Abstract
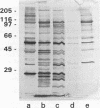
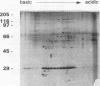
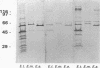
Rhoptry organelles from sporozoites of the apicomplexan parasite Eimeria tenella contain at least 60 independent polypeptides that can be resolved by two-dimensional gel electrophoresis. Rhoptries from three species of Eimeria that infect chickens share very few antibody cross-reactive epitopes, and there is poor conservation of epitopes among three distinct asexual generations of zoites within the developmental life cycle of a single parasite, E. tenella.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannister L. H., Mitchell G. H., Butcher G. A., Dennis E. D. Lamellar membranes associated with rhoptries in erythrocytic merozoites of Plasmodium knowlesi: a clue to the mechanism of invasion. Parasitology. 1986 Apr;92(Pt 2):291–303. doi: 10.1017/s0031182000064064. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Miller L. H., Hudson D., Franco E. L., Andrysiak P. M. Monoclonal antibody characterization of Plasmodium falciparum antigens. Am J Trop Med Hyg. 1984 Nov;33(6):1051–1054. doi: 10.4269/ajtmh.1984.33.1051. [DOI] [PubMed] [Google Scholar]
- Dalrymple B. P., Casu R. E., Peters J. M., Dimmock C. M., Gale K. R., Boese R., Wright I. G. Characterisation of a family of multi-copy genes encoding rhoptry protein homologues in Babesia bovis, Babesia ovis and Babesia canis. Mol Biochem Parasitol. 1993 Feb;57(2):181–192. doi: 10.1016/0166-6851(93)90194-3. [DOI] [PubMed] [Google Scholar]
- Doury J. C., Bonnefoy S., Roger N., Dubremetz J. F., Mercereau-Puijalon O. Analysis of the high molecular weight rhoptry complex of Plasmodium falciparum using monoclonal antibodies. Parasitology. 1994 Apr;108(Pt 3):269–280. doi: 10.1017/s0031182000076113. [DOI] [PubMed] [Google Scholar]
- Etzion Z., Murray M. C., Perkins M. E. Isolation and characterization of rhoptries of Plasmodium falciparum. Mol Biochem Parasitol. 1991 Jul;47(1):51–61. doi: 10.1016/0166-6851(91)90147-x. [DOI] [PubMed] [Google Scholar]
- Jaikaria N. S., Rozario C., Ridley R. G., Perkins M. E. Biogenesis of rhoptry organelles in Plasmodium falciparum. Mol Biochem Parasitol. 1993 Feb;57(2):269–279. doi: 10.1016/0166-6851(93)90203-a. [DOI] [PubMed] [Google Scholar]
- Jenkins M. C., Lillehoj H. S., Barta J. R., Danforth H. D., Strohlein D. A. Eimeria acervulina: cloning of a cDNA encoding an immunogenic region of several related merozoite surface and rhoptry proteins. Exp Parasitol. 1990 Apr;70(3):353–362. doi: 10.1016/0014-4894(90)90117-u. [DOI] [PubMed] [Google Scholar]
- Jensen J. B., Edgar S. A. Possible secretory function of the rhoptries of Eimeria magna during penetration of cultured cells. J Parasitol. 1976 Dec;62(6):988–992. [PubMed] [Google Scholar]
- Kawazoe U., Tomley F. M., Frazier J. A. Fractionation and antigenic characterization of organelles of Eimeria tenella sporozoites. Parasitology. 1992 Feb;104(Pt 1):1–9. doi: 10.1017/s003118200006073x. [DOI] [PubMed] [Google Scholar]
- Leriche M. A., Dubremetz J. F. Characterization of the protein contents of rhoptries and dense granules of Toxoplasma gondii tachyzoites by subcellular fractionation and monoclonal antibodies. Mol Biochem Parasitol. 1991 Apr;45(2):249–259. doi: 10.1016/0166-6851(91)90092-k. [DOI] [PubMed] [Google Scholar]
- Lillehoj H. S., Trout J. M. CD8+ T cell-coccidia interactions. Parasitol Today. 1994 Jan;10(1):10–14. doi: 10.1016/0169-4758(94)90347-6. [DOI] [PubMed] [Google Scholar]
- Long P. L., Millard B. J., Joyner L. P., Norton C. C. A guide to laboratory techniques used in the study and diagnosis of avian coccidiosis. Folia Vet Lat. 1976 Jul-Sep;6(3):201–217. [PubMed] [Google Scholar]
- Machado R. Z., McElwain T. F., Suarez C. E., Hines S. A., Palmer G. H. Babesia bigemina: isolation and characterization of merozoite rhoptries. Exp Parasitol. 1993 Nov;77(3):315–325. doi: 10.1006/expr.1993.1089. [DOI] [PubMed] [Google Scholar]
- McDonald V., Wisher M. H., Rose M. E., Jeffers T. K. Eimeria tenella: immunological diversity between asexual generations. Parasite Immunol. 1988 Nov;10(6):649–660. doi: 10.1111/j.1365-3024.1988.tb00251.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Perkins M. E. Rhoptry organelles of apicomplexan parasites. Parasitol Today. 1992 Jan;8(1):28–32. doi: 10.1016/0169-4758(92)90308-o. [DOI] [PubMed] [Google Scholar]
- Ridley R. G., Takacs B., Etlinger H., Scaife J. G. A rhoptry antigen of Plasmodium falciparum is protective in Saimiri monkeys. Parasitology. 1990 Oct;101(Pt 2):187–192. doi: 10.1017/s0031182000063228. [DOI] [PubMed] [Google Scholar]
- Roger N., Dubremetz J. F., Delplace P., Fortier B., Tronchin G., Vernes A. Characterization of a 225 kilodalton rhoptry protein of Plasmodium falciparum. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):135–141. doi: 10.1016/0166-6851(88)90033-3. [DOI] [PubMed] [Google Scholar]
- Saavedra R., de Meuter F., Decourt J. L., Hérion P. Human T cell clone identifies a potentially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J Immunol. 1991 Sep 15;147(6):1975–1982. [PubMed] [Google Scholar]
- Sadak A., Taghy Z., Fortier B., Dubremetz J. F. Characterization of a family of rhoptry proteins of Toxoplasma gondii. Mol Biochem Parasitol. 1988 Jun;29(2-3):203–211. doi: 10.1016/0166-6851(88)90075-8. [DOI] [PubMed] [Google Scholar]
- Suarez C. E., Palmer G. H., Hines S. A., McElwain T. F. Immunogenic B-cell epitopes of Babesia bovis rhoptry-associated protein 1 are distinct from sequences conserved between species. Infect Immun. 1993 Aug;61(8):3511–3517. doi: 10.1128/iai.61.8.3511-3517.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez C. E., Thompson S. M., McElwain T. F., Hines S. A., Palmer G. H. Conservation of oligopeptide motifs in rhoptry proteins from different genera of erythroparasitic protozoa. Exp Parasitol. 1994 Mar;78(2):246–251. doi: 10.1006/expr.1994.1025. [DOI] [PubMed] [Google Scholar]
- Tomley F. Antigenic diversity of the asexual developmental stages of Eimeria tenella. Parasite Immunol. 1994 Aug;16(8):407–413. doi: 10.1111/j.1365-3024.1994.tb00368.x. [DOI] [PubMed] [Google Scholar]