Abstract
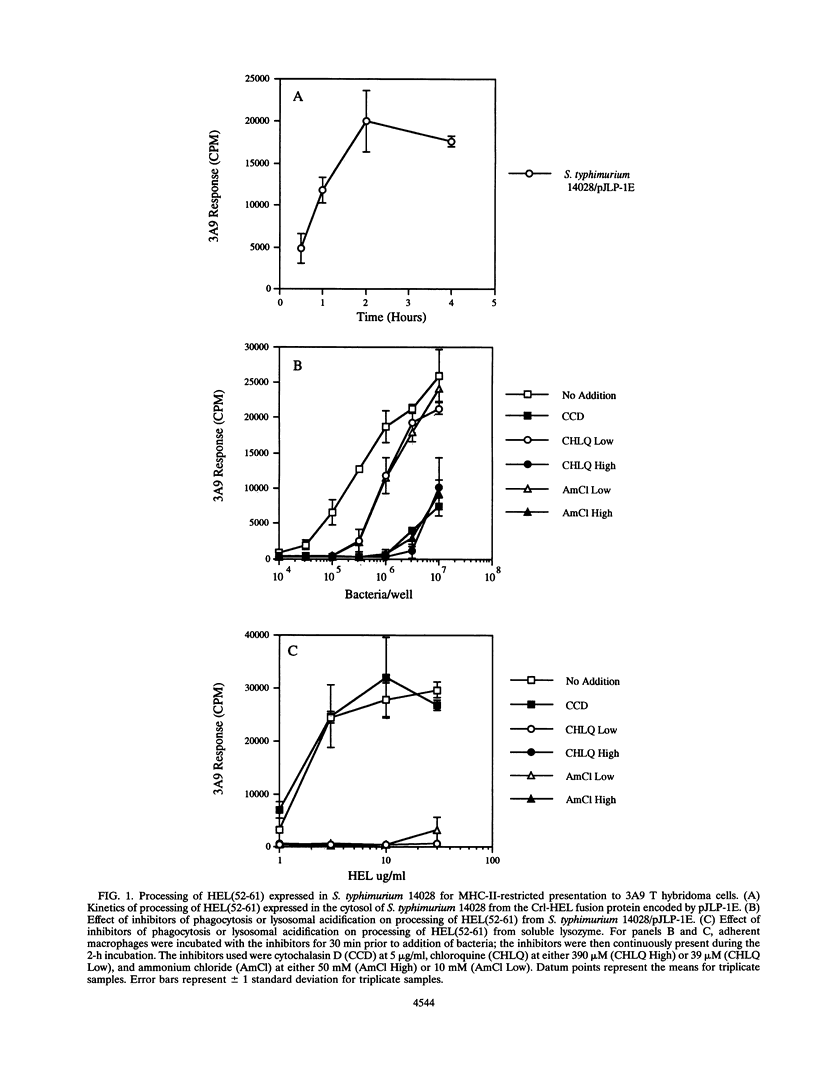
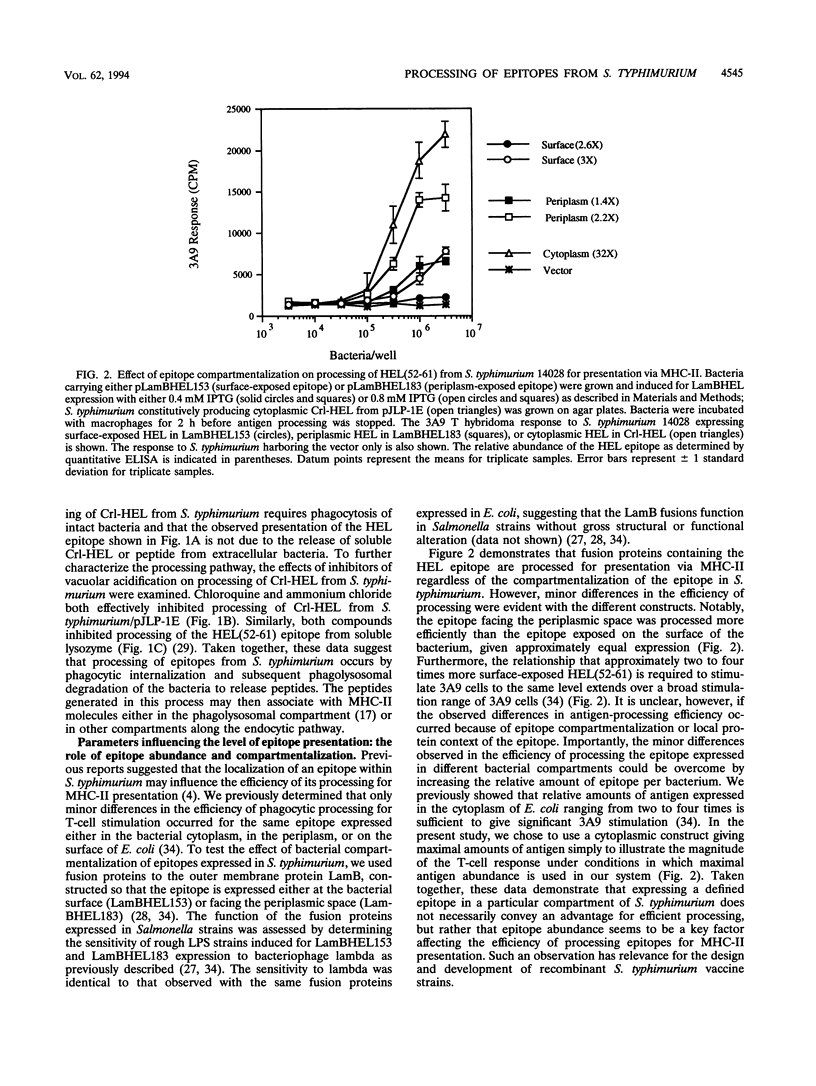
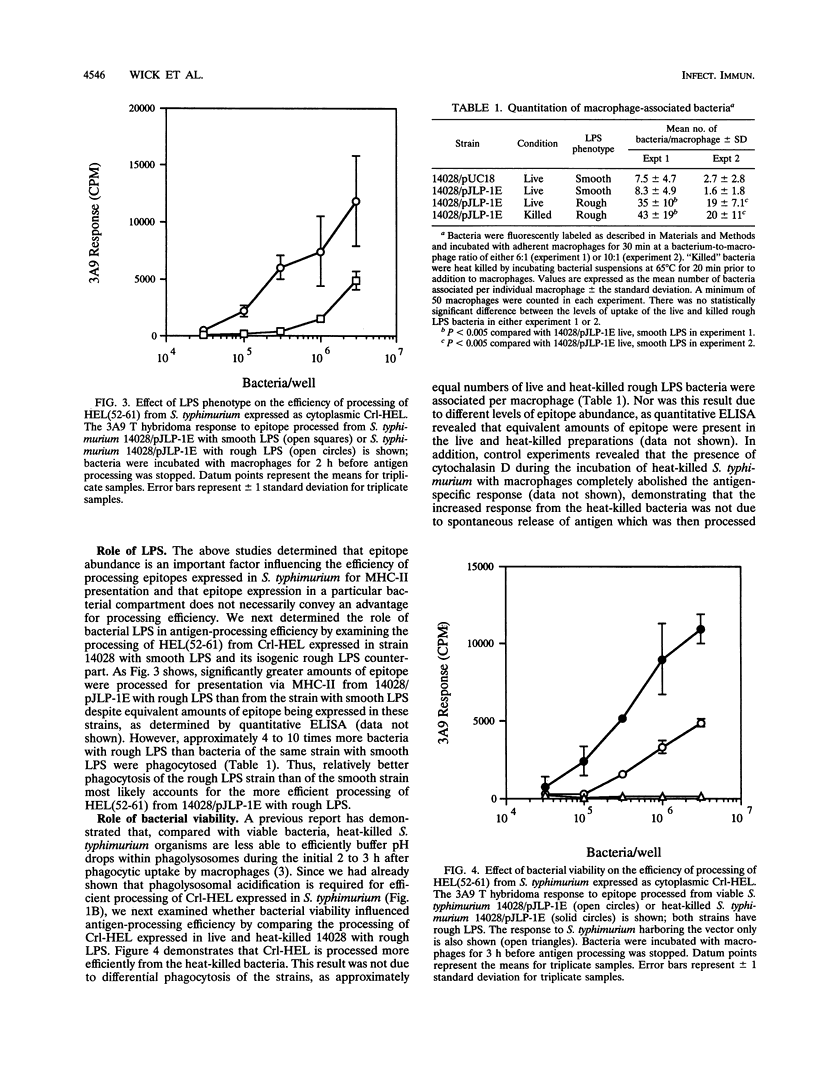
We investigated parameters that affect the efficiency with which antigenic epitopes from Salmonella typhimurium are processed for presentation to T lymphocytes. As a model system, the hen egg white lysozyme 52-61 [HEL(52-61)] epitope, which binds the murine major histocompatibility complex class II (MHC-II) molecule I-Ak, was expressed in soluble fusion proteins in S. typhimurium. Murine peritoneal macrophages mediated phagocytic processing of viable S. typhimurium expressing fusion proteins of the HEL epitope for presentation via I-Ak regardless of the bacterial compartment in which the epitope was contained (i.e., surface exposed, facing the periplasmic space, or in the cytoplasm). Minor differences in processing efficiency observed with different epitope compartmentalizations could be overcome by altering the relative expression level, indicating that epitope abundance is an important factor for efficient processing of epitopes from S. typhimurium. This processing pathway required phagocytosis of bacteria followed by passage through an acidic compartment, suggesting a pathway involving phagolysosomal degradation of the bacteria to liberate epitopes that bind MHC-II. HEL(52-61) was processed more efficiently from heat-killed S. typhimurium than from viable bacteria, and in addition, the HEL epitope was processed more efficiently from a rough lipopolysaccharide (LPS) strain than from its isogenic smooth LPS counterpart, most likely because of enhanced phagocytosis of the rough LPS strain. These data suggest that the efficiency of epitope processing from S. typhimurium for presentation via MHC-II is affected by bacterial viability, epitope abundance, and LPS phenotype, factors which may be important to consider in development of recombinant S. typhimurium vaccine strains.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal A., Kumar S., Jaffe R., Hone D., Gross M., Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990 Oct 1;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen P. M., Unanue E. R. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J Immunol. 1984 Mar;132(3):1077–1079. [PubMed] [Google Scholar]
- Alpuche Aranda C. M., Swanson J. A., Loomis W. P., Miller S. I. Salmonella typhimurium activates virulence gene transcription within acidified macrophage phagosomes. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10079–10083. doi: 10.1073/pnas.89.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Hormaeche C. E., Demarco de Hormaeche R., Winther M., Dougan G., Maskell D. J., Stocker B. A. An attenuated aroA Salmonella typhimurium vaccine elicits humoral and cellular immunity to cloned beta-galactosidase in mice. J Infect Dis. 1987 Jan;155(1):86–92. doi: 10.1093/infdis/155.1.86. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Lipps C. J., So M. Y., Heffron F. Recombination-deficient mutants of Salmonella typhimurium are avirulent and sensitive to the oxidative burst of macrophages. Mol Microbiol. 1993 Mar;7(6):933–936. doi: 10.1111/j.1365-2958.1993.tb01184.x. [DOI] [PubMed] [Google Scholar]
- Bullas L. R., Ryu J. I. Salmonella typhimurium LT2 strains which are r- m+ for all three chromosomally located systems of DNA restriction and modification. J Bacteriol. 1983 Oct;156(1):471–474. doi: 10.1128/jb.156.1.471-474.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields P. I., Groisman E. A., Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science. 1989 Feb 24;243(4894 Pt 1):1059–1062. doi: 10.1126/science.2646710. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn J. L., Weiss W. R., Norris K. A., Seifert H. S., Kumar S., So M. Generation of a cytotoxic T-lymphocyte response using a Salmonella antigen-delivery system. Mol Microbiol. 1990 Dec;4(12):2111–2118. doi: 10.1111/j.1365-2958.1990.tb00572.x. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Margulies D. H. The biochemistry and cell biology of antigen processing and presentation. Annu Rev Immunol. 1993;11:403–450. doi: 10.1146/annurev.iy.11.040193.002155. [DOI] [PubMed] [Google Scholar]
- Harding C. V. Cellular and molecular aspects of antigen processing and the function of class II MHC molecules. Am J Respir Cell Mol Biol. 1993 May;8(5):461–467. doi: 10.1165/ajrcmb/8.5.461. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Geuze H. J. Antigen processing and intracellular traffic of antigens and MHC molecules. Curr Opin Cell Biol. 1993 Aug;5(4):596–605. doi: 10.1016/0955-0674(93)90128-d. [DOI] [PubMed] [Google Scholar]
- Harding C. V., Geuze H. J. Class II MHC molecules are present in macrophage lysosomes and phagolysosomes that function in the phagocytic processing of Listeria monocytogenes for presentation to T cells. J Cell Biol. 1992 Nov;119(3):531–542. doi: 10.1083/jcb.119.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding C. V., Roof R. W., Unanue E. R. Turnover of Ia-peptide complexes is facilitated in viable antigen-presenting cells: biosynthetic turnover of Ia vs. peptide exchange. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4230–4234. doi: 10.1073/pnas.86.11.4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Mekalanos J. J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990 May;172(5):2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson C. A., Roof R. W., McCourt D. W., Unanue E. R. Identification of the naturally processed form of hen egg white lysozyme bound to the murine major histocompatibility complex class II molecule I-Ak. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7380–7383. doi: 10.1073/pnas.89.16.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton S. M., Jacob C. O., Stocker B. A. Immune response to cholera toxin epitope inserted in Salmonella flagellin. Science. 1989 Apr 7;244(4900):70–72. doi: 10.1126/science.2468182. [DOI] [PubMed] [Google Scholar]
- Palva E. T., Liljeström P., Harayama S. Cosmid cloning and transposon mutagenesis in Salmonella typhimurium using phage lambda vehicles. Mol Gen Genet. 1981;181(2):153–157. doi: 10.1007/BF00268420. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Roberts R. L., Findlay K., Normark S. J., Harding C. V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Nature. 1993 Jan 28;361(6410):359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- Pfeifer J. D., Wick M. J., Russell D. G., Normark S. J., Harding C. V. Recombinant Escherichia coli express a defined, cytoplasmic epitope that is efficiently processed in macrophage phagolysosomes for class II MHC presentation to T lymphocytes. J Immunol. 1992 Oct 15;149(8):2576–2584. [PubMed] [Google Scholar]
- Poirier T. P., Kehoe M. A., Beachey E. H. Protective immunity evoked by oral administration of attenuated aroA Salmonella typhimurium expressing cloned streptococcal M protein. J Exp Med. 1988 Jul 1;168(1):25–32. doi: 10.1084/jem.168.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner S. J., Carbone F. R., Strugnell R. A. Salmonella typhimurium delta aroA delta aroD mutants expressing a foreign recombinant protein induce specific major histocompatibility complex class I-restricted cytotoxic T lymphocytes in mice. Infect Immun. 1993 Dec;61(12):5374–5380. doi: 10.1128/iai.61.12.5374-5380.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Wick M. J., Pfeifer J. D., Findlay K. A., Harding C. V., Normark S. J. Compartmentalization of defined epitopes expressed in Escherichia coli has only a minor influence on efficiency of phagocytic processing for presentation by class I and class II major histocompatibility complex molecules to T cells. Infect Immun. 1993 Nov;61(11):4848–4856. doi: 10.1128/iai.61.11.4848-4856.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson R. G., Gemski P., Jr, Stocker B. A. Non-smooth mutants of Salmonella typhimurium: differentiation by phage sensitivity and genetic mapping. J Gen Microbiol. 1972 May;70(3):527–554. doi: 10.1099/00221287-70-3-527. [DOI] [PubMed] [Google Scholar]


