Abstract
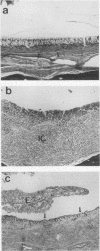
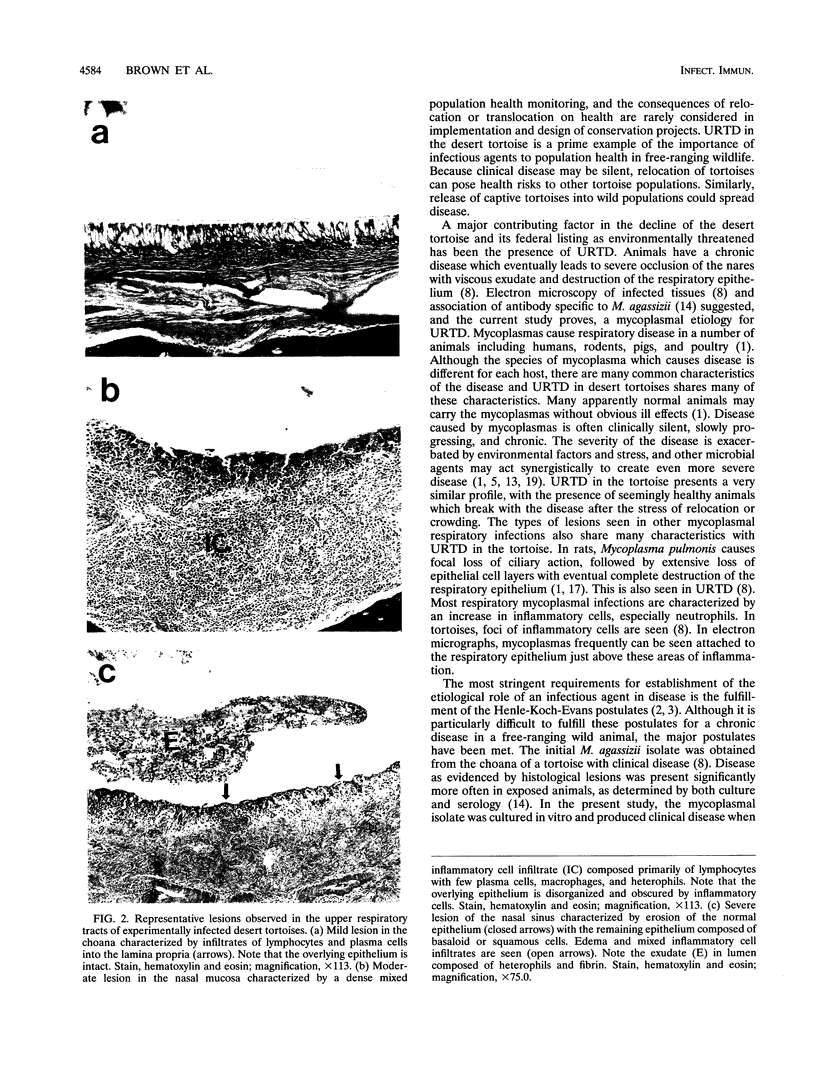
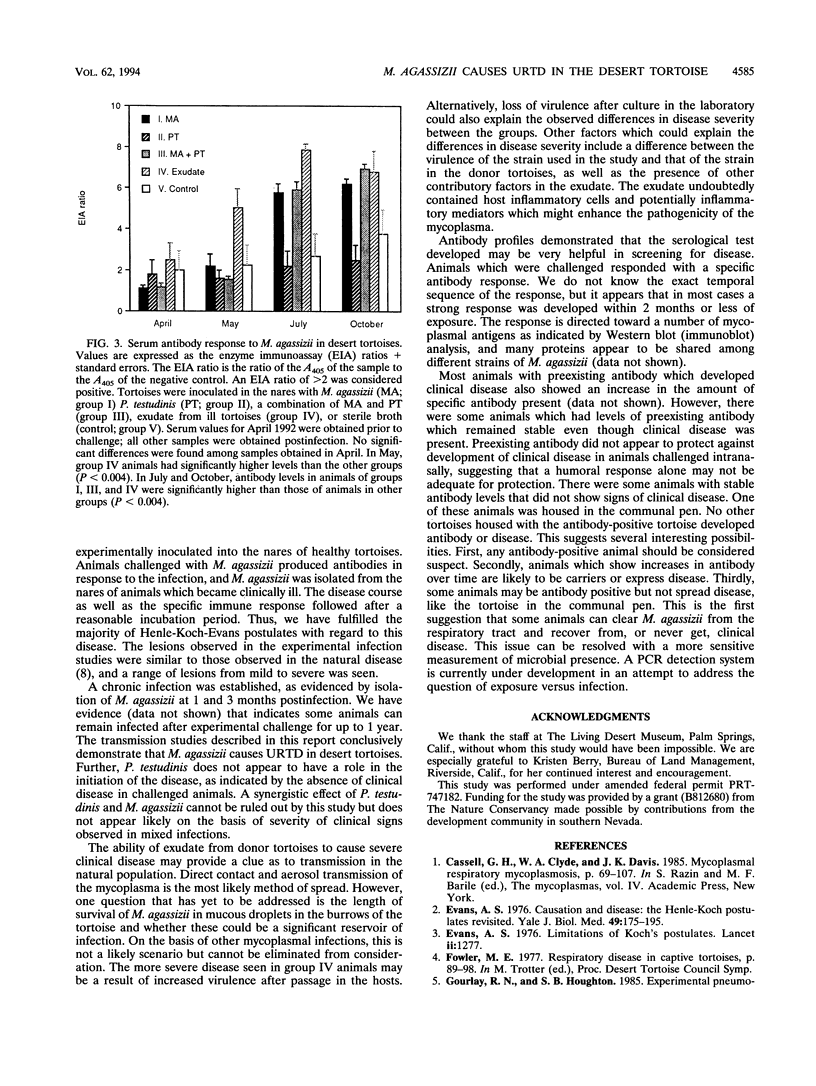
The desert tortoise is listed by the United States government as a threatened species in part of its range. A major contributing factor in the decline of this animal has been the presence of an upper respiratory tract disease (URTD) which is characterized by a chronic disease which eventually leads to severe occlusion of the nares with viscous exudate and destruction of the respiratory epithelium. Electron microscopy of infected tissues demonstrated the presence of a mycoplasma-like organism attached to the respiratory surfaces. The mycoplasma was isolated and designated as a new species, with the proposed name Mycoplasma agassizii. The current study was designed to fulfill Koch's postulates and determine if M. agassizii was the etiologic agent of URTD. Clinically healthy animals with known antibody status were infused intranasally with pooled exudate (n = 8) from ill donor animals, with M. agassizii alone (n = 9) or in combination with Pasteurella testudinis (n = 8), with P. testudinis alone (n = 9), or with sterile broth (n = 12). The pooled exudate was culture positive for M. agassizii. Tortoises which received exudate or M. agassizii alone or in conjunction with P. testudinis were significantly more likely to develop clinical disease (P < 0.0004) than animals which received P. testudinis alone or the broth controls. Tortoises demonstrated a strong immune response to M. agassizii, and seroconversion was seen in all groups with clinical disease. M. agassizii was isolated from the upper respiratory tracts of clinically ill animals up to 6 months postinfection. On the basis of the results of these transmission studies, we conclude that M. agassizii is an etiologic agent of URTD in the desert tortoise.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans A. S. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Med. 1976 May;49(2):175–195. [PMC free article] [PubMed] [Google Scholar]
- Evans A. S. Limitation of Koch's postulates. Lancet. 1977 Dec 17;2(8051):1277–1278. doi: 10.1016/s0140-6736(77)92677-0. [DOI] [PubMed] [Google Scholar]
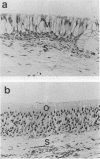
- Jacobson E. R., Gaskin J. M., Brown M. B., Harris R. K., Gardiner C. H., LaPointe J. L., Adams H. P., Reggiardo C. Chronic upper respiratory tract disease of free-ranging desert tortoises (Xerobates agassizii). J Wildl Dis. 1991 Apr;27(2):296–316. doi: 10.7589/0090-3558-27.2.296. [DOI] [PubMed] [Google Scholar]
- Lawrence K., Needham J. R. Rhinitis in long term captive Mediterranean tortoises (Testudo graeca and T hermanii). Vet Rec. 1985 Dec 21;117(25-26):662–664. doi: 10.1136/vr.117.25-26.662. [DOI] [PubMed] [Google Scholar]
- Miller R. E. Zoo veterinarians--doctors on the ark? J Am Vet Med Assoc. 1992 Mar 1;200(5):642–647. [PubMed] [Google Scholar]
- Nettles V. F., Jr Wildlife diseases and population medicine. J Am Vet Med Assoc. 1992 Mar 1;200(5):648–652. [PubMed] [Google Scholar]
- Schoeb T. R., Kervin K. C., Lindsey J. R. Exacerbation of murine respiratory mycoplasmosis in gnotobiotic F344/N rats by Sendai virus infection. Vet Pathol. 1985 May;22(3):272–282. doi: 10.1177/030098588502200310. [DOI] [PubMed] [Google Scholar]
- Schumacher I. M., Brown M. B., Jacobson E. R., Collins B. R., Klein P. A. Detection of antibodies to a pathogenic mycoplasma in desert tortoises (Gopherus agassizii) with upper respiratory tract disease. J Clin Microbiol. 1993 Jun;31(6):1454–1460. doi: 10.1128/jcm.31.6.1454-1460.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snipes K. P., Biberstein E. L., Fowler M. E. A Pasteurella sp associated with respiratory disease in captive desert tortoises. J Am Vet Med Assoc. 1980 Nov 1;177(9):804–807. [PubMed] [Google Scholar]
- Stadtländer C. T., Watson H. L., Simecka J. W., Cassell G. H. Cytopathic effects of Mycoplasma pulmonis in vivo and in vitro. Infect Immun. 1991 Nov;59(11):4201–4211. doi: 10.1128/iai.59.11.4201-4211.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully J. G., Rose D. L., Whitcomb R. F., Wenzel R. P. Enhanced isolation of Mycoplasma pneumoniae from throat washings with a newly-modified culture medium. J Infect Dis. 1979 Apr;139(4):478–482. doi: 10.1093/infdis/139.4.478. [DOI] [PubMed] [Google Scholar]
- Weinack O. M., Snoeyenbos G. H., Smyser C. F., Soerjadi-Liem A. S. Influence of Mycoplasma gallisepticum, infectious bronchitis, and cyclophosphamide on chickens protected by native intestinal microflora against Salmonella typhimurium or Escherichia coli. Avian Dis. 1984 Apr-Jun;28(2):416–425. [PubMed] [Google Scholar]