Abstract
Although much is known about extrinsic regulators of platelet function such as nitric oxide and prostaglandin I2 (PGI2), considerably less is known about intrinsic mechanisms that prevent overly robust platelet activation after vascular injury. Here we provide the first evidence that regulators of G-protein signaling (RGS) proteins serve this role in platelets, using mice with a G184S substitution in Gi2α that blocks RGS/Gi2 interactions to examine the consequences of lifting constraints on Gi2-dependent signaling without altering receptor:effector coupling. The results show that the Gi2α(G184S) allele enhances platelet aggregation in vitro and increases platelet accumulation after vascular injury when expressed either as a global knock-in or limited to hematopoietic cells. Biochemical studies show that these changes occur in concert with an attenuated rise in cyclic adenosine monophosphate levels in response to prostacyclin and a substantial increase in basal Akt activation. In contrast, basal cyclic adenosine monophosphate (cAMP) levels, agonist-stimulated increases in [Ca++]i, Rap1 activation, and α-granule secretion were unaffected. Collectively, these observations (1) demonstrate an active role for RGS proteins in regulating platelet responsiveness, (2) show that this occurs in a pathway-selective manner, and (3) suggest that RGS proteins help to prevent unwarranted platelet activation as well as limiting the magnitude of the normal hemostatic response.
Introduction
The hemostatic response to injury in humans and other species with a closed, high-pressure circulatory system represents a balance between minimizing blood loss and avoiding vascular occlusion. How this balance is achieved is only partially understood. Hemostatic thrombi are formed by a combination of fibrin and platelets. While fibrin deposition is controlled by regulating production of thrombin and subsequently by protease inhibitors, platelet activation is controlled by limiting the availability of platelet agonists and by releasing inhibitors such as prostaglandin I2 (PGI2) and nitric oxide from endothelial cells. PGI2 and nitric oxide prevent unwarranted platelet activation by raising basal cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) levels. Coming from a source outside of the platelet, they can be viewed as extrinsic regulators of platelet function. Here we asked whether platelet activation is also subject to autonomous (ie, intrinsic) regulation that limits the extent of platelet accumulation by limiting internal platelet signaling.
Although there are multiple ways that intrinsic regulation could be accomplished, most platelet agonists activate members of the G protein–coupled receptor family, making G proteins a logic target for regulation. In general, G proteins remain active until hydrolysis of Gα-bound guanosine-5′-triphosphate (GTP) restores the resting state. The rate of inactivation is determined in part by members of the regulators of G-protein signaling (RGS) family, which accelerate GTP hydrolysis by Gα.1–4 The 37 known RGS proteins have a 120 amino acid RGS domain that can interact with the switch region of activated Gα.5,6 As many as 10 RGS proteins have been reported in platelets, although many of them solely at the level of RNA transcripts.7–12 Nothing is known about the impact of these proteins on platelet activation in vitro or in vivo.
Here we asked whether RGS proteins limit platelet accumulation during thrombus formation and, if so, whether this is through an effect on the initiation or the magnitude of the platelet response to agonists. Because of uncertainty about the full repertoire of RGS proteins expressed in platelets, the paucity of available RGS protein gene knockouts, and ambiguities about the specificity of RGS/Gα interactions, we studied mice in which a substitution (G184S) in the α subunit of Gi2 renders it resistant to accelerated turn-off by all RGS proteins.13 This allowed us to focus on one particular G protein and the potential effects of unrestrained signaling downstream of that G protein mediated by either the α subunit or the βγ heterodimer. Gi2, which is only 1 of 4 Gi family members expressed in platelets, was selected because (1) platelets from Gi2α knockout mice have a distinct loss of function phenotype, suggesting the absence of functional redundancy among the family members14,15 and (2) Gi2 is the dominant signaling entity downstream of the adenosine diphosphate (ADP) receptor P2Y12, the target for several widely prescribed antiplatelet agents.16,17 RGS-resistant mutations analogous to the Gi2α(G184S) substitution have also been described in other G proteins. In each case, the mutations have been shown to impair RGS:Gα interactions without altering receptor:effector coupling.13,18,19 The biologic impact of the substitution is expected to vary depending on the signaling context of the affected G protein and the extent to which internal controls for these pathways exist.
Here we show that removing RGS protein-dependent restraints on even a single Gi2α allele produces a gain of function phenotype in platelets, shifting the dose/response curve for affected agonists to the left in vitro and increasing platelet accumulation at sites of vascular injury in vivo. The results also show that removing RGS restraints on Gi2 is sufficient to prime signaling pathways within the platelet even before an agonist is added. The increased responsiveness to agonists is not limited to ADP but retains specificity for pathways known to be mediated by Gi2. These observations indicate that platelet activation is subject to intrinsic as well as extrinsic regulation, show for the first time that RGS proteins serve this role in platelets in an agonist-selective, pathway-specific manner, and provide a novel insight into receptor:effector coupling specificity in platelets that complements earlier work that we and others have done on G-protein knockouts in platelets.
Methods
Materials
Apyrase, ADP, PGI2, prostaglandin E1 (PGE1), forskolin, 3-isobutyl-l-methyl-xanthine (IMBX), and aspirin were from Sigma-Aldrich. PAR4 agonist peptide H-AYPGKF-NH2 was from Bachem. U46619 was from CalBiochem. Collagen was from Chrono-log. Convulxin (CVX) was from Alexis Biochemicals. Horseradish peroxidase linked anti-mouse and anti-rabbit monoclonal antibodies, Lumigen PS-3 detection reagent for western blots, and the cAMP Biotrak EIA system were from GE Healthcare. The Rap1 Activation Assay kit was from Millipore. Phospho-Akt (Ser473) and Akt antibodies were from Cell Signaling. Cangrelor was a kind gift from Dr. Jayne Prats (The Medicines Company). Ready Gel 4%-15% precast gels, ImmunoBlot polyvinylidene fluoride membrane, and nonfat dry milk were from Bio-Rad Laboratories.
Mouse lines
Gi2α(+/G184S) mice backcrossed at least 10× into C57Bl/6 were described previously.13 Breeding was carried out between Gi2α(+/G184S) and Gi2α(+/+) mice from previous crosses to produce heterozygous and wild-type offspring. Experiments were performed with sex- and litter-matched mice. To prepare mice expressing the G184S substitution solely in hematopoietic cells, fetal livers were isolated from 14-16 days postcoitus (d.p.c.) wild-type or G184S/G184S fetuses and injected retro-orbitally into lethally irradiated male wild-type recipients (Cesium-137, 11 Gy, Gammacell 40 Exactor; MDS Nordion). Intravital microscopy experiments were performed at least 4 weeks after the transplant. Hematopoietic reconstitution was confirmed by measuring platelet counts before intravital experiments. All animal studies were carried out in compliance with University of Pennsylvania Institutional Animal Care and Use Committee approved protocols.
Platelet aggregation
Blood was drawn from the inferior vena cava of anesthetized mice (100 mg/kg Nembutal) using a heparinized syringe (1000 U/mL, 1:100 dilution with blood), diluted 1:1 with HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid]-Tyrode buffer, and spun at 800 rpm for 6 minutes to isolate platelet-rich plasma (PRP). PRP from one mouse of each genotype was used for aggregation experiments. Platelet counts (Beckman-Coulter, Z1) were adjusted to 2.5 × 108 platelets/mL with HEPES-Tyrode buffer. Aggregation was measured in a dual-channel Chrono-log lumi-aggregometer at 37°C with constant stirring at 800 rpm after allowing the platelets to equilibrate to 37°C for 3 minutes. For studies using apyrase (1 U/mL) and Cangrelor (100nM), the inhibitor was added to PRP 3 minutes before agonist addition. Aspirin-treated (1mM) samples were allowed to incubate for 30 minutes at room temperature before agonist addition.
α-granule secretion
Platelets from 2 mice of each genotype were resuspended in Tyrode buffer at a final concentration of 2 × 107 platelets/mL. Aliquots (100 μL) of the platelet suspension were incubated with 5 μL of fluorescein isothiocyanate–conjugated P-selectin antibody (BD Pharmingen) or an isotype-matched control. After 15 minutes at 37°C with or without AYPGKF, the platelets were diluted with 400 μL of phosphate-buffered saline before immediate analysis by flow cytometry using a BD Biosciences FACSCalibur.
Preparation of washed platelets
PRP was diluted with HEN buffer (10mM HEPES pH 6.5, 1mM EDTA [ethylenediaminetetraacetic acid], 150mM NaCl). For cAMP measurements, the HEN wash buffer was supplemented with 1mM aspirin. For Akt phosphorylation experiments, HEN buffer was supplemented with 1mM aspirin and 0.05 U/mL apyrase to prevent receptor desensitization.20 For the Rap1 activation assay, the HEN buffer was supplemented with 1μM PGE1 during the wash steps. For the Ca++ experiments, the HEN buffer was supplemented with 1 U/mL apyrase and 1μM PGE1 during wash steps. Platelets were sedimented at 1300 rpm for 15 minutes and then resuspended in HEPES-Tyrode buffer to the desired platelet counts.
Cytosolic Ca++ measurements
Washed platelets pooled from 2 mice of each genotype were resuspended in 1.5 mL of HEPES-Tyrode buffer containing 1 U/mL apyrase and 1μM PGE1 and incubated with 25 μL of Fura-2AM in DMSO (1 mg/mL) for 45 minutes in the dark. Afterward, the platelets were washed with HEN buffer and resuspended in HEPES-Tyrode buffer at 2 × 108 platelets/mL. A 384-well microtiter plate containing platelet agonists was prepared on a PerkinElmer Janus. A separate microtiter plate containing diluted platelet suspension (1 × 106 platelets/well) was prepared on a PerkinElmer Evolution. Agonists were dispensed on a Molecular Devices FlexStation. Fura2 fluorescence was measured at excitation 340/380 nm and emission 510 nm for 4 minutes in every column of the plate. The 340/380 fluorescence ratio R(t) was scaled to the mean baseline value for each well R0(t), and relative calcium concentrations were quantified as R(t)/ R0(t). All conditions were tested in replicates of 8.
cAMP measurements
Except for the basal cAMP determinations, washed platelets (2.5 × 108/mL) were incubated for 30 minutes at 37°C with 500μM IMBX to inhibit cAMP phosphodiesterase activity. Platelets were stimulated with 20μM forskolin, 15μM PGI2, and ADP for 10 minutes as indicated. The reaction was stopped with the addition of 1 volume of 10% ice-cold trichloroacetic acid after which the samples were mixed by vortexing, lysed by rapid freezing and thawing, and then spun to remove precipitates. cAMP was measured using the Biotrak EIA system from GE Healthcare. Samples used to measure basal cAMP levels were not incubated with IMBX, forskolin, PGI2, or ADP.
Akt phosphorylation
Washed platelets were prepared using blood from 2 mice of each genotype and adjusted to 2.5 × 108 platelets/mL with a total volume of 0.2 mL. After incubation with 250μM AYPGKF, 3 volumes of ice-cold sodium dodecyl sulfate sample buffer containing protease inhibitors was added. Samples were then incubated at 95°C with gel-loading buffer for 5 minutes and spun in a microcentrifuge to remove precipitates. Proteins were separated using 4%-15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gels and then transferred to polyvinylidene fluoride membranes. Nonspecific binding was prevented with 5% nonfat dry milk in Tris-buffered saline (TBS, 1 hour at room temperature). Membranes were incubated with primary antibody (1:500 in 5% milk in TBS) overnight at 4°C, washed 3 times with 0.1% Tween-20 in TBS, exposed to the appropriate secondary antibody for 2 hours at room temperature (1:5000 in 5% milk TBS), washed 5 times with Tween-20 in TBS, incubated with Lumigen PS-3 detection reagent for 5 minutes at room temperature, and then exposed to film. Samples from each genotype were run on the same gel. Quantitation of band strength was performed using ImageJ (National Institutes of Health [NIH] Version 1.42). Stripping and reprobing membranes for total Akt provided loading controls for analysis.
Rap1 activation assay
Washed platelets were prepared using blood from 2 mice of each genotype and adjusted to 4.0 × 108 platelets/mL and a total volume of 0.5 mL. After incubation with indicated amounts of ADP for 5 minutes at room temperature, platelets were lysed with Rap1 activation lysis buffer containing protease inhibitors and spun in a microcentrifuge at high speed for 10 minutes at 4°C to remove cell debris. Lysates were removed and processed as instructed by Millipore. Controls to indicate the total amount of Rap1 from each batch of platelets studied were obtained by immunoblotting platelet lysates unexposed to Ral GDS RBD agarose. Samples were immunoblotted as described previously with the Akt experiments. Samples from each genotype were run on the same gel. Quantitation of band strength was performed using ImageJ (NIH).
Intravital microscopy
Intravital microscopy was performed as previously described.21 In brief, F(ab)2 fragments of anti-CD41 antibodies (clone MWReg30; BD Biosciences) were labeled with Alexa 647 using the Alexa Fluor Protein Labeling kit (Molecular Probes). Mice were anesthetized with an intraperitoneal injection of sodium pentobarbital (90 mg/kg). Anesthesia was maintained with 5 mg/kg pentobarbital as required through a jugular vein cannula. The cremaster muscle was exteriorized and kept hydrated with buffer (135mM NaCl, 4.7mM KCl, 2.7mM CaCl2, 18mM NaHCO3, pH 7.4). Labeled anti-CD41 (0.2 μg/g bodyweight) was infused 10 minutes before the first injury. Arterioles with undisrupted flow were chosen, and injury was induced using a pulsed nitrogen dye laser at 440 nm focused through the microscope optics. Widefield fluorescence (635 nm excitation wavelength) or confocal fluorescence and brightfield images were collected alternately for up to 3 minutes after injury formation using an Olympus BX-61WI fluorescence microscope (Olympus) with a 40× water immersion objective lens (numeric aperture 0.8) and recorded using a Cooke SensiCam digital camera. Data were analyzed using SlideBook 5.0 (Intelligent Imaging Innovations). Up to 10 injuries were made in each mouse.
Flow chamber studies
Platelet adhesion and aggregation on collagen was measured in a microfluidics flow chamber as previously described.22 The chamber was coated with acid soluble collagen (0.3 mg/mL; Inamed Biomaterials). Whole blood anticoagulated with H-(D)-Phe-Pro-Arg-chloromethylketone (PPACK, 93μM final concentration; EMB Biosciences) was perfused at 1000 seconds−1 for up to 5 minutes and then increased to 10 000 seconds−1.
Results
In vivo effects of the RGS-insensitive mutation on thrombus formation
Mice that are homozygous for the Gi2α(G184S) allele exhibit reduced birth frequency and viability, growth retardation, skeletal abnormalities, splenomegaly, cardiac hypertrophy, tachycardia, and an increase in monocyte and neutrophil counts.13 Heterozygous Gi2α(+/G184S) mice lack these abnormalities. They are born with a near-expected frequency when produced in crosses between heterozygous and wild-type mice and show only an initial small reduction in body mass that resolves. Cardiac size is normal in the Gi2α(+/G184S) mice as are the platelet, erythrocyte, total leukocyte, neutrophil, and monocyte counts.
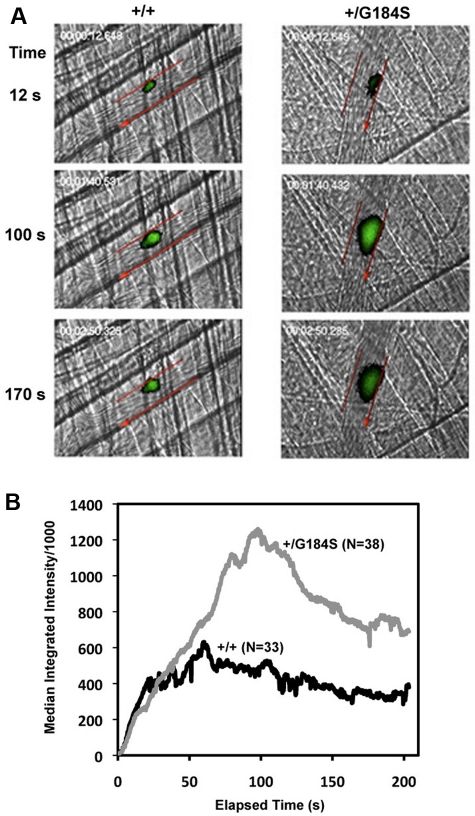
Given the physiologic abnormalities present in the homozygous mice, we focused most of our efforts on the heterozygous mice, starting by assessing platelet response to arterial injury in vivo. In the assay used, a pulsed laser was used to make localized injuries to the wall of arterioles in the cremaster muscle microcirculation. Thrombus formation was visualized in real time with fluorescently labeled platelets. This injury model is primarily (but not exclusively) thrombin driven.23 The data in Figure 1 show that the initial rate of platelet accumulation in the Gi2α(+/G184S) mice was the same as in matched controls. However, the maximum extent of platelet accumulation was approximately twice as great, as was the area under the fluorescence-versus-time curve, which is roughly proportional to the number of platelets incorporated into the thrombus. Notably, this increase in platelet accumulation was not associated with an increase in occlusive events, which rarely occur in this thrombosis model.
Figure 1.
Increased platelet accumulation in Gi2α(+/G184S) mice after laser injury in cremaster muscle arterioles. (A) Thrombus formation in Gi2α(+/G184S) mice and matched controls (+/+). Platelets labeled with fluorescently conjugated anti-CD41 Fab fragments are shown in green. Arteriole walls are outlined in red with the direction of blood flow indicated with an arrow. Video captures at selected time points are shown. Videos showing the entire period of observation are included in the online supplement. (B) Median fluorescence intensity versus elapsed time after laser injury. The dataset included 38 injuries observed in 5 Gi2α(+/G184S) mice and 33 injuries in 4 matched controls Gi2α(+/+).
To observe the effects of having 2 copies of the mutant allele and, at the same time, exclude possible effects of the mutation on cells within the vascular wall, laser injury studies were also performed in irradiated wild-type mice reconstituted with hematopoietic cells harvested from wild-type and Gi2α(G184S/G184S) embryos. A similar increase in platelet accumulation was observed (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). As with the globally expressed Gi2α(+/G184S) mice, this increase did not accompany a difference in the initial rate of platelet accumulation or frequency of occlusive events.
Ex vivo effects of the RGS-insensitive mutation
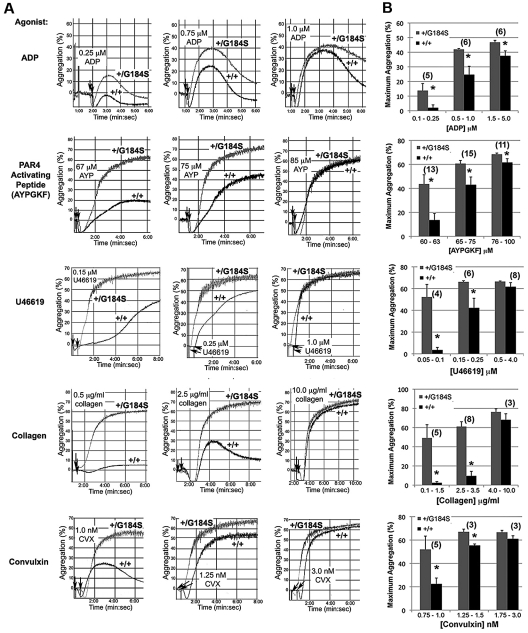
Platelet aggregation was recorded after addition of either ADP, a PAR4 thrombin receptor agonist peptide (AYPGKF), a thromboxane A2 (TxA2) mimetic (U46619), collagen or convulxin (CVX), a snake venom lectin that activates platelet glycoprotein VI collagen receptors. In each case, Gi2α(+/G184S) platelets responded significantly better than controls at low agonist concentration, reflecting a shift in the aggregation dose/response curve to the left (Figure 2). In contrast, there was no shift in the dose/response curve for α-granule secretion in response to AYPGKF (supplemental Figure 2).
Figure 2.
Increased platelet aggregation in platelets from Gi2α(+/G184S) mice. (A) Representative aggregation traces of platelets isolated from Gi2α(+/G184S) mice and matched controls. U46619 is a TxA2 receptor agonist. Convulxin is a snake venom lectin that activates the platelet collagen receptor, glycoprotein VI. (B) Maximum aggregation (%) in response to all studied agonists at dose ranges corresponding to low response, mid-response, and maximal response for the control platelets. All aggregation studies were performed as paired comparisons between platelets obtained from one mouse of each genotype. The total number of replicate observations compared in each category is shown in parentheses in the graph. The number of mouse pairs used for each agonist were 4 (ADP), 6 (AYPGKF), 3 (U46619), 3 (collagen), and 2 (convulxin). *P < .05.
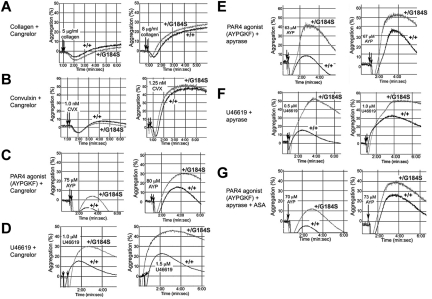
A gain of function when Gi2α(+/G184S) platelets are stimulated with ADP would be expected if RGS proteins normally constrain platelet responses downstream of Gi2-coupled P2Y12 receptors. However, collagen and CVX receptors are not coupled directly to Gi2,24 and signaling downstream of thrombin and TxA2 receptors in platelets is usually attributed to Gq and G12 family members rather than to direct activation of Gi family members.25 To determine whether the results obtained with collagen and CVX are due to secreted ADP, platelets were preincubated with Cangrelor (ARC-69931MX), a selective inhibitor of P2Y12.26 In the presence of the inhibitor, the rate and extent of platelet aggregation caused by collagen and CVX were reduced, and there was no longer a difference between the Gi2α(+/G184S) and wild-type platelets (compare Figure 3A-B with the responses to collagen and CVX in Figure 2). This shows that the gain of function with collagen and CVX observed in the absence of Cangrelor is due to the autocrine and paracrine effects of secreted ADP rather than a primary effect on collagen receptor signaling.
Figure 3.
Secreted ADP accounts for the enhanced response of Gi2α(+/G184S) platelets to collagen but not thrombin or TxA2. Platelet aggregation was measured in response to each of the agonists indicated in the presence of a P2Y12 antagonist (Cangrelor), an ADPase (apyrase), and/or a cyclo-oxygenase inhibitor, aspirin (ASA). Representative tracings are shown. The number of times each comparison was performed and the number of mouse pairs used in each part of the figure was (A) 13 times with blood from 2 pairs of mice, (B) 13 times with 2 pairs of mice, (C) 9 times with 2 pairs of mice, (D) 12 times with 4 pairs of mice, (E) 10 times with 2 pairs of mice, (F) 9 times with 2 pairs of mice, and (G) 10 times with 2 pairs of mice.
In contrast, blocking P2Y12 receptors with Cangrelor or hydrolyzing secreted ADP with apyrase did not eliminate the difference between Gi2α(+/G184S) and control platelets activated by AYPGKF or U46619 (Figure 3C-G). Although possible contributions from other secreted agonists whose receptors are coupled to Gi2α cannot be excluded, this suggests that PAR4 thrombin receptors and TxA2 receptors, unlike platelet collagen receptors, are able to couple directly to Gi2. AYPGKF-induced platelet aggregation was reduced a bit further by adding apyrase with aspirin to inhibit TxA2 production, but here again the difference between Gi2α(+/G184S) and wild-type mice persisted, providing further evidence that PAR4 couples directly to Gi2 signaling pathways.
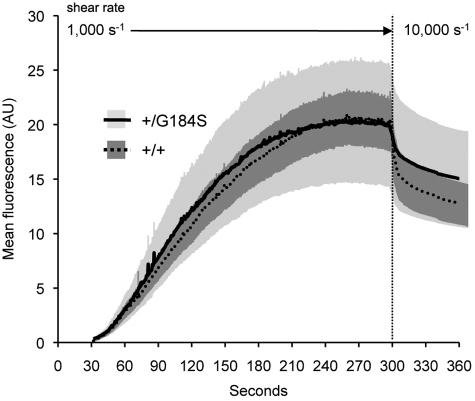
Additional evidence for the lack of a primary effect on collagen was obtained in studies measuring platelet adhesion and accumulation on a collagen-coated surface within a microfluidics flow chamber.22 The chambers were perfused with whole blood from Gi2α(+/G184S) and wild-type mice anticoagulated with the direct thrombin inhibitor, PPACK. No differences were observed in either the rate or extent of platelet accumulation (Figure 4). At the end of this period, the shear rate was increased 10-fold to challenge thrombus stability. A trend toward greater stability was observed for the Gi2α(+/G184S) platelets but did not reach statistical significance. Note, however, that these observations were performed with the flow chamber coated with a relatively high concentration of collagen. Based on the results of the aggregation studies with collagen and CVX, it is possible that reducing the collagen concentration would have revealed a greater difference between the Gi2α(+/G184S) and control platelets by increasing dependence on released ADP.27
Figure 4.
Adhesion to collagen under flow is not affected by the Gi2α(G184S) mutation. Whole blood from Gi2α(+/G184S) mice and matched controls was anticoagulated with PPACK and perfused over collagen in a microfluidics flow chamber. After 297 seconds at 1000 seconds−1, the shear was increased 10-fold to 10 000 seconds−1. The data shown are the mean ± SEM from 7 Gi2α(+/G184S) mice and 8 matched controls (+/+).
Rendering Gi2α RGS-insensitive produces a gain of function in suppression of cAMP formation and activation of Akt
Signaling pathways in platelets that are believed to be regulated via Gi2 include inhibition of cAMP formation by adenylyl cyclase, activation of the phosphatidylinositol 3-kinase pathway and, possibly, activation of the small GTPase Rap1b. There is also evidence in cells other than platelets that Gβγ derived from Gi family members can activate phospholipase Cβ, leading to phosphoinositide hydrolysis and an increase in cytosolic Ca++.28 To better understand the mechanisms underlying the observed gain of function in the Gi2α(+/G184S) platelets, we measured the impact of the Gi2α(G184S) mutation on each of these pathways.
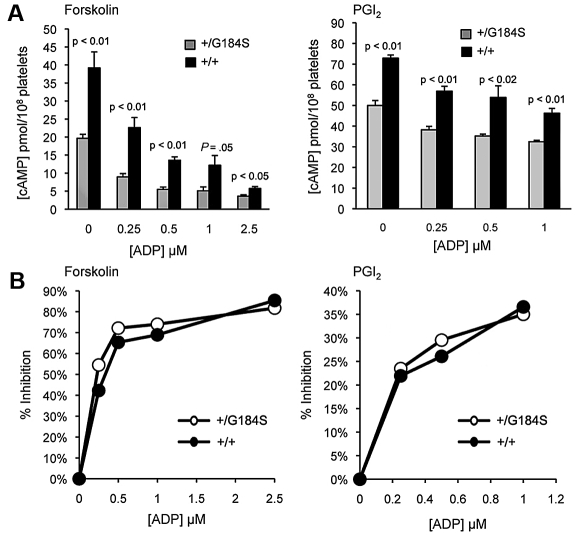
In contrast to the Gi2α knockout, which causes an increase in basal cAMP levels in platelets,15 the basal cAMP concentration in Gi2α(+/G184S) platelets was indistinguishable from matched controls: 7.0 ± 1.3 (het) and 6.5 ± 0.7 (control) pmol/108 platelets (mean ± SEM; n = 8, P = .785). However, the large increase in cAMP levels that normally occurs when platelets are incubated with either forskolin or PGI2 was attenuated in Gi2α(+/G184S) platelets (Figure 5A). Notably, the fractional inhibition of cAMP formation by ADP was unaffected (Figure 5B), indicating that receptor:effector coupling remains intact. These results suggest a role for Gi2 and RGS proteins in determining how high the cAMP levels rise in response to endothelium-derived PGI2. A diminished cAMP response to PGI2 in Gi2α(G184S)-expressing platelets would be expected to contribute to the gain of function that we observed in vivo, just as knocking out the PGI2 receptor has been shown to do in earlier studies.29
Figure 5.
cAMP formation in Gi2α(+/G184S) platelets. (A) Effect of ADP on cAMP levels after stimulation with forskolin (left) or PGI2 (right). (B) Percent inhibition of cAMP levels with increasing dosages of ADP after stimulation with forskolin (left) and PGI2 (right). Mean ± SEM. The data shown represent averaged measurements from 4 (forskolin) or 3 (PGI2) experiments. One mouse of each genotype was used in each experiment.
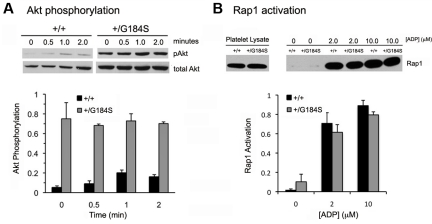
Gi-dependent signaling in platelets mediated by Gi-derived Gβγ activates phosphatidylinositol 3-kinase β isoforms, leading to phosphorylation and activation of the serine/threonine kinases Akt1 and Akt2.20,30,31 Figure 6A compares Akt activation in Gi2α(+/G184S) and matched control platelets using Ser473 phosphorylation as a marker. As seen in previous studies, resting wild-type platelets exhibited negligible Akt phosphorylation, followed by an increase in phosphorylation when AYPGKF was added. In contrast, Gi2α(+/G184S) platelets exhibited robust Akt phosphorylation even in the absence of an agonist. In fact, Akt phosphorylation in unstimulated Gi2α(+/G184S) platelets exceeded that seen in activated wild-type platelets and showed little, if any, change when AYPGKF was added. This striking increase in basal Akt phosphorylation was present even when Cangrelor, aspirin, and apyrase were added (data not shown), suggesting that it is not due to release of small amounts of ADP or formation of TxA2 during platelet handling.
Figure 6.
Akt phosphorylation and Rap1 activation in Gi2α(+/G184S) platelets. (A) Phosphorylation of Akt after addition of the PAR4 agonist peptide AYPGKF (250μM). Mean ± SEM of data from 3 experiments. One mouse of each genotype was used in each experiment. Samples from the representative pAkt blot were run on the same gel. (B) Rap1 activation in response to varying concentrations of ADP. Mean ± SEM of data from 3 experiments. Two mice of each genotype were used in each experiment. Samples from the representative Rap1 blot were run on the same gel.
The RGS-insensitive Gi2α mutant does not affect Rap1b activation or changes in cytosolic Ca++
Rap1b activation is one of the critical steps leading to αIIbβ3 activation in platelets.32–34 Loading of Rap1b with GTP has been shown to occur downstream of both Gq and Gi2, with the predominant determinant being a Gq-mediated increase in cytosolic Ca++ concentration which activates guanine nucleotide exchange by CalDAG-GEF. In contrast to Akt activation, there was no increase in Rap1b activation in response to ADP. There was also no increase in basal Rap1b-GTP levels (Figure 6B).
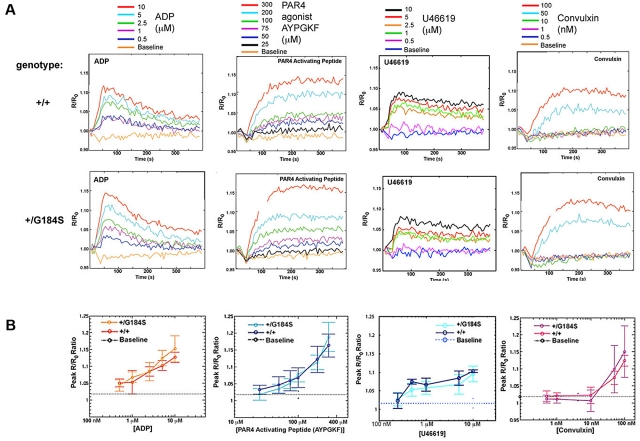
Finally, because of suggestions from the literature that Gi-derived Gβγ can activate phospholipase Cβ, we examined the effect of the Gi2α(G184S) substitution on changes in the cytosolic Ca++ concentration when Gi2α(+/G184S) and matched control platelets were incubated with ADP, AYPGKF, U46619, or CVX. A novel high throughput assay was used which allows multiple agonists to be studied in replicate at several concentrations of each agonist without requiring large amounts of blood. Each agonist tested caused an increase in cytosolic Ca++, but the extent of the increase was unaffected by the presence of the Gi2α(G184S) substitution (Figure 7). This finding is consistent with the unperturbed timing and extent of Rap1 activation in Gi2α(+/G184S) platelets. It suggests that, in this context, there is no crosstalk between Gi2- and Gq-mediated pathways in the presence of the G184S mutation. Stimulation of phospholipase C by Gβγ derived from Gi either does not occur in platelets or occurs at too low a level to dictate changes in cytosolic Ca++.
Figure 7.
Changes in cytosolic [Ca++]i upon activation of Gi2α(+/G184S) platelets. (A) Gi2α(+/G184S) and matched control platelets were loaded with Fura-2 and stimulated with several concentrations of each of the indicated agonists. Ca++-dependent Fura-2 fluorescence over time for each agonist is shown for wild-type (top) and Gi2α(+/G184S) platelets (bottom). (B) A comparison of average maximal fluorescence over the range of agonist concentrations studied for each genotype. Each curve represents the average of 8 replicates. The experiment was performed twice with similar results obtained. Blood obtained from 2 mice of each genotype was used for each experiment.
Discussion
The ability of platelets to respond to vascular injury is essential for hemostasis but carries a risk of unintended vascular occlusion, especially when arterial disease compromises endothelial barrier function. Here we have asked whether platelet accumulation in response to vascular injury is regulated by intrinsic mechanisms that limit platelet responses. In particular, we asked whether members of the RGS protein family might perform such a regulatory role, limiting signaling in a G protein–selective, pathway-specific manner. Although it has been shown that overexpression of RGS proteins in cells other than platelets can inhibit signaling,35 we used a different approach, taking advantage of a line of mice expressing a Gi2α variant that interacts poorly with RGS proteins as a class. This allowed us to focus on the consequences of signaling by a single G protein unconstrained by any of the RGS proteins that appear to be expressed in platelets.
The results show that even a single RGS-insensitive Gi2α allele is sufficient to increase platelet aggregation at low agonist concentrations and to double the extent of platelet accumulation after vascular injury. Because G protein α subunits possess intrinsic GTP hydrolysis activity that is retained in the absence of RGS proteins, an increased response at suboptimal agonist concentrations is what would be expected if RGS proteins normally restrain Gi2-dependent signaling. In investigating possible changes in specific biochemical processes that might underlie the observed phenotypic changes, we found that neither the rise in cytosolic Ca++ that usually accompanies platelet activation, Rap1b activation, which is predominantly Ca++-driven, nor α-granule secretion were altered in Gi2α(+/G184S) platelets. The basal cAMP concentration, which is elevated in platelets from Gi2α(−/−) mice,15 was also unchanged. However, the ability of PGI2 and forskolin to raise cAMP levels in platelets was attenuated in Gi2α(+/G184S) platelets, and basal Akt phosphorylation was greatly increased, even when P2Y12 was blocked and TxA2 formation suppressed.
Collectively, these observations show that the G184S substitution in Gi2α produces a pathway-selective gain of function in platelets. This gain of function is confined to Gi2-mediated events and does not spill over to events in platelets that raise the cytosolic Ca++ concentration and subsequently activate Rap1b, events that are predominantly Gq-driven in platelets. Notably, the Gi2-mediated gain of function was observable even before an agonist was added. Thus, the marked increase in basal Akt phosphorylation and the reduced cAMP concentration in platelets incubated with PGI2 and forskolin, even before ADP was added.
This suggests that the interaction of RGS proteins with Gi2α normally has 2 related roles: helping to provide a threshold to platelet activation and limiting the response to injury once platelet activation has occurred. Because platelet activation by physiologic agonists other than collagen is thought to require a combination of Gi- and Gq-dependent signaling,36 increased basal and stimulated activity in Gi-dependent pathways can account for the leftward shift in the dose/response curve for platelet aggregation that we observed ex vivo when Gi2α(+/G184S) platelets were activated by agonists coupled to Gq. It can also, along with a blunted response to locally generated PGI2, account for the increase in platelet accumulation that we observed after vascular injury in vivo.
Although it might be argued that the effects of the Gi2α(G184S) allele observed in the vascular injury model with heterozygous mice could also be due in part to the presence of Gi2α(G184S) in cells other than platelets, the corroborating ex vivo data showing a gain of function in platelet aggregation and Gi2 signaling pathway intermediates in Gi2α(+/G184S) mice suggests a uniquely platelet phenotype, as does the gain of function observed in the Gi2α(G184S/G184S) radiation chimeras. Note, however, that the chimeras do not exclude additional contributions from Gi2α(G184S) expressed in hematopoietic cells other than platelets.
Priming Gi-dependent signaling downstream of P2Y12 ADP receptors helps to account for the enhanced responsiveness of Gi2α(+/G184S) platelets that we observed, but it is unlikely to be the sole explanation. We also found that the Gi2α(G184S) substitution produced increased responses to PAR4 and TxA2 receptor agonists even when a contribution from secreted ADP was eliminated. This suggests that either there is a linkage between thrombin, TxA2, and Gi2 activation,37,38 a point that has been debated in the platelet literature39 or that PAR4 and TxA2 receptor agonists (but not collagen) cause release of a molecule other than ADP whose receptors are coupled to Gi2α. Conversely, the observation that changes in the cytosolic Ca++ concentration occurred as usual in Gi2α(+/G184S) platelets suggests that activation of phospholipase Cβ by Gi2-derived Gβγ, which does occur in neutrophils, does not contribute significantly in platelets, a conclusion that is also supported by observations on platelets from P2Y12 knockout mice.16,40 Elucidating the exact nature of this apparent cooperativity between Gq and Gi2 events provides an interesting avenue for further study.
The presence of a large number of RGS proteins in platelets with differences in their G-protein selectivity and ability to interact with the membrane, receptors, and other signaling or scaffold proteins offers a rich potential mechanism for fine tuning platelet responses to different agonists. Platelets are thought to express at least 10 different RGS protein family members,7–12 varying in their selectivity for G-protein family members and ability to interact with proteins other than Gα.35 The unique identity of specific RGS-G protein interactions, restraints provided by these interactions, and receptor-specific effects are unclear. Variants analogous to the G184S substitution in Gi2α have been established for other G proteins but have not been engineered into mice.13,18,19 It is, therefore, not yet possible to state with certainty that the observations developed here that support a regulatory interaction between RGS proteins and Gi2α also apply to the other G proteins that are expressed in platelets. However, given the presence in platelets of RGS proteins capable of interaction with Gqα, we predict that this will prove to be the case.
In summary, the present studies show for the first time that G- protein signaling pathways in platelets are normally restrained by RGS family members and that removing these restraints from Gi2-mediated events promotes platelet activation in vitro and increases platelet accumulation in vivo. The potential power of this mechanism is suggested by the readily detectable effects of taking it away even when only half of the Gi2α has been rendered RGS-resistant, leaving the other half of the Gi2α and all of the Gi1α, Gi3α, and Gzα susceptible to inhibition. Because platelet accumulation after injury in the Gi2α(+/G184S) mice usually did not continue to the point of occlusion, it is likely that other constraints exist as well, including those that might be directed against Gq-dependent signaling pathways as well as those involving extrinsic factors. Conversely, the ability of RGS proteins to restrict the duration of signaling by susceptible G protein α subunits suggests that means exist to regulate RGS:Gα interactions, allowing signaling to begin before it is shut down. Constraints mediated by RGS proteins may also be relevant at another level. Recent evidence suggests that a growing hemostatic plug comprises a core of fully active platelets overlaid with a labile layer of partially activated platelets, some of which will become fully activated and stably adherent.41,42 The balance between stable and transient attachment may be regulated in part by the rate at which RGS proteins shut down signaling downstream of platelet receptors for agonists such as ADP and TxA2. Finally, although here we have focused on the effects of a very specific, well-characterized amino acid substitution in Gi2α, it is reasonable to speculate that other sequence variations within the Gi2α switch domain or in the RGS proteins themselves may affect RGS/Gi2α interactions. Were such variations to occur naturally, they might well contribute to the spectrum of thrombotic risks that occur between individuals.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) grants HL40387 (L.F.B.), HL087385 (S.L.D. and L.F.B.), and GM39561 (R.R.N.). T.J.S. was supported by American Heart Association postdoctoral fellowship 0525630U. R.S.S. was supported by NIH T32 HL07439.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.S.S., T.J.S., K.P.F., M.S.C., P.M., and P.R.H. performed experiments; R.S.S., T.J.S., K.P.F., P.M., and M.S.C. designed research, analyzed results, and made figures; A.C., S.L.D., R.R.N., and L.F.B. designed research and contributed vital new reagents or analytical tools; and R.S.S. and L.F.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence F. Brass, MD, PhD, University of Pennsylvania, 915 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: brass@mail.med.upenn.edu.
References
- 1.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18(5):579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Sierra DA, Popov S, Wilkie TM. Regulators of G-protein signaling in receptor complexes. Trends Cardiovasc Med. 2000;10(6):263–268. doi: 10.1016/s1050-1738(00)00072-4. [DOI] [PubMed] [Google Scholar]
- 3.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54(3):527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 4.Neitzel KL, Hepler JR. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin Cell Dev Biol. 2006;17(3):383–389. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 5.Tesmer JJ, Berman DM, Gilman AG, Sprang SR. Structure of RGS4 bound to AlF4–activated G(i alpha1): stabilization of the transition state for GTP hydrolysis. Cell. 1997;89(2):251–261. doi: 10.1016/s0092-8674(00)80204-4. [DOI] [PubMed] [Google Scholar]
- 6.Xie GX, Palmer PP. How regulators of G protein signaling achieve selective regulation. J Mol Biol. 2007;366(2):349–365. doi: 10.1016/j.jmb.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berthebaud M, Riviere C, Jarrier P, et al. RGS16 is a negative regulator of SDF-1-CXCR4 signaling in megakaryocytes. Blood. 2005;106(9):2962–2968. doi: 10.1182/blood-2005-02-0526. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon AW, Murray DL, Leadley RJ. Cloning and characterization of a novel regulator of G protein signalling in human platelets. Cell Signal. 2002;14(7):595–606. doi: 10.1016/s0898-6568(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 9.Garcia A, Prabhakar S, Hughan S, et al. Differential proteome analysis of TRAP-activated platelets: involvement of DOK-2 and phosphorylation of RGS proteins. Blood. 2004;103(6):2088–2095. doi: 10.1182/blood-2003-07-2392. [DOI] [PubMed] [Google Scholar]
- 10.Kim SD, Sung HJ, Park SK, et al. The expression patterns of RGS transcripts in platelets. Platelets. 2006;17(7):493–497. doi: 10.1080/09537100600758123. [DOI] [PubMed] [Google Scholar]
- 11.Nagata Y, Oda M, Nakata H, Shozaki Y, Kozasa T, Todokoro K. A novel regulator of G-protein signaling bearing GAP activity for Galphai and Galphaq in megakaryocytes. Blood. 2001;97(10):3051–3060. doi: 10.1182/blood.v97.10.3051. [DOI] [PubMed] [Google Scholar]
- 12.Yowe D, Weich N, Prabhudas M, et al. RGS18 is a myeloerythroid lineage-specific regulator of G-protein-signalling molecule highly expressed in megakaryocytes. Biochem J. 2001;359(Pt 1):109–118. doi: 10.1042/0264-6021:3590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang X, Fu Y, Charbeneau RA, et al. Pleiotropic phenotype of a genomic knock-in of an RGS-insensitive G184S Gnai2 allele. Mol Cell Biol. 2006;26(18):6870–6879. doi: 10.1128/MCB.00314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jantzen HM, Milstone DS, Gousset L, Conley PB, Mortensen RM. Impaired activation of murine platelets lacking G alpha(i2). J Clin Invest. 2001;108(3):477–483. doi: 10.1172/JCI12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J, Wu J, Jiang H, et al. Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J Biol Chem. 2002;277(48):46035–46042. doi: 10.1074/jbc.M208519200. [DOI] [PubMed] [Google Scholar]
- 16.Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107(12):1591–1598. doi: 10.1172/JCI12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollopeter G, Jantzen HM, Vincent D, et al. Identification of the platelet ADP receptor targeted by antithrombotic drugs. Nature. 2001;409(6817):202–207. doi: 10.1038/35051599. [DOI] [PubMed] [Google Scholar]
- 18.DiBello PR, Garrison TR, Apanovitch DM, et al. Selective uncoupling of RGS action by a single point mutation in the G protein alpha-subunit. J Biol Chem. 1998;273(10):5780–5784. doi: 10.1074/jbc.273.10.5780. [DOI] [PubMed] [Google Scholar]
- 19.Fu Y, Zhong H, Nanamori M, et al. RGS-insensitive G-protein mutations to study the role of endogenous RGS proteins. Methods Enzymol. 2004:389229–243. doi: 10.1016/S0076-6879(04)89014-1. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Jin J, Kunapuli SP. Akt activation in platelets depends on Gi signaling pathways. J Biol Chem. 2004;279(6):4186–4195. doi: 10.1074/jbc.M306162200. [DOI] [PubMed] [Google Scholar]
- 21.Stalker TJ, Wu J, Morgans A, et al. Endothelial cell specific adhesion molecule (ESAM) localizes to platelet-platelet contacts and regulates thrombus formation in vivo. J Thromb Haemost. 2009 doi: 10.1111/j.1538-7836.2009.03606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neeves KB, Maloney SF, Fong KP, et al. Microfluidic focal thrombosis model for measuring murine platelet deposition and stability: PAR4 signaling enhances shear-resistance of platelet aggregates. J Thromb Haemost. 2008;6(12):2193–2201. doi: 10.1111/j.1538-7836.2008.03188.x. [DOI] [PubMed] [Google Scholar]
- 23.Vandendries ER, Hamilton JR, Coughlin SR, Furie B, Furie BC. Par4 is required for platelet thrombus propagation but not fibrin generation in a mouse model of thrombosis. Proc Natl Acad Sci U S A. 2007;104(1):288–292. doi: 10.1073/pnas.0610188104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson SP. Collagen receptor signaling in platelets and megakaryocytes. Thromb Haemost. 1999;82(2):365–376. [PubMed] [Google Scholar]
- 25.Offermanns S, Simon MI. Genetic analysis of mammalian G-protein signalling. Oncogene. 1998;17:1375–1381. doi: 10.1038/sj.onc.1202173. (11 Reviews) [DOI] [PubMed] [Google Scholar]
- 26.Ingall AH, Dixon J, Bailey A, et al. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42(2):213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 27.Maloney SF, Brass LF, Diamond SL. P2Y12 or P2Y1 inhibitors reduce platelet deposition in a microfluidic model of thrombosis while apyrase lacks efficacy under flow conditions. Integr Biol (Camb) 2010;2(4):183–192. doi: 10.1039/b919728a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camps M, Hou C, Sidiropoulos D, Stock JB, Jakobs KH, Gierschik P. Stimulation of phospholipase C by guanine-nucleotide-binding protein beta gamma subunits. Eur J Biochem. 1992;206(3):821–831. doi: 10.1111/j.1432-1033.1992.tb16990.x. [DOI] [PubMed] [Google Scholar]
- 29.Murata T, Ushikubi F, Matsuoka T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388(6643):678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, De S, Damron DS, Chen WS, Hay N, Byzova TV. Impaired platelet responses to thrombin and collagen in AKT-1-deficient mice. Blood. 2004;104(6):1703–1710. doi: 10.1182/blood-2003-10-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woulfe D, Jiang H, Morgans A, Monks R, Birnbaum M, Brass LF. Defects in secretion, aggregation, and thrombus formation in platelets from mice lacking Akt2. J Clin Invest. 2004;113(3):441–450. doi: 10.1172/JCI20267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by G(i) family members in platelets. J Biol Chem. 2002;277(26):23382–23390. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 33.Franke B, Akkerman JW, Bos JL. Rapid Ca2+-mediated activation of Rap1 in human platelets. EMBO J. 1997;16(2):252–259. doi: 10.1093/emboj/16.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115(3):680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siderovski DP, Willard FS. The GAPs, GEFs, and GDIs of heterotrimeric G-protein alpha subunits. Int J Biol Sci. 2005;1(2):51–66. doi: 10.7150/ijbs.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci U S A. 1998;95(14):8070–8074. doi: 10.1073/pnas.95.14.8070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brass LF. G protein regulators of platelet activation. Prog Clin Biol Res. 1988:283441–492. [PubMed] [Google Scholar]
- 38.Ushikubi F, Nakamura K, Narumiya S. Functional reconstitution of platelet thromboxane A2 receptors with Gq and Gi2 in phospholipid vesicles. Mol Pharmacol. 1994;46(5):808–816. [PubMed] [Google Scholar]
- 39.Dorsam RT, Kim S, Jin J, Kunapuli SP. Coordinated signaling through both G12/13 and G (i) pathways is sufficient to activate GPIIb/IIIa in human platelets. J Biol Chem. 2002;277(49):47588–47595. doi: 10.1074/jbc.M208778200. [DOI] [PubMed] [Google Scholar]
- 40.Andre P, Delaney SM, LaRocca T, et al. P2Y12 regulates platelet adhesion/activation, thrombus growth, and thrombus stability in injured arteries. J Clin Invest. 2003;112(3):398–406. doi: 10.1172/JCI17864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson SP, Nesbitt WS, Westein E. Dynamics of platelet thrombus formation. J Thromb Haemost. 2009;(7 Suppl):117–20. doi: 10.1111/j.1538-7836.2009.03401.x. [DOI] [PubMed] [Google Scholar]
- 42.Nesbitt WS, Westein E, Tovar-Lopez FJ, et al. A shear gradient-dependent platelet aggregation mechanism drives thrombus formation. Nat Med. 2009;15(6):665–673. doi: 10.1038/nm.1955. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.