protein concentrations were determined by Micro BCA Protein Assay Reagent kit (Pierce), and the effect of gene silencing by RUNX1 siRNA was examined by immunoblot analysis using antibodies against RUNX1, MLC, and β-actin antibodies (Santa Cruz Biotechnology Inc).
Cell-spreading assays and microscopy
Green fluorescent protein (GFP)–cotransfected and siRNA-cotransfected HEL cells were seeded from suspension onto glass coverslips coated with 50 μg/mL of collagen I or 100 μg/mL of human fibrinogen for 120 minutes and assayed for cell spreading as described.41 Briefly, adherent cells were fixed in 3.7% formaldehyde and permeablized with 0.1% Triton X-100, and cellular actin was labeled with 5 μg/mL of rhodamine-phalloidin (Invitrogen) for 30 minutes. Cells were mounted on slides with ProLong Gold mounting medium (Invitrogen), and coverslips were imaged with a Plan Fluor 40× oil immersion lens (n.a. = 1.3) on a Nikon E800 microscope fitted with a SPOT charge-coupled device camera (Diagnostic Instruments). The area of GFP-positive cells was measured using ImageJ software Version 1.38x (National Institutes of Health).
Bioinformatics
Potential binding sites for transcription factors were analyzed using the computer program TFSEARCH (available at http://mbs.cbrc.jp/research/db/TFSEARCH.html).
Results
Binding of RUNX1 to MYL9 promoter by ChIP
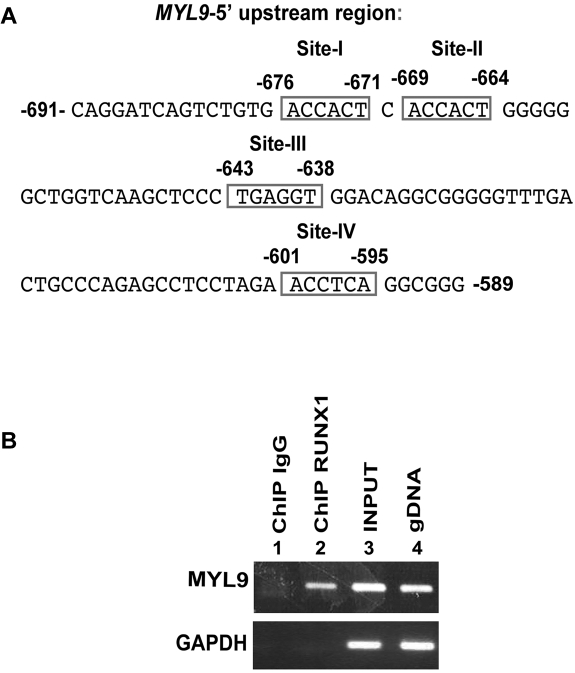
TFSEARCH on MYL9 promoter fragment (up to approximately 1000 bp) revealed 4 RUNX1 consensus sites within a roughly 100-bp narrow region: at −676/−671 (site I); −669/−664 (site II); −643/−638 (site III); and −601/−595 (site IV; Figure 1A). We performed immunoprecipitations on chromatin samples from HEL cells using an anti-RUNX1 antibody to identify an endogenous interaction between RUNX1 and the MYL9 gene. We amplified by PCR the MYL9 region −729/−542 that encompasses all 4 RUNX1 sites. The MYL9 fragment was enriched by RUNX1 antibody but not by control IgG (Figure 1B). These studies indicate that RUNX1 binds to MYL9 promoter region in vivo.
Figure 1.
ChIP analysis showing interaction of RUNX1 with MYL9 promoter. (A) MYL9 upstream region showing 4 consensus sites for RUNX1. (B) ChIP analysis of endogenous RUNX1 binding in megakaryocytic HEL cells. Immunoprecipitation was performed using control IgG (lane 1) or anti-RUNX1 antibody (lane 2). Samples were analyzed by PCR using primers for MYL9 region −729/−542 nucleotides and GAPDH. Lanes 3 and 4 show PCR amplification of total input DNA and genomic DNA, respectively.
RUNX1 binding to sites I (−676/−671) and II (−669/−664) by EMSA
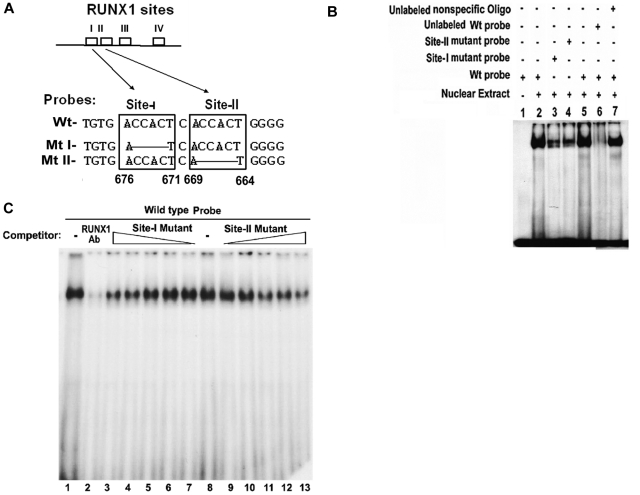
Site I and site II are separated by a single nucleotide. Electrophoretic mobility shift assay (EMSA) was performed using HEL cell extracts and Wt oligo with both sites I and II, mutant-I oligo with mutation in site I, and mutant-II oligo with mutation in site II (Figure 2A). Protein binding occurred with Wt oligo (Figure 2B, lanes 2 and 5). This was substantially reduced but not absent with mutant I and mutant II oligos (lanes 3 and 4, respectively), indicating the involvement of sites I and II in the binding. Protein binding to Wt oligo was impeded by competition with excess unlabeled oligo (lane 6), but was not altered by competition with nonspecific oligo (lane 7). To examine the involvement of RUNX1 in the protein complex, a supershift experiment with RUNX1 antibody was performed (Figure 2C). Binding to Wt oligo (lane 1) was abolished by competition with RUNX1 antibody (lane 2), indicating that RUNX1 is involved in the protein-DNA complex. Involvement of site I and site II in protein binding to the Wt oligo was further assessed by competition studies using oligos with site I or site II mutations (Figure 2C). Binding to Wt oligo (lane 8) was affected by competition with increasing amounts of unlabeled probes with mutations in site I (lanes 7 to 3) or site II (lanes 9 to 13), suggesting that both sites I and II are involved in the observed RUNX1 binding.
Figure 2.
EMSA using Wt oligonucleotide probe with sites I (−676/−671) and II (−669/−664) in MYL9 promoter and nuclear extract from PMA-treated HEL cells. (A) Wt −680/−660 bp) probe and Mt probes containing mutated RUNX1 sites I and II. (B) EMSA using Wt and Mt oligos (lanes 1-7). Lane 1, no extract; lanes 2 and 5, protein binding to Wt probe; lanes 3-4, reduced binding with Mt I and Mt II probes; lane 6, loss of binding by competition with unlabeled Wt probe; lane 7, no loss of binding by competition with nonspecific oligo. (C) Effect of anti-RUNX1 antibody (lanes 1 and 2) and of increasing amounts of unlabeled mutant probes I (lanes 7 to 3) and II (lanes 9 to 13) on protein binding to Wt oligo (−680/−660 bp). lane 1, protein binding to Wt probe; lane 2, loss of binding with anti-RUNX1 antibody. Lane 8 shows binding to Wt probe. Lanes 7 to 3, competition with increasing amounts of site I mutant probe; lanes 9-13, competition with increasing amounts of site II mutant probe. There is decreasing protein binding to Wt probe with increasing amount of either mutant I or mutant II probe, indicating that both sites are involved in protein binding to DNA.
RUNX1 binding to site III (−643/−638) by EMSA
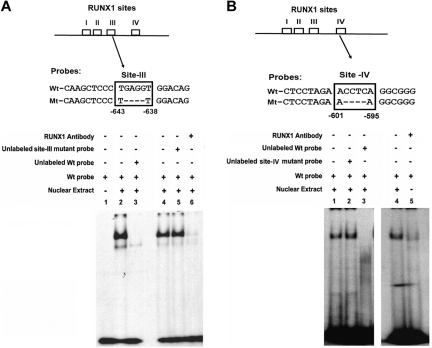
Figure 3A shows nuclear protein binding to Wt oligo-containing site III (lanes 2 and 4), which was abolished by competition with excess unlabeled identical oligo (lane 3) but not with an unlabeled mutant oligo with the RUNX1 site mutated (lane 5). Protein binding was abolished with RUNX1 antibody (lane 6). These results indicate that RUNX1 binds to site III.
Figure 3.
EMSA using Wt oligonucleotide probes with site III (−643/−638 bp) and site IV (−601/−595 bp) in MYL9 promoter and nuclear extract from PMA-treated human erythroleukemia (HEL) cells. (A) Top panel shows Wt probe (−652/−632) and Mt probe with site III mutated. Bottom panel shows EMSA using Wt and Mt probes: lane 1, no extract; lane 2, protein binding to Wt probe; lane 3, loss of binding with excess unlabeled Wt probe; lane 4, protein binding to Wt probe; lane 5, no loss of binding on competition with unlabeled mutant probe; lane 6, loss of binding with anti-RUNX1 antibody. (B) Top panel shows Wt probe (−601/−595) and Mt probe with mutated site IV. Bottom panel shows EMSA using Wt and Mt probes. Lane 1, protein binding to Wt probe; lane 2, no loss of binding by competition with unlabeled mutant probe; lane 3, loss of binding with excess unlabeled Wt probe; lane 4, protein binding to Wt probe; lane 5, loss of binding with anti-RUNX1 antibody.
RUNX1 binding to site IV (−601/−595) by EMSA
In Figure 3B, protein binding was observed with Wt oligo-bearing site IV (lane 1). No competition occurred with the mutant oligo (lane 2), but binding was abolished by excess Wt oligo (lane 3). The binding to Wt oligo (lane 4) was inhibited by RUNX1 antibody (lane 5). These results indicate that RUNX1 binds to site IV.
Binding studies with purified recombinant RUNX1
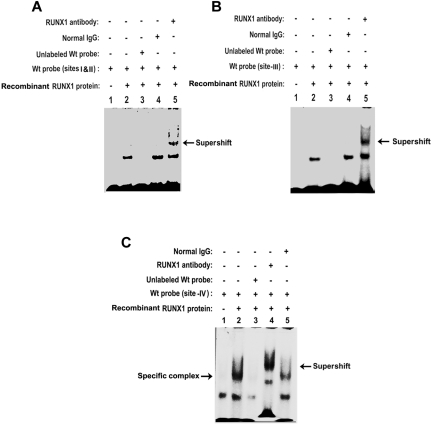
To provide further evidence of RUNX1 binding to MYL9 we performed studies using recombinant RUNX1 protein (Figure 4). Figure 4A shows protein binding to the probe containing sites I and II (lane 2). This binding was competed by excess unlabeled probe (lane 3), not affected by normal IgG (lane 4), and supershifted by anti-RUNX1 antibody (lane 5). Studies with probes containing site III (Figure 4B) and site IV (Figure 4C) also showed evidence of RUNX1 binding. In each case, specific protein binding was observed that was supershifted by RUNX1 antibody but not by nonspecific IgG. In studies with probes containing site IV, a nonspecific band was observed with the probe alone (lane 1) that was not present when bovine serum albumin was omitted from the reaction mixture. The prominent DNA protein complex observed in the presence of RUNX1 protein (lane 2) was abolished by excess unlabeled probe (lane 3) and supershifted by anti-RUNX1 antibody (arrow), but not by IgG. These studies indicate that RUNX1 binds to all 4 sites.
Figure 4.
EMSA using recombinant RUNX1 protein and oligonucleotide probes with RUNX1 binding sites. (A) RUNX1 protein binding to probe containing sites I and II (−680/−660 base pairs [bp]): lane 1, probe alone; lane 2, protein binding to Wt probe; lane 3, loss of binding with excess unlabeled Wt probe; lane 4, no loss of binding with normal IgG; lane 5, supershift with anti-RUNX1 antibody. (B) Binding to Wt probe containing RUNX1 site III (−643/−638 bp): lane 1, probe alone; lane 2, protein binding to the probe; lane 3, loss of binding with excess unlabeled probe; lane 4, no loss of binding with normal IgG; lane 5, supershift with anti-RUNX1 antibody. (C) Binding to probe containing RUNX1 site IV (−601/−595); lane 1, probe alone; lane 2, protein binding to probe; lane 3, loss of binding with excess unlabeled probe; lane 4, supershift with anti-RUNX1 antibody; lane 5, no loss of binding with normal IgG.
Transient-ChIP analysis on Wt and Mt MYL9 promoter
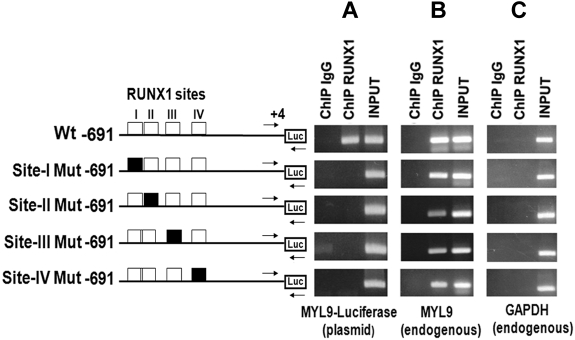
To further investigate RUNX1 recruitment to MYL9 promoter in vivo, the promoter region −691/+4 bp bearing the 4 sites and the same region with individual mutated RUNX1 sites were cloned into luciferase reporter vector (Figure 5). HEL cells were transfected individually with either the Wt or mutant-luciferase vector and ChIP was performed with anti-RUNX1 antibody. The immunoprecipitated samples were subjected to PCR using a primer set (shown by arrows in Figure 5) where the forward primer corresponded to the MYL9 promoter (−140/−107) and the reverse primer was specific to the luciferase vector backbone gene as described in “Transient ChIP assay.” This PCR amplification would reflect RUNX1 binding to the luciferase reporter vectors with Wt or mutant promoter. MYL9-luciferase plasmid fragment was enriched and amplified in the anti-RUNX1 antibody-precipitated DNA from cells transfected with Wt construct, but not from cells transfected with any of the mutant constructs (Figure 5A). This indicates that disruption of any of the 4 sites prevented RUNX1 recruitment to the promoter region and, importantly, that all 4 sites interact with each other in the observed RUNX1 binding. As a control, endogenous native MYL9 fragment −729/−542 encompassing all 4 RUNX1 sites was also amplified in parallel from chromatin precipitated from cells transfected with the Wt or mutant constructs. The primers used were specific to endogenous MYL9 promoter and the same as previously used in the ChIP experiments shown in Figure 1B. These showed, as expected, enrichment in cells transfected with Wt and Mt constructs (Figure 5B). No amplification was noted with respect to GAPDH (Figure 5C lane 2).
Figure 5.
Transient ChIP analysis using Wt and Mt MYL9 promoter (−691/+4)-luciferase reporter constructs in PMA-treated HEL cells. Cells were transfected with reporter constructs containing WtMYL9 promoter region (−691/+4) with RUNX1 sites (open boxes) or with RUNX1 sites I-IV individually mutated (filled boxes) as shown. ChIP was performed on these cells using anti-RUNX1 antibody or control IgG. PCR was performed using primers (shown by arrows), where the forward primer was specific to the MYL9 region (−140/−107) and the reverse primer was specific to the luciferase gene (Panel A; MYL9-luciferase plasmid). As a control, PCR was performed using a second set of primers (not shown) where both primers were specific to the endogenous MYL9 sequence (Panel B; endogenous MYL9). In addition, GAPDH was amplified (C). Each panel shows PCR products obtained from amplification of IgG- or anti-RUNX1 antibody-immunoprecipitated samples, and of input DNA. (A) Enrichment (RUNX1 antibody) of the MYL9-luciferase plasmid is observed from the cells transfected with Wt plasmid containing intact RUNX1 sites. This was abolished with mutations in any of the 4 RUNX1 sites. (B) As expected, Wt endogenous MYL9 sequence was amplified from chromatin immunoprecipitated by anti-RUNX1 antibody from all transfections, Wt or Mt. (C) GAPDH was not amplified from immunoprecipitated chromatin from any of the transfections.
Luciferase-reporter studies on MYL9 promoter
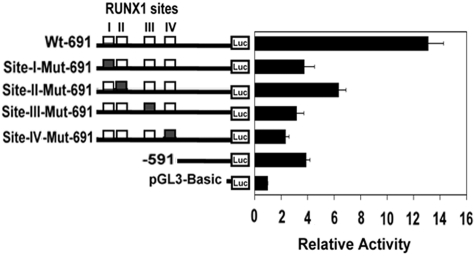
The role of RUNX1 in regulating MYL9 promoter was tested by luciferase reporter assays using Wt and Mt constructs (Figure 6). Wt construct with all 4 RUNX1 sites showed approximately 14-fold activity. Mutations in individual RUNX1 sites resulted in marked reduction in activity. A truncated construct (−591/+4) carrying no RUNX1 sites also showed markedly reduced activity (Figure 6).
Figure 6.
Luciferase-reporter studies on MYL9 promoter in PMA-treated HEL cells. Luciferase activity with Wt construct (□) and constructs with RUNX1 sites I-IV mutated (■) are shown. Mutation of any of the 4 RUNX1 sites resulted in a decrease in reporter activity, suggesting that all 4 RUNX1 sites are functional. A truncated construct (−591/+4) with no RUNX1 sites is also shown. The means ± SE are shown for 3 independent experiments in triplicates.
Enhancement of MYL9 protein expression and promoter activity by RUNX1 overexpression
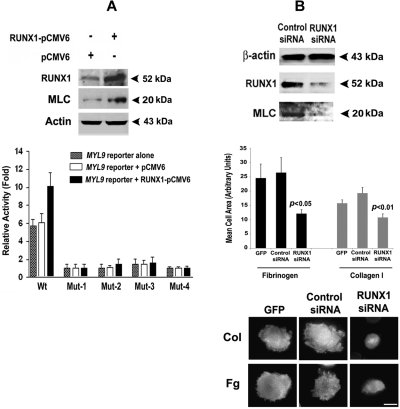
Immunoblot analysis of HEL cells transfected with RUNX1-pCMV6 expression plasmid showed overexpression of RUNX1 with an increase in MLC (Figure 7A). The promoter activity of the Wt MYL9 construct containing all 4 RUNX1 sites was markedly enhanced by RUNX1 overexpression; no increase was noted with mutations in individual RUNX1 sites (Figure 7A). These results are consistent with the conclusion that all 4 RUNX1 sites are involved in the regulation of MYL9 in megakaryocytic cells.
Figure 7.
Effect of overexpression of RUNX1 on expression of MLC protein and MYL9 promoter activity, and effect of RUNX1 siRNA on MLC protein and cell spreading in collagen and fibrinogen. (A) Immunoblot analysis of endogenous RUNX1, MLC, and actin after overexpression of RUNX1 in HEL cells (top panel). Effect of RUNX1 overexpression on MYL9 promoter activity (bottom panel). Wt and Mt constructs corresponding to sites I-IV were cotransfected with RUNX1-pCMV6 expression vector (black bars), empty vector pCMV6 (open bars), or neither (stippled bars) in HEL cells. Reporter activity was measured at 48 hours. Bar graphs show activity as mean (± S.E) of 3 independent experiments in triplicate. (B) Top panel: inhibition of MYL9 by RUNX1 siRNA. HEL cells were transfected with RUNX1 or mock siRNA. Shown are immunoblots of lysates for RUNX1, MLC, and β-actin, representative of 3 experiments. RUNX1 siRNA reduced endogenous RUNX1 and MLC protein expression. Middle and bottom panels: inhibition of cell spreading on collagen and fibrinogen by RUNX1 siRNA. Cells were transfected with GFP alone or with GFP and siRNA, as shown, and treated with PMA. Cell suspensions were seeded for 120 minutes on coverslips coated with either 50 μg/mL collagen I or 100 μg/mL fibrinogen. Adhered cells were fixed and labeled with rhodamine-phalloidin to decorate actin filaments. Cell spreading was measured as the mean cell surface area of at least 25 GFP-positive cells per sample, shown ± SEM. Spreading was significantly reduced (middle panel). P values show comparison to control siRNA. Cortical actin was absent (bottom panel) in RUNX1 knockdown cells exposed to collagen and fibrinogen. Bar in bottom panel, 10 mm.
Inhibition of MYL9 and cell spreading by RUNX1 siRNA
We investigated MYL9 knockdown by RUNX1 siRNA. HEL cells were transfected with either a control nonsilencing siRNA or RUNX1 siRNA. Western blot analyses showed that both RUNX1 and MLC protein were decreased by RUNX1 siRNA (Figure 7B). Because of the role of myosins and MLC in cell spreading and cytoskeletal reorganization20,21,36 and to obtain evidence of a functional effect, we studied cell spreading on collagen and fibrinogen. The siRNA knockdown of RUNX1 led to decreased cell spreading on collagen and fibrinogen, and loss of the cortical actin that was observed in control cells (Figure 7B). This is attributable to an effect on MYL9, but a concomitant effect on other cytoskeletal genes also regulated by RUNX118 cannot be ruled out.
Discussion
RUNX1 is a major hematopoietic transcription factor whose haplodeficiency is associated with familial thrombocytopenia, platelet dysfunction, and a predisposition to leukemia.9,10 The major novel finding in our studies is the evidence that RUNX1 regulates MYL9 expression in megakaryocytic cells. We have shown that RUNX1 binds to MYL9 promoter region −729/−542 in vivo (Figure 1) and that it binds to each of the 4 consensus sites in this region (Figures 2–3). In addition, we have shown that recombinant RUNX1 binds to the RUNX1 consensus sites in this region (Figure 4). In vivo assay using transient-ChIP analysis revealed that these 4 RUNX1 sites are involved in RUNX1 recruitment to MYL9 promoter (Figure 5). Mutation of any of the sites disrupted RUNX1 binding to the promoter, which suggests that they interact with each other (Figure 5). In reporter gene expression studies, mutation of each RUNX1 site caused reduction in the promoter activity (Figure 6). Overexpression of RUNX1 induced an increase in RUNX1 and MLC protein levels, and markedly enhanced MYL9 promoter activity, which was abolished by mutation in RUNX1 sites (Figure 7A). RUNX1 siRNA inhibited MLC protein expression in HEL cells (Figure 7B), associated with decreased cell spreading on collagen and fibrinogen, and loss of cortical actin (Figure 7B). Together with our previously reported findings of decreased MLC transcript levels12 and diminished MLC-phosphorylation in our patient platelets,17 these findings indicate that MYL9 is regulated at the transcriptional level by RUNX1, and this is altered in RUNX1 haplodeficiency.
One of the major hallmarks of RUNX1 haplodeficiency is thrombocytopenia. The evidence that RUNX1 regulates MYL9 has direct and important relevance to this aspect. In platelets the myosin II complexes are made up of 2 heavy chains (MYH-IIA), each of which is associated with a myosin regulatory light chain. Phosphorylated MLC regulates the actin-dependent myosin motor activity.20 Both MLC and phosphorylated MLC increase during MK differentiation.36 Megakaryocytes extend long cytoplasmic projections (proplatelets) and generate nascent platelets at the proplatelet tips, involving actin and microtubule cytoskeletons as well as myosin. Multiple lines of evidence indicate myosin II is a major player in platelet production and that the Rho-ROCK–myosin II–MLC axis in megakaryocytes is a negative regulator of proplatelet formation, a role essential to the orderly temporal and spatial processes leading to the production and release of normal platelets.35–37 The overall model culminating from these studies is that the myosin II-Rho–ROCK pathway restricts the premature proplatelet formation from maturing MK and thereby prevents release of structurally, functionally, or structurally and functionally aberrant platelets. This inhibitory effect involving myosin is critical, as shown by the studies in the May-Hegglin anomaly and related disorders with mutations in myosin heavy chain IIA coded by MYH9; the observed thrombocytopenia and abnormally increased platelet size in these patients are attributed to the enhanced PPF and premature platelet release.35,36,42 Moreover, interaction of MK with type I collagen suppresses PPF, and this is abolished by a myosin IIA antagonist.37 MLC and its phosphorylation also play a regulatory role in PPF36; inhibition of MLC phosphorylation using inhibitors of Rho, ROCK, and myosin light chain kinase (MLCK) increases proplatelet formation.36 We have previously documented12,17 that platelets from our patient with a RUNX1 mutation have decreased expression of MYL9 and that agonist-induced platelet MLC-phosphorylation is impaired. We now show that MYL9 is regulated by RUNX1. It is likely that the loss of negative regulation of PPF applies to RUNX1 mutations via the decreased expression of regulatory MLC; this would explain the consistent thrombocytopenia in such patients. Our studies show also that cell interaction with collagen is impaired with down-regulation of RUNX1 and MLC (Figure 7). This finding becomes relevant to the pathogenesis of thrombocytopenia because collagen suppresses premature PPF formation, an effect mediated by myosin IIA.36,37 Thus, we propose that in RUNX1 haplodeficiency one mechanism for thrombocytopenia is the loss of the critical negative modulating effect of myosin on PPF arising from decreased expression of the regulatory MLC, as contrasted to the defect in myosin heavy chain in MYH9 syndromes. While this is an important mechanism, it may not be the complete story in RUNX1 mutations because the primary deficiency is in a transcription factor, a mechanism that may alter expression of other genes as well. While MK maturation appears to be normal in the monogenic MYH9-related disorders,35,42 there is an evidence that megakaryopoiesis is abnormal in RUNX1 haplodeficiency,9 and in one report platelets had decreased Mpl expression.15
A second feature observed in patients with RUNX1 mutations is impaired platelet function. Several studies have documented abnormal platelet aggregation and secretion10,17,43 responses in these patients. In our patient, aggregation, dense granule secretion, and activation of GPIIb-IIIa were impaired in response to activation with several agonists; moreover, phosphorylation of MLC and pleckstrin was decreased.17 We have shown that platelet PKC-θ is decreased,12 and that this enzyme is regulated by RUNX1.44 Our current studies provide a mechanism in our patient for the decreased expression of MYL912 and the previously observed decreased MLC phosphorylation, which we attribute to diminished levels of MLC.17 Aside from regulating platelet production, MLC phosphorylation plays an important role in several platelet function responses, including shape change and secretion.28–34 It is likely that MLC phosphorylation defect contributes also to the altered platelet responses observed in patients with RUNX1 mutations. Thus, the functional defect in these patients is complex, arising from multiple mechanisms mediated by alterations in distinct gene products regulated by RUNX1 in megakaryocytes/platelets. We have shown that these proteins include 12-lipoxygenase,45 PKC-θ,44 and PF4.19
Promoters of several genes regulated by RUNX1 have multiple consensus sites for RUNX1 binding,46–49 including GPIIb46 and ALOX-1245 in megakaryocytic cells. RUNX1 proteins can stabilize their interaction with DNA either by homodimerizing in adjacent sites or heterodimerizing with other proteins binding to the DNA.50 RUNX1 physically interacts with GATA-1 in megakaryocytic cells to enhance the expression of GPIIb46 and enhances megakaryocytic induction. RUNX1 binding at multiple sites can modify promoter architecture and chromatin organization.49 Our studies show 4 consensus sites for RUNX1 on the MYL9 proximal promoter that interact and are involved collectively in RUNX1 binding, as shown by the transient ChIP experiment (Figure 5), and these sites are functional in megakaryocytic cells (Figure 6A).
In summary, our studies reveal that MYL9 is regulated by transcription factor RUNX1 in megakaryocytes and platelets. RUNX1 dysregulation of MYL9 gene in megakaryocytes is an important aspect of the abnormal platelet production and function associated with human RUNX1 mutations. We propose that alterations in MLC (MYL9) are also associated with congenital thrombocytopenia, as has been reported with mutations in myosin heavy chain (MYH9).35,36,42 The key finding that MYL9 expression was decreased in this patient came from studies using platelet expression profiling.17 Our studies provide support to the concept that platelet expression profiling can yield hitherto unavailable new insights into molecular mechanisms in patients with platelet disorders.
Acknowledgments
The authors thank Denise Tierney for assisting in manuscript preparation.
This work was supported by National Institutes of Health grants R01HL85422 (A.K.R.), R01HL56724 (A.K.R.), and HL093416 (L.G.). G.K. was supported by National Institutes of Health T32 training grant HL007777.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: G.J. performed the research, analyzed the data, and wrote the paper; G.M. and G.K. performed some of the project studies; L.E.G. performed the studies on cell spreading; D.N.D. contributed to the data analyses; and A.K.R. designed the research, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: A. Koneti Rao, Sol Sherry Thrombosis Research Center, Temple University School of Medicine, 3400 N Broad St, OMS-300, Philadelphia, PA 19140; e-mail: koneti@temple.edu.
References
- 1.Speck NA, Stacy T, Wang Q, et al. Core-binding factor: a central player in hematopoiesis and leukemia. Cancer Res. 1999;59(7 suppl):1789s–1793s. [PubMed] [Google Scholar]
- 2.de Bruijn MF, Speck NA. Core-binding factors in hematopoiesis and immune function. Oncogene. 2004;23(24):4238–4248. doi: 10.1038/sj.onc.1207763. [DOI] [PubMed] [Google Scholar]
- 3.Mikhail FM, Sinha KK, Saunthararajah Y, Nucifora G. Normal and transforming functions of RUNX1: a perspective. J Cell Physiol. 2006;207(3):582–593. doi: 10.1002/jcp.20538. [DOI] [PubMed] [Google Scholar]
- 4.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84(2):321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 5.Wang Q, Stacy T, Binder M, Marin-Padilla M, Sharpe AH, Speck NA. Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc Natl Acad Sci U S A. 1996;93(8):3444–3449. doi: 10.1073/pnas.93.8.3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichikawa M, Asai T, Saito T, et al. AML-1 is required for megakaryocytic maturation and lymphocytic differentiation, but not for maintenance of hematopoietic stem cells in adult hematopoiesis. Nat Med. 2004;10(3):299–304. doi: 10.1038/nm997. [DOI] [PubMed] [Google Scholar]
- 7.Michaud J, Simpson KM, Escher R, et al. Integrative analysis of RUNX1 downstream pathways and target genes. BMC Genomics. 2008;9:363. doi: 10.1186/1471-2164-9-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto F, Lubbert M, Stock M. Upstream and downstream targets of RUNX proteins. J Cell Biochem. 2003;89(1):9–18. doi: 10.1002/jcb.10491. [DOI] [PubMed] [Google Scholar]
- 9.Song WJ, Sullivan MG, Legare RD, et al. Haploinsufficiency of CBFA2 causes familial thrombocytopenia with propensity to develop acute myelogenous leukaemia. Nat Genet. 1999;23(2):166–175. doi: 10.1038/13793. [DOI] [PubMed] [Google Scholar]
- 10.Michaud J, Wu F, Osato M, et al. In vitro analyses of known and novel RUNX1/AML1 mutations in dominant familial platelet disorder with predisposition to acute myelogenous leukemia: implications for mechanisms of pathogenesis. Blood. 2002;99(4):1364–1372. doi: 10.1182/blood.v99.4.1364. [DOI] [PubMed] [Google Scholar]
- 11.Ho CY, Otterud B, Legare RD, et al. Linkage of a familial platelet disorder with a propensity to develop myeloid malignancies to human chromosome 21q22.1-22.2. Blood. 1996;87(12):5218–5224. [PubMed] [Google Scholar]
- 12.Sun L, Mao G, Rao AK. Association of CBFA2 mutation with decreased platelet PKC-theta and impaired receptor-mediated activation of GPIIb-IIIa and pleckstrin phosphorylation: proteins regulated by CBFA2 play a role in GPIIb-IIIa activation. Blood. 2004;103(3):948–954. doi: 10.1182/blood-2003-07-2299. [DOI] [PubMed] [Google Scholar]
- 13.Buijs A, Poddighe P, van Wijk R, et al. A novel CBFA2 single-nucleotide mutation in familial platelet disorder with propensity to develop myeloid malignancies. Blood. 2001;98(9):2856–2858. doi: 10.1182/blood.v98.9.2856. [DOI] [PubMed] [Google Scholar]
- 14.Walker LC, Stevens J, Campbell H, et al. A novel inherited mutation of the transcription factor RUNX1 causes thrombocytopenia and may predispose to acute myeloid leukaemia. Br J Haematol. 2002;117(4):878–881. doi: 10.1046/j.1365-2141.2002.03512.x. [DOI] [PubMed] [Google Scholar]
- 15.Heller PG, Glembotsky AC, Gandhi MJ, et al. Low Mpl receptor expression in a pedigree with familial platelet disorder with predisposition to acute myelogenous leukemia and a novel AML1 mutation. Blood. 2005;105(12):4664–4670. doi: 10.1182/blood-2005-01-0050. [DOI] [PubMed] [Google Scholar]
- 16.Matheny CJ, Speck ME, Cushing PR, et al. Disease mutations in RUNX1 and RUNX2 create nonfunctional, dominant-negative, or hypomorphic alleles. Embo J. 2007;26(4):1163–1175. doi: 10.1038/sj.emboj.7601568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabbeta J, Yang X, Sun L, McLane MA, Niewiarowski S, Rao AK. Abnormal inside-out signal transduction-dependent activation of glycoprotein IIb-IIIa in a patient with impaired pleckstrin phosphorylation. Blood. 1996;87(4):1368–1376. [PubMed] [Google Scholar]
- 18.Sun L, Gorospe JR, Hoffman EP, Rao AK. Decreased platelet expression of myosin regulatory light chain polypeptide (MYL9) and other genes with platelet dysfunction and CBFA2/RUNX1 mutation: insights from platelet expression profiling. J Thromb Haemost. 2007;5(1):146–154. doi: 10.1111/j.1538-7836.2006.02271.x. [DOI] [PubMed] [Google Scholar]
- 19.Aneja K, Jalagadugula G, Mao G, Rao AK. Mechanism of platelet factor (PF4) deficiency with RUNX1 mutations: RUNX1 is a transcriptional regulator of PF4 [abstract]. Blood. 2009;114:98–99. doi: 10.1111/j.1538-7836.2010.04154.x. Abstract 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR. Nonmuscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol. 2009;10(11):778–790. doi: 10.1038/nrm2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krendel M, Mooseker MS. Myosins: tails (and heads) of functional diversity. Physiology (Bethesda) 2005;20:239–251. doi: 10.1152/physiol.00014.2005. [DOI] [PubMed] [Google Scholar]
- 22.Marigo V, Nigro A, Pecci A, et al. Correlation between the clinical phenotype of MYH9-related disease and tissue distribution of class II nonmuscle myosin heavy chains. Genomics. 2004;83(6):1125–1133. doi: 10.1016/j.ygeno.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Hathaway DR, Eaton CR, Adelstein RS. Regulation of human platelet myosin light chain kinase by the catalytic subunit of cyclic AMP-dependent protein kinase. Nature. 1981;291(5812):252–256. doi: 10.1038/291252a0. [DOI] [PubMed] [Google Scholar]
- 24.Paul BZ, Daniel JL, Kunapuli SP. Platelet shape change is mediated by both calcium-dependent and -independent signaling pathways. Role of p160 Rho-associated coiled-coil-containing protein kinase in platelet shape change. J Biol Chem. 1999;274(40):28293–28300. doi: 10.1074/jbc.274.40.28293. [DOI] [PubMed] [Google Scholar]
- 25.Bodie SL, Ford I, Greaves M, Nixon GF. Thrombin-induced activation of RhoA in platelet shape change. Biochem Biophys Res Commun. 2001;287(1):71–76. doi: 10.1006/bbrc.2001.5547. [DOI] [PubMed] [Google Scholar]
- 26.Bauer M, Retzer M, Wilde JI, et al. Dichotomous regulation of myosin phosphorylation and shape change by Rho-kinase and calcium in intact human platelets. Blood. 1999;94(5):1665–1672. [PubMed] [Google Scholar]
- 27.Nakai K, Suzuki Y, Kihira H, et al. Regulation of myosin phosphatase through phosphorylation of the myosin-binding subunit in platelet activation. Blood. 1997;90(10):3936–3942. [PubMed] [Google Scholar]
- 28.Johnson GJ, Leis LA, Krumwiede MD, White JG. The critical role of myosin IIA in platelet internal contraction. J Thromb Haemost. 2007;5(7):1516–1529. doi: 10.1111/j.1538-7836.2007.02611.x. [DOI] [PubMed] [Google Scholar]
- 29.Fox JE, Phillips DR. Role of phosphorylation in mediating the association of myosin with the cytoskeletal structures of human platelets. J Biol Chem. 1982;257(8):4120–4126. [PubMed] [Google Scholar]
- 30.Daniel JL, Molish IR, Rigmaiden M, Stewart G. Evidence for a role of myosin phosphorylation in the initiation of the platelet shape change response. J Biol Chem. 1984;259(15):9826–9831. [PubMed] [Google Scholar]
- 31.Hartwig JH, Bokoch GM, Carpenter CL, et al. Thrombin receptor ligation and activated Rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82(4):643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe Y, Ito M, Kataoka Y, et al. Protein kinase C-catalyzed phosphorylation of an inhibitory phosphoprotein of myosin phosphatase is involved in human platelet secretion. Blood. 2001;97(12):3798–3805. doi: 10.1182/blood.v97.12.3798. [DOI] [PubMed] [Google Scholar]
- 33.Nishikawa M, Tanaka T, Hidaka H. Ca2+-calmodulin-dependent phosphorylation and platelet secretion. Nature. 1980;287(5785):863–865. doi: 10.1038/287863a0. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki Y, Yamamoto M, Wada H, et al. Agonist-induced regulation of myosin phosphatase activity in human platelets through activation of Rho-kinase. Blood. 1999;93(10):3408–3417. [PubMed] [Google Scholar]
- 35.Chen Z, Naveiras O, Balduini A, et al. The May-Hegglin anomaly gene MYH9 is a negative regulator of platelet biogenesis modulated by the Rho-ROCK pathway. Blood. 2007;110(1):171–179. doi: 10.1182/blood-2007-02-071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y, Aurade F, Larbret F, et al. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood. 2007;109(10):4229–4236. doi: 10.1182/blood-2006-04-020024. [DOI] [PubMed] [Google Scholar]
- 37.Balduini A, Pallotta I, Malara A, et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6(11):1900–1907. doi: 10.1111/j.1538-7836.2008.03132.x. [DOI] [PubMed] [Google Scholar]
- 38.Cupit LD, Schmidt VA, Gnatenko DV, Bahou WF. Expression of protease activated receptor 3 (PAR3) is upregulated by induction of megakaryocyte phenotype in human erythroleukemia (HEL) cells. Exp Hematol. 2004;32(10):991–999. doi: 10.1016/j.exphem.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Lavrrar JL, Farnham PJ. The use of transient chromatin immunoprecipitation assays to test models for E2F1-specific transcriptional activation. J Biol Chem. 2004;279:46343–46349. doi: 10.1074/jbc.M402692200. [DOI] [PubMed] [Google Scholar]
- 40.Dignam JD, Lebovitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goldfinger LE, Ptak C, Jeffery ED, Shabanowitz J, Hunt DF, Ginsberg MH. RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration. J Cell Biol. 2006;174(6):877–888. doi: 10.1083/jcb.200603111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pecci A, Malara A, Badalucco S, et al. Megakaryocytes of patients with MYH9-related thrombocytopenia present an altered proplatelet formation. Thromb Haemost. 2009;102(1):90–96. doi: 10.1160/TH09-01-0068. [DOI] [PubMed] [Google Scholar]
- 43.Arepally G, Rebbeck TR, Song W, Gilliland G, Maris JM, Poncz M. Evidence for genetic homogeneity in a familial platelet disorder with predisposition to acute myelogenous leukemia (FPD/AML) [letter]. Blood. 1998;92(7):2600–2602. [PubMed] [Google Scholar]
- 44.Jalagadugula GS, Mao G, Dhanasekaran DN, Kaur G, Rao AK. RUNX1 regulates platelet/megakaryocyte PKC-theta: decreased platelet PKC-θ expression in human RUNX1 haplodeficiency [abstract]. J Thromb Haemost. 2009;(suppl 2):95. [Google Scholar]
- 45.Kaur G, Jalagadugula G, Mao G, Rao AK. RUNX1/core binding factor A2 regulates platelet 12-Lipoxygenase gene (ALOX12): studies in human RUNX1 haplodeficiency. Blood. 2010;115:3128–3135. doi: 10.1182/blood-2009-04-214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elagib KE, Racke FK, Mogass M, Khetawat R, Delehanty LL, Goldfarb AN. RUNX1 and GATA-1 coexpression and cooperation in megakaryocytic differentiation. Blood. 2003;101(11):4333–4341. doi: 10.1182/blood-2002-09-2708. [DOI] [PubMed] [Google Scholar]
- 47.Huang G, Zhang P, Hirai H, et al. PU. 1 is a major downstream target of AML1 (RUNX1) in adult mouse hematopoiesis. Nat Genet. 2008;40(1):51–60. doi: 10.1038/ng.2007.7. [DOI] [PubMed] [Google Scholar]
- 48.Hug BA, Ahmed N, Robbins JA, Lazar MA. A chromatin immunoprecipitation screen reveals protein kinase Cbeta as a direct RUNX1 target gene. J Biol Chem. 2004;279(2):825–830. doi: 10.1074/jbc.M309524200. [DOI] [PubMed] [Google Scholar]
- 49.Javed A, Gutierrez S, Montecino M, et al. Multiple Cbfa/AML sites in the rat osteocalcin promoter are required for basal and vitamin D-responsive transcription and contribute to chromatin organization. Mol Cell Biol. 1999;19(11):7491–7500. doi: 10.1128/mcb.19.11.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li D, Sinha KK, Hay MA, Rinaldi CR, Saunthararajah Y, Nucifora G. RUNX1-RUNX1 homodimerization modulates RUNX1 activity and function. J Biol Chem. 2007;282(18):13542–13551. doi: 10.1074/jbc.M700074200. [DOI] [PubMed] [Google Scholar]