Abstract
The membrane-type matrix metalloproteinases (MT-MMPs) are essential for pericellular matrix remodeling in late stages of development, as well as in growth and tissue homeostasis in postnatal life. Although early morphogenesis is perceived to involve substantial tissue remodeling, the roles of MT-MMPs in these processes are only partially characterized. Here we explore the functions of 2 prominently expressed MT-MMPs, MT1-MMP and MT2-MMP, and describe their roles in the process of placental morphogenesis. The fetal portion of the placenta, in particular the labyrinth (LA), displays strong overlapping expression of MT1-MMP and MT2-MMP, which is critical for syncytiotrophoblast formation and in turn for fetal vessels. Disruption of trophoblast syncytium formation consequently leads to developmental arrest with only a few poorly branched fetal vessels entering the LA causing embryonic death at embryonic day 11.5. Through knockdown of MMP expression, we demonstrate that either MT1-MMP or MT2-MMP is crucial specifically during development of the LA. In contrast, knockdown of MT-MMP activity after LA formation is compatible with development to term and postnatal life. Taken together these data identify essential but interchangeable roles for MT1-MMP or MT2-MMP in placental vasculogenesis and provide the first example of selective temporal and spatial MMP activity required for development of the mouse embryo.
Introduction
After embryo implantation and decidualization, a critical step in placental development is the formation of the placental labyrinth (LA), which enables nutrient and gas exchange between the embryonic vasculature and the maternal blood supply.1–5 LA formation is associated with substantial tissue remodeling and cell differentiation, as well as ingrowth of the embryonic vasculature through the chorion to a point of immediate proximity with the maternal blood supply. During this process, chorionic trophoblasts (CHs) differentiate into 2 perivascular cell populations that form distinct bilaminar envelopes of syncytiotrophoblasts in immediate contact with the fetal vascular endothelium.6 Multiple transcription factors, growth factors, adhesion molecules, and gap junction molecules are known to influence the formation of the LA.7 Interestingly, few if any proteolytic enzymes have so far been proven essential for LA formation although tissue remodeling is considered an integral part of this morphogenetic process. Several of the matrix metalloproteinases (MMPs), cathepsins, and serine proteinases are expressed in the placenta during development, however to date none have proven indispensable for development of the LA and in turn development of the embryo to term.8–12 Among the 6 known membrane-type MMP (MT-MMP) molecules in the mouse, MT1-MMP and MT3-MMP possess pericellular collagenase activity and are required for both prenatal and postnatal remodeling of the major fibrillar collagen types, cell surface receptors, and signaling molecules.13–15 Ablation of MT1-MMP deprives cells of the ability to migrate through and process several both permanent and provisional extracellular matrices, and in vivo leads to severe defects in postnatal remodeling of connective tissues.16–19 Moreover, MT1-MMP is required in the stromal compartment for efficient dissemination of malignant epithelial cells in mouse mammary carcinoma.20 MT1-MMP deficiency is partially mitigated by the activity of the molecular relative, MT3-MMP, which shares at least some overlapping substrate specificity with MT1-MMP. Accordingly, incremental loss of alleles encoding each molecule markedly exacerbates the cellular matrix remodeling deficit in a gene dosage dependent manner, and double deficiency results in severe developmental deficits and perinatal death, but importantly, not preterm loss of embryos.15 As expected from these observations, MT1-MMP and MT3-MMP are frequently coexpressed in the same tissue compartments. The placental LA however is devoid of MT3-MMP expression but displays conspicuous expression of another MT-MMP family member, MT2-MMP.11,21 So far, the function of MT2-MMP has been determined biochemically and in cell-based assays. Under these conditions, MT2-MMP displays the ability to process basement membrane components and fibrillar collagen matrices whereas its role in vivo has remained unexplored.22,23 Here we demonstrate that MT2-MMP deficiency in the mouse is compatible with development and postnatal growth, however, the combined loss of 2 MT-MMPs, MT1-MMP and MT2-MMP, leads to arrest of gestation at embryonic day (E)10.5. The double-deficient mice die in utero due to a failure of trophoblasts to form the syncytial portion of the trilaminar structure that constitute the labyrinthine interchange between the fetal vasculature and the maternal blood supply in the developing placenta.6 By time dependent ablation of MT-MMP activity in vivo, we specify the temporal requirement for MT-MMPs in LA formation. Loss of either or both MT-MMPs after placental LA formation is compatible with development to term as is postnatal loss of both molecules. These observations demonstrate that either MT1-MMP or MT2-MMP activity is irreplaceable in an early developmental program responsible for placental development and shows, for the first time, that MT-MMP activity is an obligate requirement for development of the mouse embryo to term.
Methods
Animal experiments
Laboratory animal experiments in this study were conducted with the approval of the National Institute of Dental and Craniofacial Research (NIDCR) animal use and care committee.
In situ hybridization
Formaldehyde-fixed placental tissue sections were deparaffinized and hybridized to [α-33P] uridine-5′-triphosphate radiolabeled antisense and sense probes specific for MT1-MMP and MT2-MMP as previously described.24
Real-time PCR
Fetal portions of placentas were dissected and DNase-free RNA was prepared using an RNAqueous-4PCR kit (Ambion) according to the manufacturer's protocol.
One microgram of total RNA was transcribed into cDNA using an iScript cDNA Synthesis Kit (Bio-Rad). cDNA (0.5 μL) was subsequently amplified by real-time polymerase chain reaction (PCR) using SYBR Green PCR Master Mix (Bio-Rad) on a MyIQ thermocycler (Bio-Rad) initially at 95°C for 10 minutes, then 40 cycles of 95°C for 10 seconds and 62°C for 30 seconds. Melting curves were established by 80 cycles of heating from 55°C to 95°C for 10 seconds. Each sample was analyzed in triplicate with threshold levels set automatically. Cycle threshold (Ct) values were normalized to the Ct values for 29S ribosomal protein mRNA. Relative expression values were calculated by formula 2[Ct(29S)-Ct(MT1-MMP)] × 1000. Primer sequences were kindly provided by Dr Matthew Hoffman (NIDCR) and are available upon request.
Tamoxifen treatment of mice
Presence of a copulation plug in the morning was considered E0.5 for purposes of developmental staging. Females in different stages of pregnancy were dosed orally with 8 mg of tamoxifen (TMX) and 4 mg of progesterone in vegetable oil once daily by gavage from 3 to 5 days. Embryos were collected 24 hours after the final TMX treatment. Placentas were dissected, fixed in 4% formaldehyde/phosphate-buffered saline (PBS), and processed for histology. Embryos were frozen and later used for mRNA extraction to examine the efficiency of MT1-MMP deletion. For administration of TMX to suckling pups, lactating females were dosed orally with 8 mg of TMX in vegetable oil daily for 5 days. The pups were allowed to reach the desired age and then killed. The hearts, normally a site of abundant MT1-MMP expression, were used for RNA extraction to examine the efficiency of MT1-MMP deletion. Adult mice (6-10 weeks old) were dosed orally with 8 mg of TMX daily for 5 days. They were observed and killed 4 to 5 months later for analysis.
Transmission electron microscopy
The placentas were fixed in 2% glutaraldehyde in 0.1M sodium cacodylate buffer pH 7.4 overnight at 4°C, washed in cacodylate buffer and subsequently postfixed with 2% osmium tetroxide for 2 hours. The tissue was washed again with 0.1M sodium cacodylate buffer, serially dehydrated in ethanol and propylene oxide and embedded in EMBed 812 resin (Electron Microscopy Sciences). Thin sections, approximately 80 nm, were obtained using the Leica Ultracut-UCT ultramicrotome (Leica), placed onto 300 mesh copper grids, and stained with saturated uranyl acetate in 50% methanol and then with lead citrate. The grids were viewed in a JEM-1200EXII electron microscope (JEOL Ltd) at 80 kV and images were recorded on a XR611M, midmounted, 10.5 megapixel, charge-coupled device (CCD) camera (Advanced Microscopy Techniques).
Immunohistochemistry
For immunohistochemical staining, paraffin slides were dewaxed, rehydrated through graded ethanol and washed in PBS. The antigen was retrieved by microwaving for 20′ in 10mM Na-citrate buffer pH 6.0, except for detection of laminin. The slides were incubated for 30′ in blocking solution (2% goat serum, 1% bovine serum albumin, 0.1% Triton X-100, 0.05% Tween 20, and 0.05% sodium azide in PBS), then in primary antibody at 1:50 dilution for 1 hour and washed in PBS. For 3,3′-diaminobenzidine staining, endogenous peroxidase activity was blocked by incubation in 3% H2O2 in PBS for 10 minutes and the slides were then reacted with secondary biotinylated antibody diluted 1:400 for 30 minutes and washed, incubated with avidin-biotin complex (Vector Laboratories) for 30 minutes and washed. Bound avidin-biotin complex was detected with 3,3′-diaminobenzidine and the slides were rinsed in water and counterstained briefly in hematoxylin, then dehydrated and mounted. For immunofluorescence, the slides were reacted with Alexa 488–conjugated secondary antibody (Molecular Probes) 1:100 for 1 hour, washed in PBS, and stained in 0.2% Sudan black in 70% ethanol for 30 minutes as described.25 Type I collagen was detected using Abcam 34710, Laminin (Sigma-Aldrich L9393) and type IV collagen (Chemicon AB 756P).
Microscopy and imaging
Whole mount darkfield images were captured on an Olympus SZH dissection scope equipped with a Q-imaging Micropublisher RTV CCD camera (Q-Imaging) and a 0.5× PLFL lens at 3.75-32× magnification. Bright and darkfield images of histologic sections were captured on a Zeiss Axioplan 2 (Carl Zeiss Microimaging) upright microscope equipped with an Axiocam MRc CCD camera using Zeiss Plan-NEOFLUAR 2.5×/NA 0.075; 5×/NA 0.15; 10×/NA 0.30; and 20×/NA 0.5. Immunofluorescence was imaged as gray scale on the fluorescein isothiocyanate and DAPI (4′,6-diamidino-2-phenylindole) channels and pseudocolored using Zeiss Axiovision Version 4.6 imaging software (Carl Zeiss Microimaging).
Results
MT1-MMP and MT2-MMP are coexpressed in the placenta
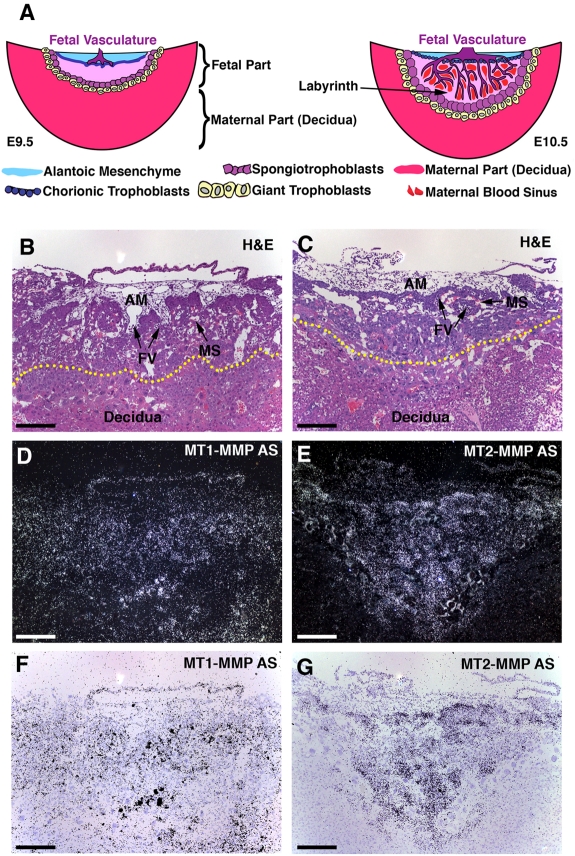
To dissect the role of MT-MMP activity in early development we initially analyzed placental tissues for expression of MT-MMPs. In situ hybridization of placenta sections demonstrated a particularly pronounced expression of both MT1-MMP and MT2-MMP at E10.5 when development of the LA occurs (Figure 1A). MT1-MMP was expressed throughout the fetal portions of the E10.5 placenta, including the allantoic mesenchyme (AM), CHs and differentiated as well as undifferentiated trophoblasts of the prospective LA. Moreover, a significant MT1-MMP signal was detected in the decidual part of the placenta (Figure 1B,D,F). MT2-MMP was likewise detected in the fetal portion of the placenta with intense signal in the CHs and in the prospective LA. Unlike the signal for MT1-MMP, however, the MT2-MMP signal in the decidua was largely absent (Figure 1C,E,G).
Figure 1.
Mouse placental structure and expression of MT-MMPs. (A) Schematic representation of mouse placental development between E9.5 and E10.5. At E9.5, the fetal portion of the placenta is of limited size, but CHs rapidly expand the fetal portion of the placenta at this time. Fetal vessels (FVs) make their way into the prospective LA, and after differentiation of CHs, the vessels are ensheathed in 2 layers of syncytiotrophoblasts that separate the FVs from the adjacent maternal blood sinuses (MSs). This structure facilitates nutrient and gas exchange. (B-C) Hematoxylin and eosin (H&E)–stained cross sections of wild-type mouse placentas at E10.5 with abundant FVs containing nucleated red cells and MSs with enucleated erythrocytes. The yellow dotted lines in panels B-C represent the approximate border between the fetal portion of the placenta and the decidua (maternal portion). (D) Darkfield image of section serial with that shown in panel B hybridized to antisense probe (AS) for MT1-MMP. Note the abundant signal in the embryonic mesenchyme, the LA and the decidua. (E) Darkfield section serial with that shown in panel C hybridized to MT2-MMP AS. Note the signal in the trophoblasts and the limited or absent signal in decidua. (F-G) Brightfield images of sections shown in panels D-E. Note that the signal shown here is pseudocoloration of the darkfield signal projected onto the brightfield image. Scale bar (B-G): 200 μm.
Loss of MT2-MMP is compatible with normal development, growth, and reproduction
To establish the biologic role of MT2-MMP in development, we cloned part of the sequence encoding this MT-MMP from a mouse genomic lambda phage library. A targeting vector deleting the sequences between the 3′ part of exon 2 through exon 5, including the catalytic site, was constructed and used for generation of mice carrying this deletion in its germ line. When mice heterozygous for this deleted MT2-MMP allele (2-) were interbred, homozygous offspring henceforth referred to as (2−/−), were recorded with a frequency consistent with unperturbed Mendelian distribution of the wild-type and mutated alleles. Homozygous mutant mice expressed no mRNA for MT2-MMP when tested by reverse transcription PCR (supplemental Figure 1C, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and based on this result we concluded that the (2−/−) locus was functionally a null allele.
Neither female or male mice with the (2−/−) genotype displayed any significant difference in bodyweight compared with wild-type and heterozygous littermates. Consistent with the normal bodyweight, (2−/−) mice likewise displayed no overt aberrations in size and general appearance. The lifespan, behavior, and grooming habits were normal and the (2−/−) mice reproduced normally and reared litters to weaning age without impediments.
MT-MMP activity is required for placentogenesis and development to term
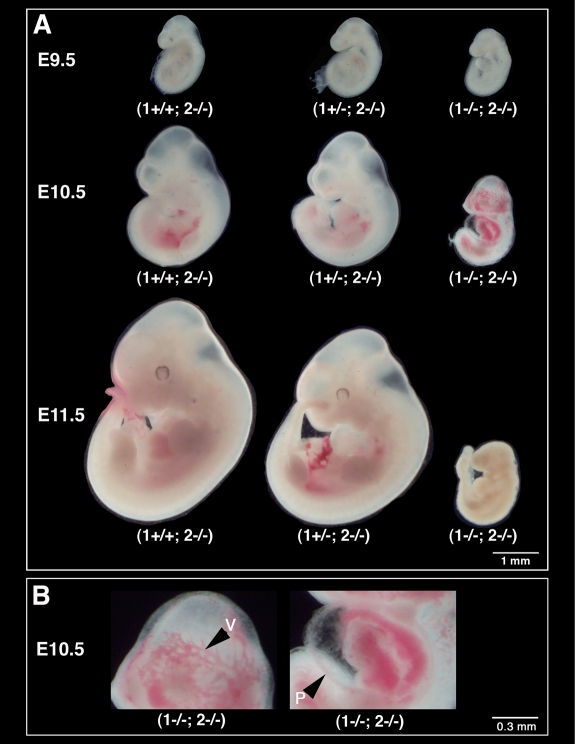
Based on the lack of overt physiologic consequences of MT2-MMP-deficiency, we subsequently tested if MT2-MMP was completely redundant or if the function of MT2-MMP is wholly or partly compensated for by other proteases of related molecular structure, expression pattern and substrate specificity. Since MT1-MMP, by virtue of its overlapping expression pattern, related molecular structure and relative expression level,11 could be compensating for the loss of MT2-MMP in the placenta and vice versa, we crossed the MT2-MMP null allele, (2−), into our MT1-MMP–deficient background,16 henceforth referred to as (1−/−). Mice with the (1−/−) genotype do not reproduce and we therefore first established (1+/−; 2+/−) mice, which appeared healthy and unaffected by the loss of one MT1-MMP and one MT2-MMP allele. Subsequent interbreeding of (1+/−; 2+/−) mice surprisingly yielded no offspring with the (1−/−; 2−/−) genotype while the remaining genotype combinations appeared with expected Mendelian frequency. To eliminate the possibility that the double-deficient mice had perished before weaning or that some random skewing of the genotype distribution could be the cause of the lack of double mutant pups, we set up breeding between (1+/−; 2−/−) parents expecting a 25% frequency of (1−/−; 2−/−) pups. However, among more than 100 offspring not a single (1−/−; 2−/−) pup was identified and we therefore concluded that (1−/−; 2−/−) embryos were lost in utero. Using timed pregnancies, we initiated genotyping of embryos at E9.5. After genotyping, we documented the presence of (1−/−; 2−/−) embryos with approximately 25% frequency in 170 embryos and these were indistinguishable in size and appearance from either (1+/+; 2−/−) or (1+/−; 2−/−) siblings. At E10.5 the number of (1−/−; 2−/−) embryos did not deviate significantly from the expected number in 311 samples, but they were easily identified by their smaller size, prominent dilation of their vasculature (Figure 2A-B) and enlargement of the pericardium. Despite the expected frequency of the (1−/−; 2−/−) genotype in 166 embryos at E11.5, none were found alive and all were uniformly small and partially involuted. Consistent with these findings, only 3 (1−/−; 2−/−) were found in 91 E12.5 age embryos (χ2 = 25.00, P < .0001). We concluded based on these results that the combined loss of MT1-MMP and MT2-MMP caused abrogation of embryogenesis shortly after E10.5 and subsequent involution of the embryo.
Figure 2.
Loss of MT1-MMP and MT2-MMP leads to embryonic demise. (A) Whole mount darkfield images of mouse embryos. Double-deficient embryos (1−/−; 2−/−) at E9.5 are indistinguishable from their control littermates; at E10.5, however, they are easily distinguished by their retarded growth. (B) Double-mutant embryos moreover display dilated vasculature (v) and enlarged pericardia (P). All double-deficient embryos are dead at E11.5. Scale bars: (A) 1 mm; (B) 0.3 mm.
Whole mount preparations and histologic sections of the double mutant E9.5 embryos revealed no overt difference compared with the control littermates in either embryo or placenta. However, at E10.5 dilated vasculature and an enlarged pericardium in the double mutant embryos (Figure 2) suggested placental abnormalities as previously reported in Fra-1 deficient mice.26 We then sought to establish the cause of the premature demise of (1−/−; 2−/−) embryos. Analysis of the placentas from double-deficient mice revealed that vascularization of the fetal portion of the tissues that eventually establish the labyrinthine layer of the embryonic and maternal vascular interchange was retarded (Figure 3B,D,F,H,J) compared with placentas from control littermates (Figure 3A,C,E,G,I). Specifically, the number of FVs extending from the AM through the CH layer and into the prospective LA was diminished (outline, Figure 3E-F). Moreover, when FVs did penetrate they invariably displayed a greatly reduced degree of branching (Figure 3G-H, LA and asterisks).
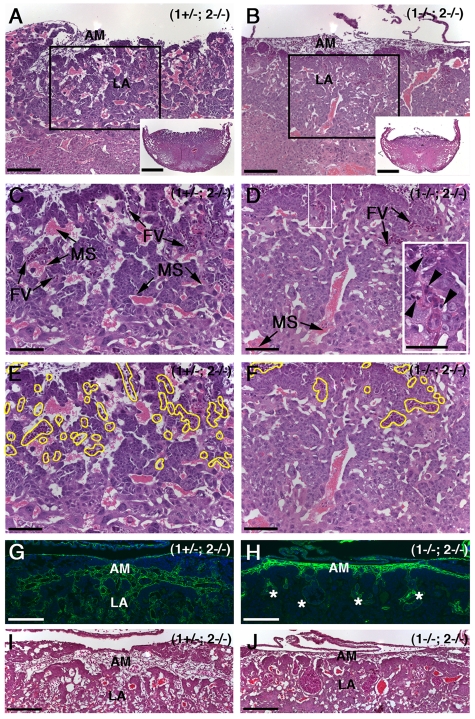
Figure 3.
Loss of MT1-MMP and MT2-MMP leads to defective LA formation. (A) Cross section of H&E-stained normal placentas at E10.5 showing the AM and the placental LA with abundant FVs and MSs. Insets in (A) and (B) show entire placentas in cross section at low magnification. (B) Placenta from (1−/−; 2−/−) embryo displaying a more compact structure of the labyrinth with sparse FVs and MSs. (C) Area framed in panel A shown at high magnification. Note the abundant FVs with nucleated red cells and the adjacent MSs with enucleated red cells. (D) Area framed in panel B demonstrating the underdeveloped LA of (1−/−; 2−/−) placentas. FVs are sparse and penetrate only to a shallow depth. Top white frame is enlarged in right bottom corner and displays apoptotic bodies and dead cells surrounding the fetal vessel (arrowheads). (E-F) Same images as in panels C-D with FVs outlined in yellow. (G) Immunohistochemical localization of collagen type IV outlining FVs of the elaborately branched LA. (H) Collagen type IV–specific stain of placenta from (1−/−; 2−/−) littermate showing diminished vessel branching and penetration (asterisks) into the prospective LA. (I-J) H&E stains of sections serial with sections shown in panels G-H. Scale bars: (A-B) 200 μm; (A-B inset) 1 mm; (B-J) 100 μm.
Arrest of development in (1−/−; 2−/−) embryos is not associated with accumulation of extracellular matrix in the placental LA
Because MT1-MMP is essential for remodeling of extracellular matrix molecules, such as collagen, in postnatal life, we probed if a defect in matrix remodeling leading to matrix accumulation could be documented in double-deficient placentas. We therefore analyzed tissue for detectable imbalances in the amount of extracellular matrix proteins present, including basement membrane components. We performed immunohistochemistry for type IV collagen (Figure 3G-H), laminin (supplemental Figure 4G-H), and collagen type I (supplemental Figure 4C-D). In addition, we assessed the content of type III collagen by reticulin stain (supplemental Figure 4E-F) as well as fibronectin (supplemental Figure 4I-J). All immunohistochemical stains demonstrated that extracellular matrix components were predominantly localized to the AM and fetal vessel basement membrane (collagen type IV and laminin) or associated perivascular tissue (collagen type I). Aside from the difference in fetal vessel density, we saw no overt change in the distribution of these potential MT-MMP substrates that would suggest a defect in processing. When assessed in detail by confocal microscopy (supplemental Figures 6-8), there was a slight increase of fluorescence intensity in the AM of (1−/−; 2−/−) placentas. Importantly, however, the matrix content around vessels or prospective vessels in the LA was reduced compared with control littermates suggesting that matrix accumulation was not taking place there. Moreover, we recorded no accumulation of extracellular matrix components in the prospective LA where FVs in double-deficient placentas failed to advance. Reticulin fibers were present principally in the AM and in the decidua, while none were detected in the prospective LA irrespective of genotype.
We further analyzed the levels of mRNA of basement membrane components by real-time PCR analysis, but in accordance with the immunohistochemistry for type IV collagen and laminin we found no significant deviations from control placenta (1+/−; 2+/−) mRNA levels for these 2 molecules. Additionally mRNA levels for nidogen, perlecan, and fibronectin did not deviate from those found in the controls (supplemental Figure 2A). Furthermore, we found no significant change in the level of MT1-MMP mRNA in the absence of MT2-MMP and vice versa. Likewise, neither partial or complete loss of MT1-MMP and MT2-MMP resulted in any detectable alteration in MT3-MMP expression levels (supplemental Figure 2B). Moreover, we ascertained if loss of MT1-MMP and MT2-MMP would affect the mRNA expression levels of several collagen types. Consistent with our immunohistochemistry results, the mRNA levels for type I and type IV collagen were identical to that in control tissue and type III and type XVIII collagen were likewise unchanged in response to loss of one or both of the MT-MMPs (supplemental Figure 2C). Finally, to broaden our search for potential substrates that were unprocessed in the absence of MT1-MMP and MT2-MMP, we performed expression array analysis and mass-spectrometry analysis on the fetal portion of the placenta isolated from E10.5 embryos. Both analyses revealed no differences pointing to accumulation of known structural substrates and we concluded that any candidate molecule was likely to be present in quantities below the detection limit for these assays.
MT1-MMP or MT2-MMP is required for placental syncytium formation
Next we questioned if the observed defect in the placenta was associated with decreased viability of cells in the placenta by terminal deoxynucleotidyl transferase deoxyuridine-triphosphatase nick end labeling staining (data not shown), but only recorded cell death in the immediate vicinity of the few FVs present, thereby confirming our earlier observations of apoptotic bodies in the fetal vessel perimeter of mutant placentas (Figure 3D inset, outline). Bromodeoxyuridine labeling (data not shown) did not demonstrate diminished cell proliferation and we concluded that the placental defect observed did not stem from a major deficit in cell proliferation or viability.
We next considered if cell differentiation could be affected in the absence of MT1-MMP and MT2-MMP. To ascertain if trophoblasts differentiated from the chorionic state to syncytiotrophoblasts, sections were stained for hepatocyte growth factor activator inhibitor (HAI-1), which stains only CHs and differentiated syncytiotrophoblasts.27 Because these 2 types of cells are morphologically distinct in the placenta, HAI-1 very clearly delineates their distribution and in turn the degree of tissue differentiation. The staining for HAI-1 demonstrated that trophoblasts in (1−/−; 2−/−) were present mostly as compact undifferentiated CHs whereas far fewer were present as syncytiotrophoblasts in the LA compared with the control samples (Figure 4A-B outline). This suggests that the apparent defect in the placenta is the inability of trophoblasts to differentiate from the chorionic state to the syncytiotrophoblast state.
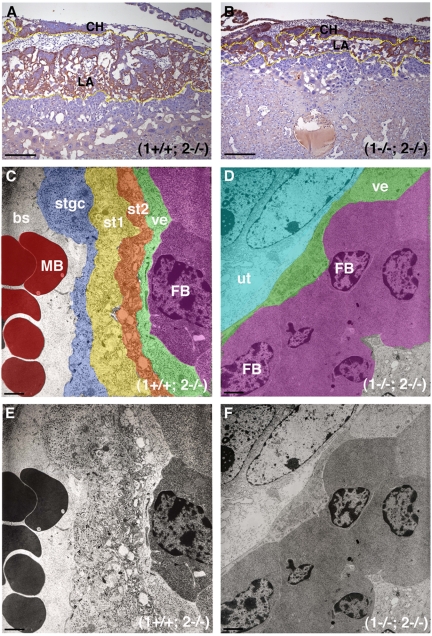
Figure 4.
MT1-MMP/MT2-MMP deficiency leads to disruption of LA architecture. (A) Control placenta from E10.5 embryo stained for HAI-1. Note the localization of brown immunoreactivity in CHs and in the differentiated trophoblasts of the LA outlined in yellow. (B) The staining pattern in a (1−/−; 2−/−) littermate is confined to a more restricted area due to the poor development of the LA (yellow outline). (C) Ultrastructure of the LA from a control placenta demonstrating the trilaminar structure of the fetal vasculature. Pseudocolors show the fetal-maternal interface composed of the fetal vascular endothelium (ve, green), 2 layers of syncytial trophoblasts (st2, orange and st1, yellow) and the STGC lining the MSs (stgc, blue). Fetal blood cell (FB, purple), maternal blood cell (MB, red). Note fetal red cells are nucleated in contrast to maternal cells. (D) Pseudocolored electron microscope image shows that the 2 syncytial layers are missing in the double-deficient placenta and the MSs are not in proximity. The fetal vascular endothelium in green (ve) is surrounded by undifferentiated trophoblasts in cyan (ut). Fetal blood cells (FB, purple). (E) Unaltered version of image shown in panel C. (F) Unaltered version of image shown in panel D. Scale bars: (A-B) 200 μm; (C-F) 2 μm.
To gain a more detailed understanding of the defect leading to lack of LA formation, we performed transmission electron microscopy analysis of control (1+/+; 2−/−) and mutant (1−/−; 2−/−) placentas (Figure 4C-F). In control placentas (Figure 4C), the interchange between maternal blood sinuses (MSs) and FVs displayed a morphology of 4 distinct layers consisting of a mononuclear sinus trophoblast giant cell (STGC) layer lining the MSs and 2 peripheral cell compartments (st1 and st2) constituted by trophoblast syncytia, which enveloped the fetal vascular endothelium (ve; Figure 4C,E).6 In the mutant placenta (Figure 4D,F), the MSs and the occasional FVs invariably were farther apart due to the diminished branching of the FVs. Moreover, the ultrastructural analysis revealed a striking absence of the double layer of syncytiotrophoblasts, reducing the fetal perivascular microenvironment to the undifferentiated trophoblast (ut) species lining the vascular endothelium (ve, Figure 4D). The major defect associated with combined loss of MT1-MMP and MT2-MMP activity thus appeared to be inability of trophoblasts to form the syncytia required for the development of a functional LA.
MT-MMP activity is required for LA formation and is dispensable thereafter
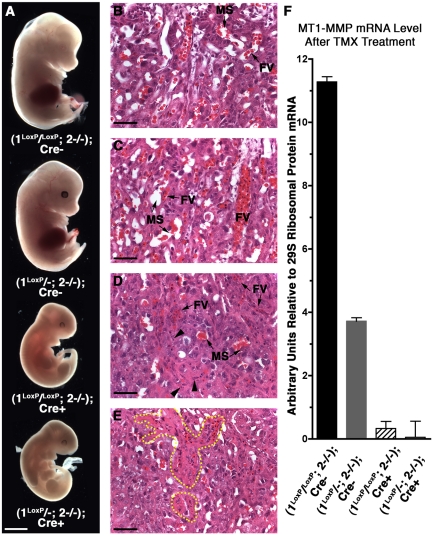
To further refine our observations, we addressed the temporal requirement for the activity of MT1-MMP and MT2-MMP with a time-dependent inactivation of MT1-MMP using a TMX-sensitive Cre allele28 and a floxed MT1-MMP allele (1loxP). Briefly, the gene product of the beta-actin/CreERT allele used here is retained in the cytoplasm in the absence of TMX and only dissociated from heat shock protein 90 and translocated to the nucleus after TMX administration. This strategy enabled recombination and knockdown of a floxed MT1-MMP allele (1loxP) at will (supplemental Figure 3) and enabled time-dependent analysis of MT1-MMP function. We generated (1loxP/−; 2−/−); Cre+ mice and (1loxP/−; 2−/−); Cre− littermates, and evaluated the requirement for MT1-MMP during different time points of gestation in an MT2-MMP deficient background by TMX administration. Pregnant female mice were first administered TMX starting at day 7.5 postcoitum (pc) and repeatedly dosed at 24-hour intervals for 5 days. At day 12.5 pc, the embryos were collected (Figure 5A) and analyzed for the levels of MT1-MMP message by real-time PCR. In addition, placentas were sectioned and stained to evaluate development after induced loss of MT1-MMP in an MT2-MMP-deficient background (Figure 5B-E). As observed earlier, the placental LA failed to develop in the absence of MT1-MMP and MT2-MMP and accordingly the placentas in (1LoxP/−; 2−/−); Cre+ mice displayed gross evidence of cell demise around the few FVs as previously seen with the unconditional deletion of MT1-MMP and MT2-MMP (Figure 5D-E). Notably, (1LoxP/−; 2−/−); Cre− embryos developed unimpeded thereby demonstrating that the observed defects in development of the (1LoxP/−; 2−/−); Cre+ embryos were a direct consequence of MT1-MMP deficiency rather than TMX toxicity (Figure 5B-C).
Figure 5.
Early conditional loss of MT1-MMP in an MT2-MMP–deficient background replicates unconditional loss of both genes and LA disruption. (A) Whole mount images of embryos collected at E12.5 with double-deficiency induced before the formation of the LA via TMX-induced Cre-mediated excision from E7.5 to 11.5. (B-E) Corresponding LA structure of embryos in panel A. (B) Normal LA with abundant FVs and MSs juxtaposed. (C) Equivalent LA structure to that shown in panel B except the embryo is heterozygous for the unconditional MT1-MMP allele. (D) LA from Cre+ placenta displaying a LA morphology equivalent to that found in (1−/−; 2−/−) placentas featuring a compact structure and sparse vessels. Note the many dead cells (arrowheads) and the large distance between FVs and MSs. (E) LA from another Cre+ placenta demonstrating multiple dead cells around the FVs (yellow outline). (F) Relative MT1-MMP mRNA level measured by real-time PCR of embryonic tissues shown in panel A after TMX treatment. Note that TMX treatment in Cre− mice does not affect development. Scale bars: (A) 1 mm; (B-E) 50 μm.
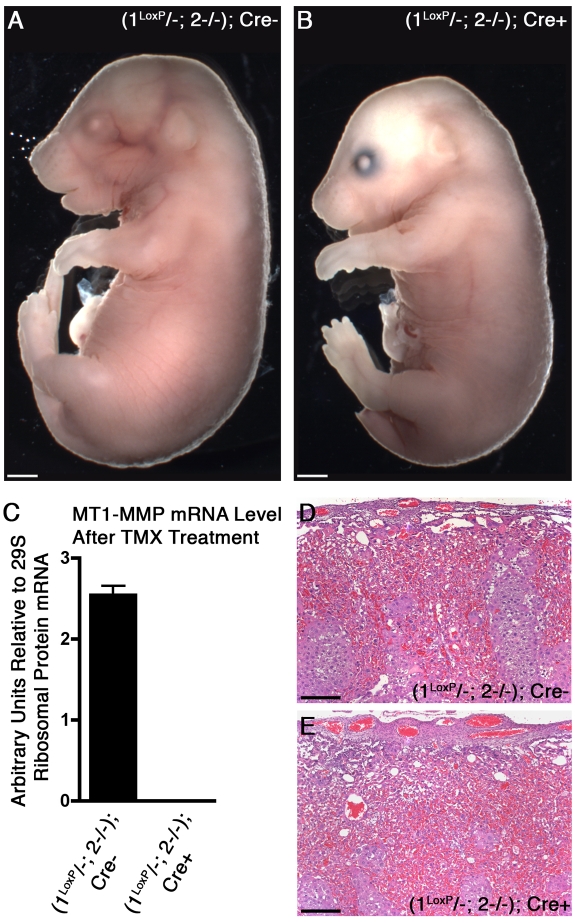
To evaluate the need for MT1-MMP and MT2-MMP after LA development we next administered TMX to pregnant females starting at day 12.5 pc when the placental LA is fully established and collected the embryos at day 17.5 pc. Despite complete loss of MT1-MMP mRNA after TMX administration in Cre+ mice, all embryos regardless of genotype were largely normal in appearance and displayed only the phenotype described previously for embryos deficient for MT1-MMP (Figure 6A-C).16 Notably, when the placentas were analyzed, they uniformly displayed a normal appearance with extensive and elaborate LAs (Figure 6D-E). To further explore the viability of double-deficient mice, several litters were brought to term with no evidence of excess mortality. We further probed the postnatal viability of (1LoxP/−; 2−/−); Cre+ mice by inducing double gene deficiency at various time points. These experiments demonstrated that double deficiency was tolerated at all times after birth although generating a phenotype equivalent to that described previously for unconditional MT1-MMP deletion when the gene was deleted prenatally or early postnatally.16 Finally, to evaluate if a possible defect in basement membrane remodeling was the root cause of the placental defect, we tested the ability of double-deficient female mice to undergo mammary gland involution. A general failure of basement remodeling in the involution of the lactating gland was anticipated to cause a partial or complete arrest of the process in which the both MT1-MMP and MT2-MMP are abundantly coexpressed.24 Despite loss of both proteases after TMX administration, we recorded no differences in involution between double-deficient Cre+ mice and control Cre− mice and therefore concluded that a systemic inability to remodel basement membrane components was an unlikely cause of the placental defect (supplemental Figure 5).
Figure 6.
Conditional loss of MT1-MMP in an MT2-MMP deficient background after LA formation is compatible with development to term. (A) Whole mount preparation of a Cre− embryo treated with TMX after the LA was formed (from E12.5 to 16.5). (B) Cre+ littermate with identical gross appearance despite MT1-MMP and MT2-MMP-deficiency after TMX treatment. (C) Relative expression level of MT1-MMP mRNA evaluated by real-time PCR on embryonic tissue (from A and B) demonstrates complete ablation of MT1-MMP after treatment with TMX in the presence of Cre. (D-E) H&E–stained cross-sections of the placentas corresponding to the embryos shown in panels A and B, respectively. Note the abundant vascularization of both placental LAs despite the conditional loss of both MT1-MMP and MT2-MMP in (E). Compare with the control placenta after formation of the LA in panel D. Scale bars: (A-B) 1 mm; (D-E) 200 μm.
Taken together our experiments show that either MT1-MMP or MT2-MMP is stringently required during a narrow window of development for syncytiotrophoblast formation, coincident with the establishment of the placental LA, and that a deficiency of one or both of these molecules after LA formation is compatible with development to term and postnatal life.
Discussion
Retardation of placental development, and specifically establishment of the LA, is observed in several mouse mutant strains with genetic alterations affecting matrix molecules, signaling molecules, and molecules important in cell-cell interaction, but interestingly, with very few proteolytic enzymes.7 Based on both well established and proposed functions of MT1-MMP and MT2-MMP, we considered the most likely cause of the placental defect in double-deficient mice to be either disrupted processing of known structural substrates such as collagens and other extracellular matrix macromolecules or a defect in processing of uncharacterized low abundance substrates.
MT1-MMP is the principal collagenase in mice and is required for sustained collagen remodeling in late development and in postnatal life.29 In contrast, we demonstrate here that the molecular relative MT2-MMP is largely dispensable in the presence of MT1-MMP and offers little in the way of a possible physiologic function despite its prominent expression in distinct tissue compartments of diverse species.11,21,24 Notwithstanding the seemingly inconsequential nature of MT2-MMP deficiency, this molecule becomes an obligate requirement for placental development and in turn successful gestation to term in the absence of MT1-MMP and vice versa. Based on the interchangeable functions of MT1-MMP and MT2-MMP in the placenta it is difficult to determine whether one or the other serves a dedicated function here. The LA formation in the presence of only one MT-MMP-type is indistinguishable from that found in wild-type mice. Given the inability of MT1-MMP-deficient mice to breed, the selective pressure on germline null mutations in this locus precludes propagating such alleles.16,19 Consequently, MT1-MMP is invariably expressed in the placenta and can facilitate LA morphogenesis. One may thus ponder if MT2-MMP is functionally relevant in the placenta in the presence of MT1-MMP, or confers competitive advantages in other aspects of life such as pathogen challenge or behavioral adaptation, which is not easily mimicked in an experimental environment. Although we demonstrate here that the activity of either MT1-MMP or MT2-MMP is needed for establishment of the placental LA, both gene products are dispensable for the development of the mouse embryo to term after establishment of the LA. These findings point to a very limited yet essential requirement for at least one of the proteinases, and constitute a novel observation linking the morphogenetic process of LA development to the 2 MT-MMPs analyzed here. Imbalance in proteolytic activity after ablation of HAI-1 has previously been reported to result in defective LA formation, highlighting the liability of excess proteolytic activity.27 Morphogenetic defects in placenta have likewise been observed after overexpression of cysteine proteinases.30 To our knowledge, this is the first report of proteolytic activity being required in the formation of the placental LA, and supports the widely held notion that placental development is associated with substantial tissue remodeling. However, in the evaluation of the specific cause of this developmental defect, 2 notable observations stand out. First, we document no obvious accumulation of either established or potential extracellular matrix substrates in the prospective LA, either by gross analysis or by universal expression analysis and mass spectrometry.33 Nor did ultrastructural analysis reveal aberrant accumulation of potential substrates in detectable quantities in the LA of double mutants. Second, vasculogenic or angiogenic processes are unaffected by single deficiencies of either MT1-MMP or MT2-MMP, despite the expression of MT1-MMP in both endothelial cells and smooth muscle actin–positive pericytes.31 In further support of this observation, vessel formation was unaffected in developmental stages of embryogenesis subsequent to LA formation in the conditional double-deficient embryos. Moreover, pregnant double-deficient female mice displayed no impairment in the processes leading to basement membrane remodeling during mammary gland involution, which had been considered a possibility based on the coexpression of the 2 MT-MMPs in involuting glands.24 Collectively these observations point to a defect other than impaired basement membrane remodeling, but rather disrupted vessel formation secondary to absence of syncytium formation. This defect in vessel formation, however, is only evident in the special environment of the prospective LA where a low abundance substrate may be the target of MT1-MMP and MT2-MMP. This suggestion is supported by the observation that merely one functional allele out of 4 confers unimpeded LA formation, gestation to term and postnatal viability. In contrast, the processing of high abundance fibrillar collagen substrates requires multiple gene copies. Accordingly, incremental loss of alleles characteristically leads to a proportional reduction of viability in mice deficient for alleles of MT1-MMP and MT3-MMP.15
Our ultrastructural analysis points to a defect in syncytiotrophoblast formation, which is an integral part of fetal perivascular tissue. This unique structure is the terminal state of an incompletely characterized process in which CHs differentiate into a bilaminar structure consisting of an outer (ST-I) and inner (ST-II) syncytium. This anatomical structure envelops the fetal vascular endothelium and facilitates nutrient, waste and gas exchange with the adjacent MS, which is lined by a STGC.6 The absence of both ST layers in MT1-MMP/MT2-MMP double-deficient mice point to a common defect in cell fusion, which affects both syncytial layers. Given the unimpeded differentiation and cell migration observed in early development of double-deficient embryos and later in conditionally deficient embryos after LA formation, we infer that the more likely defect is in the specific process of cell fusion whether related to fusogenic ligands, accessory factors or the presently unknown cognate receptor(s) of the mouse fusogenic proteins.32
In summary, we present here the first evidence for the requirement of MT-MMP activity in development by demonstrating that MT-MMP activity is not only critical in late embryonic development and postnatal life, but also catalyzes essential processes in early development required for the formation of syncytiotrophoblasts in the placental LA.
Supplementary Material
Acknowledgments
We thank Larry Fisher, Marian Young, and Pamela Gehron Robey of NIDCR for critical reading of the manuscript, and Ivan Rebustini and Matthew Hoffman of NIDCR for technical assistance.
This study was supported by the Division of Intramural Research, NIDCR of the Intramural Research Program, National Institutes of Health. M.S. was supported in part by postdoctoral fellowship funds from the Korea Science and Engineering Foundation.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L.S. designed and performed the research, analyzed the data, and wrote the manuscript; M.S. performed the research and analyzed the data; J.S. performed research; S.Y. performed the research, analyzed data, and wrote the manuscript. S.C. and M.S. performed research; P.Z. and W.S. performed research and analyzed data; and K.H. designed and performed the research, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kenn Holmbeck, National Institutes of Health, Bldg 30, Rm 125, 30 Convent Dr, MSC 4380, Bethesda, MD 20892-4380; e-mail: kenn.holmbeck@nih.gov.
References
- 1.Abrahamsohn PA, Zorn TM. Implantation and decidualization in rodents. J Exp Zool. 1993;266(6):603–628. doi: 10.1002/jez.1402660610. [DOI] [PubMed] [Google Scholar]
- 2.Cross JC, Nakano H, Natale DR, Simmons DG, Watson ED. Branching morphogenesis during development of placental villi. Differentiation. 2006;74(7):393–401. doi: 10.1111/j.1432-0436.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 3.Rinkenberger JL, Cross JC, Werb Z. Molecular genetics of implantation in the mouse. Dev Genet. 1997;21(1):6–20. doi: 10.1002/(SICI)1520-6408(1997)21:1<6::AID-DVG2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 4.Salamonsen LA, Dimitriadis E, Jones RL, Nie G. Complex regulation of decidualization: a role for cytokines and proteases–a review. Placenta. 2003;24(Suppl A):S76–S85. doi: 10.1053/plac.2002.0928. [DOI] [PubMed] [Google Scholar]
- 5.Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat Rev Genet. 2006;7(3):185–99. doi: 10.1038/nrg1808. [DOI] [PubMed] [Google Scholar]
- 6.Simmons DG, Natale DR, Begay V, Hughes M, Leutz A, Cross JC. Early patterning of the chorion leads to the trilaminar trophoblast cell structure in the placental labyrinth. Development. 2008;135(12):2083–2091. doi: 10.1242/dev.020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson ED, Cross JC. Development of structures and transport functions in the mouse placenta. Physiology (Bethesda) 2005;20:180–193. doi: 10.1152/physiol.00001.2005. [DOI] [PubMed] [Google Scholar]
- 8.Ishida M, Ono K, Taguchi S, et al. Cathepsin gene expression in mouse placenta during the latter half of pregnancy. J Reprod Dev. 2004;50(5):515–523. doi: 10.1262/jrd.50.515. [DOI] [PubMed] [Google Scholar]
- 9.Varanou A, Withington SL, Lakasing L, Williamson C, Burton GJ, Hemberger M. The importance of cysteine cathepsin proteases for placental development. J Mol Med. 2006;84(4):305–317. doi: 10.1007/s00109-005-0032-2. [DOI] [PubMed] [Google Scholar]
- 10.Mason RW. Emerging functions of placental cathepsins. Placenta. 2008;29(5):385–390. doi: 10.1016/j.placenta.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 11.Nuttall RK, Sampieri CL, Pennington CJ, Gill SE, Schultz GA, Edwards DR. Expression analysis of the entire MMP and TIMP gene families during mouse tissue development. FEBS Lett. 2004;563(1–3):129–134. doi: 10.1016/S0014-5793(04)00281-9. [DOI] [PubMed] [Google Scholar]
- 12.Szabo R, Bugge TH. Type II transmembrane serine proteases in development and disease. Int J Biochem Cell Biol. 2008;40(6–7):1297–1316. doi: 10.1016/j.biocel.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Basile JR, Holmbeck K, Bugge TH, Gutkind JS. MT1-MMP controls tumor-induced angiogenesis through the release of semaphorin 4D. J Biol Chem. 2007;282(9):6899–6905. doi: 10.1074/jbc.M609570200. [DOI] [PubMed] [Google Scholar]
- 14.Kajita M, Itoh Y, Chiba T, et al. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J Cell Biol. 2001;153(5):893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shi J, Son MY, Yamada S, et al. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev Biol. 2008;313(1):196–209. doi: 10.1016/j.ydbio.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holmbeck K, Bianco P, Caterina J, et al. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99(1):81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 17.Hotary KB, Yana I, Sabeh F, et al. Matrix metalloproteinases (MMPs) regulate fibrin-invasive activity via MT1-MMP-dependent and -independent processes. J Exp Med. 2002;195(3):295–308. doi: 10.1084/jem.20010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabeh F, Ota I, Holmbeck K, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167(4):769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Z, Apte SS, Soininen R, et al. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc Natl Acad Sci U S A. 2000;97(8):4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabova L, Chrysovergis K, Yamada SS, Holmbeck K. MT1-MMP is required for efficient tumor dissemination in experimental metastatic disease. Oncogene. 2008;27(23):3274–3281. doi: 10.1038/sj.onc.1210982. [DOI] [PubMed] [Google Scholar]
- 21.Bjorn SF, Hastrup N, Larsen JF, Lund LR, Pyke C. Messenger RNA for membrane-type 2 matrix metalloproteinase, MT2-MMP, is expressed in human placenta of first trimester. Placenta. 2000;21(2–3):170–176. doi: 10.1053/plac.1999.0447. [DOI] [PubMed] [Google Scholar]
- 22.d'Ortho MP, Will H, Atkinson S, et al. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250(3):751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 23.Hotary K, Li XY, Allen E, Stevens SL, Weiss SJ. A cancer cell metalloprotease triad regulates the basement membrane transmigration program. Genes Dev. 2006;20(19):2673–2686. doi: 10.1101/gad.1451806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Szabova L, Yamada SS, Birkedal-Hansen H, Holmbeck K. Expression pattern of four membrane-type matrix metalloproteinases in the normal and diseased mouse mammary gland. J Cell Physiol. 2005;205(1):123–132. doi: 10.1002/jcp.20385. [DOI] [PubMed] [Google Scholar]
- 25.Romijn HJ, van Uum JF, Breedijk I, Emmering J, Radu I, Pool CW. Double immunolabeling of neuropeptides in the human hypothalamus as analyzed by confocal laser scanning fluorescence microscopy. J Histochem Cytochem. 1999;47(2):229–236. doi: 10.1177/002215549904700211. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber M, Wang ZQ, Jochum W, Fetka I, Elliott C, Wagner EF. Placental vascularisation requires the AP-1 component fra1. Development. 2000;127(22):4937–4948. doi: 10.1242/dev.127.22.4937. [DOI] [PubMed] [Google Scholar]
- 27.Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26(11):1546–1556. doi: 10.1038/sj.onc.1209966. [DOI] [PubMed] [Google Scholar]
- 28.Hayashi S, McMahon AP. Efficient recombination in diverse tissues by a tamoxifen-inducible form of Cre: a tool for temporally regulated gene activation/inactivation in the mouse. Dev Biol. 2002;244(2):305–318. doi: 10.1006/dbio.2002.0597. [DOI] [PubMed] [Google Scholar]
- 29.Holmbeck K, Bianco P, Yamada S, Birkedal-Hansen H. MT1-MMP: a tethered collagenase. J Cell Physiol. 2004;200(1):11–19. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 30.Screen M, Dean W, Cross JC, Hemberger M. Cathepsin proteases have distinct roles in trophoblast function and vascular remodelling. Development. 2008;135(19):3311–3320. doi: 10.1242/dev.025627. [DOI] [PubMed] [Google Scholar]
- 31.Yana I, Sagara H, Takaki S, et al. Crosstalk between neovessels and mural cells directs the site-specific expression of MT1-MMP to endothelial tip cells. J Cell Sci. 2007;120(Pt 9):1607–1614. doi: 10.1242/jcs.000679. [DOI] [PubMed] [Google Scholar]
- 32.Dupressoir A, Marceau G, Vernochet C, et al. Syncytin-A and syncytin-B, two fusogenic placenta-specific murine envelope genes of retroviral origin conserved in Muridae. Proc Natl Acad Sci U S A. 2005;102(3):725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel V, Hood BL, Molinolo AA, et al. Proteomic analysis of laser-captured paraffin-embedded tissues: a molecular portrait of head and neck cancer progression. Clin Cancer Res. 2008;14(4):1002–1014. doi: 10.1158/1078-0432.CCR-07-1497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.