Abstract
Structures of the type IV pili secretin complexes from Neisseria gonorrhoeae and Neisseria meningitidis, embedded in outer membranes were investigated by transmission electron microscopy. Single particle averaging revealed additional domains not observed previously. Secretin complexes of N. gonorrhoeae showed a double ring structure with a 14–15-fold symmetry in the central ring, and a 14-fold symmetry of the peripheral ring with 7 spikes protruding. In secretin complexes of N. meningitidis, the spikes were absent and the peripheral ring was partly or completely lacking. When present, it had a 19-fold symmetry. The structures of the complexes in several pil mutants were determined. Structures obtained from the pilC1/C2 adhesin and the pilW minor pilin deletion strains were similar to wild-type, whereas deletion of the homologue of N. meningitidis PilW resulted in the absence of secretin structures. Remarkably, the pilE pilin subunit and pilP lipoprotein deletion mutants showed a change in the symmetry of the peripheral ring from 14 to 19 and loss of spikes. The pilF ATPase mutant also lost the spikes, but maintained 14-fold symmetry. These results show that secretin complexes contain previously unidentified large and flexible extra domains with a probable role in stabilization or assembly of type IV pili.
Introduction
Neisseria species are Gram-negative β-proteobacteria, whose pathogenic members Neisseria meningitidis, which normally inhabits the human nasopharynx, and Neisseria gonorrhoeae, which normally colonizes urogenital mucosal surfaces, are responsible for bacterial meningitis and septicemia, and the sexually transmitted disease gonorrhea, respectively. During the infection process, several factors contribute to the interaction with the host cells [1]. Among these factors are type IV pili which mediate binding of the bacteria to the host cells. Type IV pili are long fibrous structures extending from the bacterial surface which can be extended and retracted [2], [3]. They are involved in a variety of processes; not only do they mediate cellular attachment to tissue receptors [1], [4], but they are also involved in several other processes, including bacterial auto-agglutination [5], [6], twitching motility [7], biofilm formation [3], [8], [9], [10], and natural competence for DNA uptake [11], [12], [13].
Type IV pili are dynamic structures which consist of approximately 500–2000 subunits of the major pilin protein, PilE [14] and which are assembled and disassembled by a complex machinery of approximately 10 conserved core proteins and several additional proteins [2], [3], [15]. This machinery shows similarity to the complexes involved in secretion of proteins via the type II secretion pathway [16], [17]. The nomenclature of components of type IV pili systems often differs between organisms. In this manuscript we will refer to the N. gonorrhoeae genes and proteins if not indicated otherwise. The first step of pilus assembly is the insertion of the pilin into the cytoplasmic membrane. After membrane insertion, the leader peptide is both cleaved at the cytosolic side of the membrane and methylated on the N-terminal amino acid by the pre-pilin peptidase PilD [18], [19]. The PilE subunits are assembled and extruded from the inner membrane by the PilF hexameric ATPase (a homologue of GspE, and a member of the AAA chaperone/mechanico-enzyme family) with the aid of a polytopic inner membrane protein, PilG [20]. Remarkably, PilT which is a similar ATPase to PilF, is involved in the disassembly of the PilE subunits at the cytoplasmic membrane. Disassembly takes place at a rate of approximately 700 pilin subunits/s, resulting in retraction of the pilus with a force of over 100 pN [21], [22]. Several other proteins, called pseudo-pilins or minor pilins, are similarly processed by PilD and can also be integrated into the growing pilus, and were proposed to affect pilus dynamics by influencing the membrane-localization and/or polymerization state [23]. The pilus passes the outer membrane through PilQ [24], [25]. PilQ is one of the most abundant Neisserial outer membrane proteins and it has previously been estimated that PilQ comprises 10–13% of the total outer membrane proteins [26]. PilQ is a member of the GspD secretin superfamily of integral outer membrane proteins involved in type IV pili and in type II and type III secretion systems [27].
Transmission electron microscopy (TEM) of purified members of the secretin superfamily, such as XcpQ and PilQ from Pseudomonas aeruginosa [28], PulD from Klebsiella oxytoca [29], [30], [31], the pIV filamentous phage protein [32], GspD from Vibrio cholerae [33] and PilQ of N. meningitidis [24], [25], [34], [35], indicated that these proteins form a multimeric ring-like structure. A 3D structure of the N. meningitidis PilQ (Nme PilQ) was determined by using single particle averaging methods applied to transmission electron microscopy (EM) images of the purified multimer visualized by cryo-negative EM staining. This structure showed 4-fold rotational symmetry (and 12 fold quasi-symmetry) with four ‘arm’-like structures extending from the structure and a large central cavity which was closed on both sides. [24], [25], [34], [35]. The observed structure was flexible, and showed conformational changes upon interaction with isolated pili [25] and purified NMe PilP [36]. A higher resolution 3D structure of a secretin was obtained for K. oxytoca PulD [29], [30], [31]. This complex consists of a dodecameric structure composed of a closed disc with ring-like structures on both sides. The two rings form chambers on either side of a central plug that is part of the middle disc. A recently published dodecameric cryo-EM structure of the purified GspD secretin of Vibrio cholera shows a 200 Å long complex with a periplasmic domain, an outer membrane domain and a unique extracellular cap. The structure was obtained in its “closed” state and has an outer diameter of 155 Å. It has a prominent periplasmic gate and a conserved constricted region. It was proposed that this region interacts with the substrate and renders conformational changes to the structure for toxin secretion [33].
Members of the secretin superfamily often interact with small lipoproteins, also known as pilotins, or pilot proteins. These lipoproteins are involved in oligomerization, stabilization, and/or outer membrane localization of the secretin. For example, the K. oxytoca PulD requires the PulS pilotin for proper outer membrane association; in its absence, PulD remains associated with the inner membrane [37]. Similarly the Shigella flexneri MxiM and Yersinia enterocolitica YscW pilotins are required for outer membrane localization of the Type III secretion secretins MxiD and YscC, respectively [38], [39]. The interaction between MxiM and MxiD has been studied using NMR spectroscopy [40]. It has been demonstrated that Nme PilP and Nme PilW interact with Nme PilQ. Purified Nme PilP was shown to interact with Nme PilQ and was proposed to localize to the cap region of the Nme PilQ structure [36]. Although Nme PilQ does not need Nme PilP for its stabilization or membrane localization, Nme pilP mutants showed a loss of piliation and of natural competence [36]. In a Nme pilW deletion mutant, the total amount of outer membrane localization of Nme PilQ monomers was not changed, but the stability of the Nme PilQ multimer was strongly affected [41]. Another protein that has been proposed to interact with PilQ is PilC. Two copies of pilC (pilC1 and pilC2) are found in pathogenic Neisseria species. In N. gonorrhoeae, each copy can function as a pilus tip adhesin, while in N. meningitidis, only PilC1 promotes adhesion [42]. The Nme PilC proteins are associated with the outer membrane but can also be recovered from purified pili, where they seem to be located at the top of the pilus [43].
Although the observations made by different authors have been useful in establishing that secretins adopt ring-like structures with 12–14 fold symmetry, there are still many remaining questions. All structural information about the secretins obtained to date has been obtained from purified proteins, and structural information about the interaction of the secretins with other components is lacking. Multi-subunit membrane complexes can loose subunits during the purification procedure [44]. Therefore we set out to study the structure of the PilQ secretin within the membrane using Transmission Electron Microscopy and single particle averaging. To obtain further information on the PilQ complex, we studied the structure of the complex in membranes derived from different pil deletion mutants. Implications for the assembly and structure of the observed PilQ mega-complex are discussed.
Results
Transmission electron microscopy on isolated membranes and whole cells of Neisseria gonorrhoeae
To study the structure of the PilQ secretin of N. gonorrhoeae in its native environment, total membranes were isolated and separated on a sucrose gradient. Fractions containing the highest amount of PilQ (from 45 to 51% (w/v) sucrose) were collected and concentrated. This fraction contained both inner and outer membranes as determined by antibodies against SecY and DsbA inner membrane markers) and Omp85 and Imp (outer membrane markers). Although several methods, including different disruption methods in combination with a large variety of density gradients were tested to separate inner and outer membranes, no complete separation was obtained. PilQ containing fractions were analyzed with transmission electron microscopy. Both inner membranes, which appear as vesicles, and outer membranes, which appear as flattened sheets, could be identified [45]. Roughly 25% of the vesicles seem to be derived from inner membranes. The membranes form intact closed vesicles because upon air-dried negative staining the membranes collapse and become superimposed. This can be seen at the edges where a white rim marks the curvature (Figure 1A). The outer membranes contained prominent stain-filled indentations (Figure 1A) which were absent in the inner membranes, with an average density of 350 indentations per µm2
Figure 1. Overview of negatively stained N. gonorrhoeae membranes.
(A) Membranes isolated from strain MS11. (B) Membranes isolated from a pilQ deletion mutant strain. (C) Immungold labeling of membranes from strain MS11 with PilQ antibody. The scale bar is 1000 Å.
Since these stain-filled indentations were most likely formed by the PilQ secretin, a pilQ deletion strain was constructed. Comparison of the outer membrane enriched samples of MS11 and the pilQ deletion strain confirmed both the abundance of PilQ in the outer membrane samples, and the absence of PilQ in the deletion strain (see Figure S1A). Isolated membranes of the pilQ deletion strain were analyzed by electron microscopy (Figure 1B). The stain filled indentations were absent in the membranes of the pilQ deletion strain demonstrating that they are indeed related to the presence of PilQ. Interestingly, the stain filled indentations were also evident on whole cells of Neisseria gonorrhoeae when observed using electron microscopy (Figure 2). While comparing piliated and non piliated cells, some of the thin, long type IV pili structures on the piliated cells seemed to extend from the stain filled indentations. These results further demonstrated the outer membrane localization of the stain filled indentations (Figure 2A and 2B). To further confirm that these indentations contain PilQ, nanogold labeling was performed on the outer membrane enriched fractions using a N.meningitidis PilQ monoclonal antibody [46]. This monoclonal antibody specifically cross reacts with N.gonorrhoeae PilQ (see Figure S1B). Here we observed labeling of the indentations in PilQ containing membranes (See Figure 1C). When similar experiments were performed in the absence of the PilQ antibody only very low levels of nano-gold labeling were observed (see Figure S3A). Similar very low levels of nano-gold labeling were observed for inner membranes and for membranes derived from the pilQ deletion strain (Figure S3B). To-gether this showed that labeling is specific for the presence of PilQ in the indentations. Since the gold labeling influences the alignment procedure, single particle averaging was not performed on gold labeled particles instead, about a hundred images were visually analyzed (Figure 1C, see also Figure S2). Labeling was strongly reduced for the observed inner membranes, or when control experiments were performed in the absence of the PilQ antibody (Figure 1C).
Figure 2. Secretin structures observed by negative stain imaging of whole cells.
(A) Piliated and (B) non-piliated cells of N. gonorrhoeae strain were imaged by electron microscopy. The scale bar is 1000 Å.
Projection structure of the N. gonorrhoeae PilQ complex
To further analyze this PilQ-containing structure, a large data set of about 20,000 single projections of the stain-filled indentations was obtained from EM images and analyzed by single particle analysis. After several cycles of multi-reference alignment, multivariate statistical analysis and classification, final class sums from all analyzed particles were obtained (Figure 3A). The 2D map shows a circular particle composed of a double ring with extending spike-like densities. The central ring has a diameter of 150 Å and has a large central cavity, whereas the peripheral ring has a diameter of 210 Å. The spikes further extend the diameter to 310 Å. The second ring has a 14-fold symmetry, while the spike-like densities show a 7-fold symmetry. After applying 7-fold symmetry, the features of both the second ring and the spikes improve (Figure 3B). When this figure was used for further improvement as a reference in a next alignment procedure, it appears that the spike features became stronger, but at the cost of the resolution of the peripheral ring, which now becomes less well defined (Figure 3C). This suggests that the structure has some flexibility between the second ring and the spikes. The symmetry of the central ring could not be resolved from either the class averages without symmetry applied or from the class averages with 7-fold symmetry applied. In an attempt to determine the symmetry of the central ring, the second ring was masked out during analysis. After repeated alignment and classification, the final projection map showed two striking features. First, densities in the central ring come into focus (Figure 3D). At least 11 densities are well separated, with a average center-to-center distance of about 25 Å (red bars, Figure 3E). However, in two areas the features are not well resolved (blurred red bars), despite the fact that we increased the number of analyzed projections from 20,000 to 36,000. This indicates that at the current resolution of this map, which is in the range of the 25 Å of the center-to-center distance of the central ring densities, we cannot prove the symmetry. However, it appears most likely that the symmetry is 14 or 15. By imposing 14-fold symmetry, as performed in Figure 3F, the features become stronger as compared with any other imposed symmetry between 12 and 16. A second result from this analysis is the total disappearance of the features of the second ring. This can either be caused by a symmetry mismatch between the central and peripheral rings or by a flexible association of the central and peripheral rings.
Figure 3. Class averages of single particle electron microscopy images of the PilQ complex from N. gonorrhoeae.
(A) wild-type projection map (B) with 7-fold symmetry imposed on the peripheral spikes (C) with 7-fold symmetry imposed using the class average of (B) as template (D) class average of the central ring, after masking out the second ring and spikes (E) central ring 2D map with positions of densities indicated (F) central ring 2D map with imposed 14-fold symmetry. The projection map of the PilQ complex in frame A has a resolution of 20 Å. The scale bar is 100 Å.
Transmission electron microscopy on isolated membranes of N. meningitides
To enable us to compare the previously published structure of the purified Nme PilQ complex with the structure observed in the membrane, membranes of N. meningitidis were isolated and analyzed by transmission electron microscopy. The membranes of N. meningitidis also showed the presence of indentations, but in much smaller numbers compared to N. gonorrhoeae. Moreover, the pores were less homogeneously distributed in the membrane, and some of them are found in small clusters (Figure 4A). Single particle analysis showed a structure composed of only one ring (Figure 4B) or with an additional second ring in about 20% of the data set (Figure 4C). Some particles (10%) showed an incomplete second ring (Figure 4D). The central and peripheral rings have the same diameter as observed in the N. gonorrhoeae structure (Figure 4E). Remarkably, there was no indication of spikes attached to the second ring. Comparison with the previously published purified Nme PilQ structure, which has a diameter of approximately 15.5 to 16.5 nm and a 6.0- to 6.5-nm-diameter cavity showed that PilQ forms the central ring of both the N. meningitidis and N. gonorrhoeae structures, whereas the peripheral ring and the spikes are formed by additional proteins. Remarkably, the symmetry number of the peripheral ring observed for N. meningitidis was substantially bigger than that observed for N. gonorrhoeae, indicating that this ring is possibly composed of a larger number of copies of the same or a smaller protein. Symmetry analysis performed to evaluate the copy number shows that imposing 2-, 3-, or 7- fold does not enhance this feature. Since the motif is smaller than in N. gonorrhoeae, symmetries above 14 were evaluated (Figure 4F–J). This approach strongly pointed to a 19-fold symmetry in the second ring. Based on the high similarities of the PilQ proteins of N. meningitidis and N. gonorrhoeae (89% identity and 91% similarity), the observed differences were unexpected. To ensure that the observed differences in the PilQ complexes in the membranes of the N. gonorrhoeae MS11 and the N. meningitidis HB-1 strains are species specific, membranes of different strains were isolated. Two additional N. meningitidis strains, strain H44/76, the wild-type parent of HB-1 (to ensure that the absence of the capsule locus did not affect PilQ appearance), and wild-type strain M986, belonging to a different clonal lineage were tested. Furthermore, N. gonorrhoeae strain FA1090 was examined. Both N. meningitidis strains gave very similar results as strain HB-1. Similarly FA1090 gave identical results as were obtained for MS11. To further exclude that the strains used in our study contained any mutations in pilP or pilQ, the entire pilP/Q operon and flanking regions were sequenced, but no differences were observed with the published sequences [47]. One of the differences between the N. meningitidis and N. gonorrhoeae PilQ is the presence of a small octapeptide (PAKQQAAA) basic repeat of which four to seven copies are present in N. meningitidis PilQ, whereas N. gonorrhoeae PilQ contains either two or three copies [48]. However, the 14-fold symmetry of the peripheral ring and the spikes were still observed in the N. gonorrhoeae strain in which the 2 repeats of MS11 were replaced with the 6 repeats of N. meningitidis strain HB1 (data not shown).
Figure 4. Electron microscopy analysis of the PilQ complexes in isolated membranes.
(A) N. meningitidis membranes negatively stained with 2% uranyl acetate. (B–D) Average of 8,000 projections of the PilQ complexes from N. meningitidis showing classes with single, double and incomplete rings, respectively. (E) Average of 10,000 projections of PilQ complex from N. gonorrhoeae showing central and the peripheral ring having 14-fold symmetry. (F-J) To investigate the number of copies of the peripheral ring, 17- to 21-fold rotational symmetry (white numbering) was imposed on the classes after completion of the analysis. The strongest rotational symmetry within the second ring is in frame H. The scale bar for frame (A) is 1000 Å, and equals 100 Å for frame (B–J).
Structure and assembly of the PilQ complex of N. gonorrhoeae
Our analysis of the structure of the PilQ complex in isolated outer membranes unequivocally shows extra domains, i.e. a peripheral ring with associated spikes, not observed in purified Nme PilQ complexes. To attempt to identify these novel features, we set out to generate deletion mutants for genes of possible candidates for the extra observed densities. PilC is a protein normally present in two copies, is involved in adhesion to epithelial cells, and is located in the outer membrane and at the tip of the pilus. Since the N. gonorrhoeae MS11 strain used for our study, contains a non functional copy of pilC1 that is not expressed due to a frame-shift mutation [49], we generated a pilC2 deletion mutant in MS11. The pilC1/C2 mutation resulted in non-piliated cells, as seen in previous studies. The pilC1 gene was sequenced in the pilC2 deletion mutant and this re-confirmed the presence of the frame-shift mutation. Hence, we conclude that a true pilC1/C2 mutant was generated. Single particle analysis was performed and 6,000 projections were analyzed. The pilC1/C2 mutant yielded projection maps showing a similar structure as observed for wild-type with the presence of central and peripheral rings and 7 extending spikes (Figure 5C). Again, these features became more visible after imposing symmetry (Figure 5D). This demonstrated that PilC is not a subunit of the observed PilQ complex.
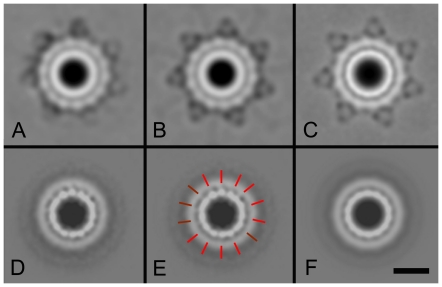
Figure 5. Single particle analysis of particles derived from membranes of different N. gonorrhoeae deletion mutant strains.
Projection maps derived from membranes isolated from (A) N. gonorrhoeae strain MS11, (B) with 7-fold symmetry imposed, (C) the pilC1/C2 deletion mutant, (D) the pilC1/C2 deletion mutant with 7-fold symmetry imposed, (E) the pilP deletion mutant, (F) the pilP deletion mutant with 19-fold symmetry imposed, (G) the pilF deletion mutant, (H) the pilF deletion mutant with 7-fold symmetry imposed, (I) the the pilE deletion mutant, (J) the pilE deletion mutant with 19-fold symmetry imposed, (K) the pilW deletion mutant, and (L) the pilW deletion mutant with 7-fold symmetry imposed, Scale bar equals 100 Å.
In a next step, we generated a deletion mutant of N. gonorrhoeae NgonM_03101, a small (28 kDa) lipoprotein containing six tetratricopeptide repeats (TPR) motifs. N. gonorrhoeae NgonM_03101 is a homologue of Nme PilW and Pseudomonas aeruginosa PilF, which have been shown to be involved in stabilization of the PilQ oligomer [41], [50], [51]. In membranes isolated from the NgonM_03101 deletion mutant, no stain filled indentations were observed demonstrating that NgonM_03101 is involved either in assembly or stabilization of the PilQ oligomer. Since no structures were observed, we cannot discriminate between the possibility that NgonM_03101 functions as a chaperone for oligomerisation of the secretin, or that it is part of the larger PilQ complex.
PilP is an 18 kDa lipoprotein shown to interact with PilQ. Both in N. meningitidis and N. gonorrhoeae pilP and pilQ are co-transcribed [36]. pilP mutants show a loss of piliation and natural competence [52]. In a previous study additional densities were observed when purified Nme PilQ was incubated with recombinant Nme PilP [36]. To study the localization of PilP in PilQ-containing complexes within the membrane, a pilP deletion mutant was created. Western blotting confirmed the expression of PilQ in the pilP deletion mutant strain. An increased degradation of full length PilQ was however observed (Figure S4) and the observed density of indentations in the membranes derived from the pilP deletion mutant was reduced to ∼30% compared to the wild-type membranes, suggesting that PilP influences the stability of PilQ multimers. Differences were observed when the class averages of 6,000 particles of the pilP mutant without (Figure 5E) and with applied symmetry (Figure 5F) were compared to the class averages obtained from wild-type membranes (Figure 5A and B). The pilP deletion mutant not only lost the extending spike-like densities, but remarkably the symmetry of the peripheral ring changed from 14 to 19. Even more surprisingly, the structure of the PilQ-containing complex in membranes derived of the N. gonorrhoeae pilP deletion mutant showed a notable similarity to the structure of the PilQ complex in N. meningitidis membranes. Based on a possible localization of PilP between the central and peripheral rings of the PilQ complex, it could be proposed that absence of PilP affects the interface between the central and peripheral ring, resulting in a reassembly of the second ring. The reassembled second ring does not appear to be able to bind the spike-like extensions.
It has been previously demonstrated that incubation of purified Nme PilQ complexes with isolated pili can induce structural changes in Nme PilQ [25]. To compare the structures of PilQ-containing complexes that have interacted with the pilus and those that have not interacted with the pilus, membranes derived from pilF and pilE deletion mutants were studied. PilF is an ATPase, localized in the inner membrane and essential for the assembly and extrusion of pilin subunits. PilE is the pilus subunit, which forms thin pilin filaments of ∼60–80 Å [53]. When the class averages of 6,000 particles obtained from membranes of the pilF and pilE deletion mutants without (Figure 5G and 5I) and with applied symmetry (Figure 5H and 5J) were compared to the class averages obtained from wild-type membranes, it appeared that the secretin structures of both mutants lost the extending spike-like densities. This suggests that the secretin complex changes its conformation upon interaction with the pilus resulting in assembly of the extending spike-like structures or that the spike-like structures are formed by a protein transported across the outer membrane along with the extension of the pilus. Interestingly, in addition to disappearance of the spike-like densities, the pilE mutant also showed a 19-fold symmetry similar to the pilP deletion mutant and the secretin of wild-type N. meningitidis. Phase variation could have changed the expression levels of PilE in the pilP mutant and in the N. meningitidis strains, and lowered levels of PilE in the pilE and pilP mutants of N. gonorrhoeae and in the N. meningitidis strains might explain the change in symmetry. To test this, the expression levels of PilE in N. gonorrhoeae strain MS11 and the pilQ, pilP and pilE deletion mutants and in the N. meningitides strain HB1, H44/76 and M986 were determined by Western blotting using a PilE-SM1 monoclonal antibody [54] (Figure S4). This demonstrated that PilE expression could be detected in all strains except in the N. gonorhoeae pilE deletion mutant and the N. meningitidis strain M986. The N. meningitidis strain M986 strain expresses a class II pilin that can not be detected with the class I pilE-SM1 antibody [55] but most likely also expresses PilE. Since the pilP mutant that has an 19 fold symmetry of the outer ring still expresses PilE, phase variation of PilE expression could be excluded as a possible reason for the structural change of the outer ring of the secretin complex. Why the absence of PilP/PilE in the structure of the N. gonorrhoeae PilQ complex results in a structural change to a complex resembling the PilQ complex in N. meningitidis remains an open question.
To examine the possible effect of the deletion of a minor pilin, a deletion mutant was created of pilW, a minor pilin located in an operon with pilV and pilX. Single particle analysis was performed and yielded projection maps showing a similar structure as observed for wild-type (Figure 5K and 5L), demonstrating that at least deletion of the pilW minor pilin has no effect on the domain structure of the secretin complex.
Discussion
In this study we analyzed the structure of the PilQ secretin within isolated outer membranes using transmission electron microscopy and single particle averaging. Several lines of evidence demonstrate that the observed stain filled indentations are formed by PilQ: I) The structures are not observed in the pilQ deletion mutant (Figure 1A and 1B). II) The structures are also not observed in the ngonM_03101 mutant. NgonM_03101 is a homologue of N. meningitidis PilW. Deletion of the N. meningitidis pilW gene was shown to abolish the formation of the PilQ oligomer [56]. III) The symmetry of the outer ring of the structure is affected in deletion mutants of several other pil genes, e.g. pilE, pilP, and pilF. It is unlikely that these mutants would affect the structures of another membrane complex than PilQ. IV) The structures are labeled using immuno-gold labeling with an antibody specific for N. gonorrhoeae PilQ (Figure 1C). V) The inner ring of strain filled indentations has the same diameter as the purified PilQ complex of N. meningitidis. VI) The observed structure is present only in outer membrane sheets. Inner and outer membranes can be easily distinguished in electron microscopy, and the structures are also seen on electron microcopy images of whole cells. VII) The abundance of the structure correlates with the abundance of PilQ in the outer membrane [26]. Our approach revealed features of a large structure not seen previously. Compared to the published structures derived from purified PilQ of N. meningidis, the complexes observed in N. meningitidis membranes contained an extra peripheral proteinous ring with a 19-fold symmetry. In our analysis, we also observed structures lacking or having a partial peripheral ring, indicating that the extra domains are not tightly attached and may be dissociated during the membrane isolation procedure. Apparently, this extra ring structure is also lost during the previously described purification of the PilQ complex [36]. Also for other purified secretins, no additional ring structures were observed. Only after purification of PulD-PulS complex from K. oxytoca radial spokes, most likely formed by PulS were observed [31]. These spokes seem however of a much small mass then the extra ring structure observed for the PilQ complex.
Remarkably, the secretin complexes observed in membranes isolated from N. gonorrhoeae appear much more stable, and showed a double ring structure with a 14-fold symmetry of the peripheral ring, from which seven external spikes protrude. These data demonstrate that the study of these multi-component membrane inserted complexes within their native lipid environment by electron microscopy can identify extra components and/or structures which are lost during purification. Compared to the previously published structures derived from purified PilQ complexes of N. meningitidis, the central ring in our structures consists of PilQ. The symmetry of the central ring of N. meningitidis has previously been determined to be 12, while the symmetries of the K. oxocyta PulD and the pIV protein of filamentous phages were 12 and 14, respectively. Unfortunately, we were unable to conclusively determine the symmetry of the central ring of N. gonorrhoeae, but our analysis indicates that it is most likely 14, and thus could differ from the central ring of N. meningitidis.
Another interesting feature of the secretin complexes investigated is the high flexibility between the different rings and the spikes. In particular, the observation that the number of protein copies in the second ring changes from 14 to 19 in the pilP and pilE mutants is intriguing. A comparison of the pilP and pilE mutants with an 19-fold symmetry to those of wild-type and the pilC1/C2 mutant with an 14-fold symmetry shows that the overall diameter of the peripheral ring is smaller in the pilP and pilE deletion mutants, whereas the size of the central ring is equal for all complexes. This indicates that it is unlikely that there is a higher copy number of the same protein in the structure with the 19-fold symmetry (which would increase the size of the peripheral ring), but instead suggests that the structure with the 19-fold symmetry either arises from processing of the peripheral ring protein(s), or that the peripheral ring protein(s) are replaced by other protein(s). It appears that the spikes can only attach to the structure with the 14-fold symmetry. Since also structure with a 14-fold symmetry without the spikes are observed it is unlikely that that the presence of spikes forces the inner ring into the 14 fold symmetry.
A comparable change in symmetry between rings has been observed for photosystem I (PSI) of Synechocystis PCC. Monomeric photosystem I (PSI) is a membrane protein complex of 330 kDa which is mainly present as trimers in cyanobacteria. Under stress conditions, it forms supercomplexes IsiA, with a 37 kDa integral membrane protein. These complexes have been extensively studied by electron microscopy [57], [58] and it was shown that IsiA can form complete and incomplete single and double rings around monomeric or trimeric PSI. The number of IsiA copies was variable; in the case of monomers the inner IsiA ring was composed of 12, 13 or 14 copies and these numbers corresponded to 19, 20 or 21 copies in the peripheral ring, respectively. On trimers with two IsiA rings, the inner ring is composed of 18 copies and the peripheral ring is formed by 25. However, the positions of IsiA in incomplete second rings with 12–19 copies were slightly different. If extrapolated to the complete rings, they appeared to consist of only 24 copies. These data illustrate how IsiA is flexibly attached to PSI ([57] and unpublished data). Similarly, it is possible that the protein(s) making the second ring around the secretin of the type IV pilus are flexible in their self-association.
Within this study we also attempted to identify the proteins located within the second ring and in the spike-like extensions. Initially, we expected that the peripheral rings and/or spikes were formed by PilC, since PilC is a large (110 kDa) protein which was shown to be located in the outer membrane and at the tip of the pilus [43], [49], and Nme pilQ mutants were shown to shed Nme PilC to the medium [52]. However, a mutant of pilC1/C2 showed similar complexes as observed in wild-type membranes demonstrating that PilC is not a component of the peripheral ring or the spike-like extensions. Another candidate was the homologue of N. menigitidis PilW (NgonM_03101), a small (28 kDa) putative lipoprotein containing six tetratricopeptide repeats (TPR) motifs, necessary for the stabilization of pili fibers but not for their assembly or surface localization. A deletion mutant of the N. gonorrhoeae homologue of NMe PilW strongly affected the stability of Nme PilQ multimers [41]. Similar results were obtained for the N. meningitidis PilW homologues of Myxococcus xanthus (Tgl) [59] and of Pseudomonas aeruginosa (PilF) [50]. In membranes of the deletion mutant of the N. gonorrhoeae homologue of N. menigitidis PilW, no secretin complexes are observed, confirming that also the N. gonorrhoeae NgonM_03101 affects the stability of PilQ. Therefore we cannot rule out that NgonM_03101 is part of the second ring or the spikes, although the small size of the protein might not account for the densities of the peripheral ring subunits.
Interestingly, our study demonstrated that the symmetry of the peripheral ring of the secretin complex in the pilP and pilE deletion mutants changed from 14 to 19, and that the structure lost the extending spike-like densities in the pilP, pilE and pilF deletion mutants. These results demonstrate that both PilE and PilP are important for the assembly of the peripheral ring. PilP is a small protein (21 kDa) previously suggested to be localized in the inner membrane, and to attach to the cap region of the PilQ complex [36]. This would place the PilP protein on the periplamic interface possibly between the central and peripheral ring. These data and the small size of PilP make it unlikely that PilP forms either the second ring or the spike-like extension, but PilP could be involved in aligning the central and peripheral ring. The effects of mutations in the pilin protein PilE, and the PilF secretion ATPase, which both inhibit formation of the pilus structure, demonstrate that pilus formation influences the PilQ complex. The changes observed in the PilQ complexes can be a direct effect of an interaction between the pilus and PilQ, or an indirect effect on the export or assembly of minor pilins or pilus associated proteins in the absence of a formed pilus. Our data cannot discriminate between these two possibilities.
Our approach has revealed that the PilQ secretin complex of the type IV pili of Neissera gonorrhoeae interacts with other proteins in the peripheral membrane to form a large multi-domain complex. The function of these extra domains is currently unknown, but they may simply be involved in anchoring the secretin stably into the outer membrane during pilus extension and retraction. Alternatively, the extra domains could be involved in attaching proteins to the pilus, modifying the pilus or play a specific role in type IV pili dependent natural transformation. It will be important to identify the protein within the extra domains and to determine whether these domains can also be found in Type II secretion systems or in the Type IV pili systems of other organism.
Materials and Methods
Strains, plasmids, primers and media
Strains used in this study are described in Table 1. N. gonorrhoeae strains were grown at 37°C in 5% CO2 on GCB (Difco) plates containing Kellog's supplement [60] or GCB liquid medium (GCBL) containing 0.042% NaHCO3 and Kellog's supplements. N. meningitidis was also grown at 37°C in 5% CO2 on GC-agar plates or in tryptic soy broth (TSB). When necessary, erythromycin was used at 5 µg/ml.
Table 1. Strains used in this study.
| Strains | Description | Reference |
| HB1 | Neisseria meningitidis strain | [68] |
| M986 | Neisseria meningitidis strain | [69] |
| MS11 | Neisseria gonorrhoeae strain | [6] |
| FA1090 | Neisseria gonorrhoeae strain | [70] |
| SJ001-MS | MS11 strain with pilQ truncation | This work |
| SJ030-MS | MS11 strain transformed with pSJ030; non polar insertion in pilC, ErmC | This work |
| SJ031-MS | MS11 strain transformed with PCR product to introduce in frame deletion of aa 1 to 181 of pilP | This work |
| SJ007-MS | MS11 strain transformed with PCR product to introduce in frame deletion of aa 1 to 145 of NgonM_03101 | This work |
| EP060 | MS11 strain transformed with pEP057; non polar Insertion in pilF, ErmC | This work |
| SJ006-FA1090 | FA1090 strain transformed with PCR product to introduce in frame deletion of aa 1 to 31 of pilE | This work |
| SJ002-MS | MS11 transformed with SBR repeat containing pilQ region of HB1 | This work |
| SJ032-MS | MS11 strain transformed with pSJ032; non polar insertion of pilW, ErmC | This work |
Construction of deletion mutant strains
Deletion mutants in pilC and pilF were made using the insertion-duplication mutagenesis method [61]. Using this method, the gene is disrupted and expression of genes downstream of the disrupted gene is driven from the erythromycin promotor. PCR products encoding 541 bp (primers PilC-for and PilC-rev), 524 bp (primers PilF-for and PilF-rev) and 452 bp (primers PilW-for and PilW-rev) fragments of pilC, pilF and pilW were amplified from isolated chromosomal DNA of N. gonorrhoeae strain MS11 (for a list of used primers, see Table 2). The pilC and pilW PCR fragment was digested with BamHI and KpnI and ligated into the BamHI/KpnI sites of pIND3 [62], resulting in plasmid pSJ030 and pSJ032 respectively. The pilF PCR fragment was digested with XhoI and KpnI and ligated into XhoI/KpnI site of pIND3, resulting in plasmid pEP057. Plasmids pSJ030, pSJ032 and pEP057 were transformed to MS11 and colonies were selected on GCB plates containing erythromycin. Correct clones were identified by performing a PCR on isolated chromosomal DNA of these colonies resulting in strains SJ030-MS, SJ032-MS and EP060, respectively (Table 1). To create marker-less non-polar deletion mutants of pilP, NgonM_03101, pilQ and pilE, PCR fragments of the flanking regions of the respective genes were annealed using the splicing by overlapping extension PCR (SOE-PCR) [63] method. To create the PCR products for pilP, NgonM_03101, pilQ and pilE, the primer combinations of PilP-for1/PilP-rev1 and PilP-for2/PilP-rev2; NgonM_03101-for1/NgonM_03101-rev1 and NgonM_03101-for2/NgonM_03101-rev2, PilQ-for1/PilQ-rev1 and PilQ-for2/PilQ-rev2 and PilE-for1/PilE-rev1 and PilE-for2/PilE-rev2 were used. The obtained PCR products were diluted and amplified with the external primers which also contained the gonoccocal DNA uptake sequence (DUS). The PCR product was transformed to strain MS11 or FA1090 and the mutant colonies were checked using colony PCR. The marker-less insertion of the SBR containing region of the N. meningitidis HB1 strain was introduced into N. gonorrhoeae MS11 by transformation of a PCR fragment carrying the extra region. The PCR product was obtained by using the SBR-for and SBR-rev primers. Correct clones were identified by performing a PCR on isolated chromosomal DNA of several colonies resulting in strains SJ031-MS, SJ007-MS, SJ001-MS, SJ006-FA1090 and SJ002-MS (Table 1). To further confirm the correct deletion of the gene, the deletion site and the flanking regions were determined by sequencing.
Table 2. Primers used in this study.
| PilC-for | 5′-TGGCGGTACCCTCGCTGCCCAAATTGAAAG-3′ |
| PilC-rev | 5′-GCGCGGATCCGTAAATACGCTATTATCATGGACG-3′ |
| NgonM_03101-for1 | 5′-GACGTCATAGTCAGCAATTACCCCTGTTGTCCGATTAAA-3′ |
| NgonM_03101-rev1 | 5′-TTCAGACGGCATGACCACGGCTACGGTTTGAG-3′ |
| NgonM_03101-for2 | 5′-ATGCCGTCTGAAGCTGTTGACGGTAATCCGCAC-3′ |
| NgonM_03101-rev2 | 5′-GCTGACTATGACGTCCCTGAATAAAGGTATATGCAGCG-3′ |
| PilF-for | 5′-ATGCTCGAGACGGCGCGACACCCATATTC-3′ |
| PilF-rev | 5′-TACGGTACCCGGCAAGCCTGTCGATTTCC-3′ |
| PilP-for1 | 5′-GGTTTCCCTAACGTAAGTTATTTTTGCTCGGCATTTTGTG-3′ |
| PilP-rev1 | 5′-ATGCCGTCTGAACAACCTATCGTAAAGGCGGCCGAATCCAA-3′ |
| PilP-for2 | 5′-TTCAGACGGCATGCCGCCAATTCGATAATGCC-3′ |
| PilP-rev2 | 5′-ACTTACGTTAGGGAAACCATGAATACCAAACTGACAAAAATC-3′ |
| PilQ-for1 | 5′-GCTGACTATGACGTCCAGACATCAAAGTTTCCTCCCTGCC-3′ |
| PilQ-rev1 | 5′-TTCAGACGGCATGCCGCCAATTCGATAATGCC-3′ |
| PilQ-for2 | 5′-ATGCCGTCTGAAACCTTTCCAAGCACCTACCC-3′ |
| PilQ-rev2 | 5′-GACGTCATAGTCAGCATGGAGTAATCCTCTTCTTAAT-3′ |
| PilE-for1 | 5′-ATGCCGTCTGAAGCCTTATTTGGCAGTTGG |
| PilE-rev1 | 5′-GCTGACTATGACGTCCCTACCAAGACTACACCGCCC |
| PilE-for2 | 5′-TTCAGACGGCATACGCTTCATCTGCCGGTTGCATAG |
| PilE-rev2 | 5′-GACGTCATAGTCAGCGGCGACAACTGCGTATTATAAAGC |
| SBR-for | 5′-GCTGACTATGACGTCCAGACATCAAAGTTTCCTCCCTGCC-3′ |
| SBR-rev | 5′-TTCAGACGGCATGCTTTGTCTTTGGCAAGCAG-3′ |
| PilW-for | 5′-GCGCGGTACCTTGTCCGCGATGCAAGAATG-3′ |
| PilW-rev | 5′-GCGCGGATCCCGACCGCATAGGCATTGACCAC-3′ |
Membrane Preparation
To isolate membranes of N. gonorrhoeae, the strain was plated on GCB plates with the appropriate antibiotic, and (when possible) piliated cells were scraped from the plate and transferred to 3 ml GCBL medium. Cells were grown to an OD660 of 0.6 and consecutively diluted into increasing volumes until a final volume of 1 liter with an OD660 of 1.0 was obtained. Cells were centrifuged at 8,000 rpm in a JLA-16.25 rotor and resuspended in 50 mM Tris-HCl pH 7.5. Cells were broken by three passes through a French press at 15 kpsi. Cell debris was removed by centrifugation at 6,000 rpm in a SS34 rotor for 10 min. The membranes were pelleted at 40,000 rpm in a Ti-45 rotor for 1 h, resuspended in 1 ml of 50 mM Tris-HCl pH 7.5 and overlaid on a 4 step (1, 1.8, 0.8 and 0.8 ml) sucrose gradient of 54, 51, 45 and 36 (w/v) sucrose) and centrifuged at 80,000 rpm in a MLA-80 rotor for 30 min. The lower two fractions were collected, diluted in 50 mM Tris-HCl pH 7.5, membranes were collected by centrifugation at 40,000 rpm in a Ti-45 rotor for 1 h, and resuspended in 1 ml 50 mM Tris-HCl pH 7.5. To isolate membranes of N. meningitidis, the strain was inoculated from an overnight GC-agar plate in 40 ml tryptic soy broth at an OD550 of 0.1 and grown for 7 h to OD550 of 4. Cells were collected by centrifugation, resuspended in 50 mM Tris/HCl, 5 mM EDTA pH 8 and frozen at −80°C. After thawing, aliquots were plated to verify killing of the bacteria. The suspensions were sonicated for 4 min (Branson 450, setting 6, output 40%) and spun at 10,000 rpm in an SS34 rotor. The resulting supernatant was spun for 8 min at 40,000 rpm in a Ti-70. Cell envelope pellets were dissolved in 2 mM Tris/HCl pH 7.6 and overlaid on a 4 step (1, 1.8, 0.8 and 0.8 ml) sucrose gradient of 54, 51, 45 and 36% (w/v) sucrose and centrifuged at 80,000 rpm in a MLA-80 rotor for 30 min. The lower two fractions were collected, diluted in 50 mM Tris-HCl pH 7.5 and membranes were collected by centrifugation at 40,000 rpm in a Ti-45 rotor for 1 h. The final membrane preparation was resuspended in 50 mM Tris-HCl, pH 7.5, and used for EM analysis.
Electron Microscopy and single particle analysis
For image processing, whole membranes from N. gonorrhoeae and N. meningitidis were negatively stained with 2% uranyl acetate on glow-discharged carbon-coated copper grids. Electron microscopy was performed on a Philips CM120 equipped with a LaB6 tip operating at 120 kV. The “GRACE” system for semi-automated specimen selection and data acquisition [64] was used to record 2048×2048 pixel images at 60,000x calibrated magnification with a Gatan 4000 SP 4K slow-scan CCD camera. About 9,000 images were recorded.
From the images we selected about 20,000 single particle projections of the PilQ complex from N. gonorrhoea, 8,000 projections of the PilQ complex from N. meningitidis, 7,000 projections of the pilC deletion mutant and approximately 5,000 of the pilE and pilP deletion mutants from N. gonorrhoea, respectively. Single particle analysis was performed using the Groningen Image Processing (“GRIP”) software packages (see http://bfcemw0.chem.rug.nl/progs-grip.html for a description) on PC clusters. Single particles of PilQ were repeatedly aligned with multireference and nonreference alignments and treated with multivariate statistical analysis and hierarchical ascendant classification [65]. In the final step, the best 50% of the class-members of the best 50% of the classes were taken for the final sums, with the correlation coefficient from alignments as a quality parameter. Rotational symmetry was analyzed in a similar way, as described previously [66].
Nanogold labeling of isolated membranes with PilQ antibodies
5 µl of the outer membrane enriched fraction of wild type N.gonorrhoea MS11A strain was immobilized on a glow-discharged carbon-coated copper grid. The grid was then incubated with N.meningitidis PilQ monoclonal antibody [46] diluted 1∶1 in wash buffer (20 mM Tris-HCl pH 7.5, 150 mM NaCl) for 1 hr. After 3 washes with wash buffer, the grid was incubated for 1 hr with 1∶10 diluted gold labeled Protein G secondary antibody (Aurion, The Netherlands). After 3 washes with wash buffer the sample was fixed with 2% glutaraldehyde for 5 minutes before staining with 2% uranyl acetate. Electron microscopy was then performed as described above. To exclude aspecific labeling, membranes were labeled using a similar protocol as described above, with the difference that the PilQ monoclonal antibody was replaced by buffer. To test whether the labeling was specific for the presence of the indentations, membranes from the outer membrane enriched fractions of the wild type N.gonorrhoea MS11A strain and the pilQ deletion mutant strain were mixed and immobilized on a grid. Labeling was then performed as above.
Electron microscopy on whole cells
Piliated and non-piliated colonies of N. gonorrhoeae strain MS11 were selected and transfered to GCB plates. After 18 hrs of growth, the cells were scraped from the surface of the plate and resuspended in 1 ml of GCBL medium. 5 µl of this suspension was incubated on a glow-discharged carbon-coated copper grid. Carbon grids were then washed three times with water before straining with uranyl acetate. The grids were analyzed by electron microscopy as described above.
SDS-PAGE and Western blotting
In order to test the cross-reactivity of the PilQ monoclonal antibody [46] directed against N.meninigitidis PilQ to that of N.gonorrhoeae PilQ; isolated outer membrane enriched samples were treated with phenol to generate momomeric PilQ as described previously [67]. Briefly, 200 µl (about 500 µg protein) was mixed with equal volume of 88% phenol and incubated at 70°C for 10 minutes. The samples were then cooled to 4°C and centrifuged at 5000× g for 10 minutes. The upper aqueous phase was discarded and the intermediate and lower phase was retained and mixed with equal volume of water. After incubation at 70°C for 10 minutes, samples were centrifuged at 5000× g for 10 minutes to remove the aqueous phase once again. The protein was then precipitated with 1 ml ice cold acetone and the pellet was resuspended in sample buffer and run on a 10% SDS-PAGE gel either for coomassie staining or for immunoblotting.
Western blotting was performed using PVDF membranes. Blots were developed by incubating with a 1∶1000 dilution of the PilQ [46] and the PilE monoclonal [54] antibody, followed by washes, and incubation with a 1.10000 dilution of anti-Mouse alkaline phosphatase-conjugated secondary antibody (Sigma). The chemiluminescence signal was obtained using the CSDP-star substrate (Roche) on a Roche Lumi-imager.
Supporting Information
PilQ is a major outer membrane protein in Neisseria species. (A) Coomassie stained PAGE gel and (B) Western blot using the monoclonal antibody raised against N.meningitidis PilQ [1] of phenol treated outer membrane enriched samples from N.meningitidis (lane 1 and 4), N.gonorrhoeae MS11 (lane 2 and 5) and the N.gonorrhoeae pilQ mutant (lane 3 and 6).
(DOCX)
Nanogold labeling of isolated N.gonorrhoeae membranes with PilQ monoclonal antibody. Membranes are labeled with PilQ antibody-gold conjugate. Besides uncoated pores (red boxes), some pores are covered with gold clusters; the fact that some of the clusters are right on top of the pores is visible from the bright circular circumference or “halo” around the gold clusters (green boxes). The scale bar is 100 nm.
(DOCX)
The PilQ monoclonal antibody specifically labels PilQ. (A) Labeling of membranes of N.gonorrhoeae MS11 (WT) with secondary antibody-gold conjugate in the absence of the primary PilQ antibody shows no gold-conjugates. (B) Labeling of mixed membranes of the N.gonorrhoeae pilQ mutant (left) and N.gonorrhoeae MS11 (WT, right) on the same electron microscopy grid with PilQ antibody, followed by detection with a secondary antibody-gold conjugate directed against the PilQ antibody labels only membranes containing PilQ (right) Both pictures are representative selections of the same grid.
(DOCX)
Immunoblots on Neisseria membranes with PilE and PilQ antibody. (A) Western blot using a monoclonal antibody raised against N.meningitidis PilE-SM1. Lanes show outer membrane enriched samples from N.gonorrhoeae MS11 (lane 1), and the pilQ (Lane 2), pilP (Lane 3) and pilE mutants (Lane 4), and N.meningitidis strains M986 (lane 5), H44/76 (lane 6) and HB1 (lane 7). (B) Western blot using the monoclonal antibody raised against N.meningitidis PilQ on phenol treated outer membrane enriched samples from N.gonorrhoeae MS11 (lane 1), and pilQ (Lane 2), pilP (Lane 3), and pilE (Lane 4) mutants.
(DOCX)
Acknowledgments
We thank Arnold J.M. Driessen and the department of molecular microbiology, in which part of these experiments have been performed and the Groningen Biomolecular Sciences and Biotechnology Institute for continuous support. We thank Lotte Søgaard-Andersen, Penelope Higgs, Stuart Huntley, Maria Zweig and Eva-Maria Heller, for discussions and critical reading of the manuscript. Anti-PilQ monoclonal antibodies were generously provided by GlaxoSmithKline Biologicals SA.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The authors have no support or funding to report.
References
- 1.Virji M. Pathogenic neisseriae: surface modulation, pathogenesis and infection control. Nat Rev Microbiol. 2009;7:274–286. doi: 10.1038/nrmicro2097. [DOI] [PubMed] [Google Scholar]
- 2.Hansen JK, Forest KT. Type IV pilin structures: insights on shared architecture, fiber assembly, receptor binding and type II secretion. J Mol Microbiol Biotechnol. 2006;11:192–207. doi: 10.1159/000094054. [DOI] [PubMed] [Google Scholar]
- 3.Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Froholm LO, Jyssum K, Bovre K. Electron microscopical and cultural features of Neisseria meningitidis competence variants. Acta PatholMicrobiolScand[B] Microbiol Immunol. 1973;81:525–537. doi: 10.1111/j.1699-0463.1973.tb02238.x. [DOI] [PubMed] [Google Scholar]
- 6.Swanson J, Kraus SJ, Gotschlich EC. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J ExpMed. 1971;134:886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merz AJ, So M, Sheetz MP. Pilus retraction powers bacterial twitching motility. Nature. 2000;407:98–102. doi: 10.1038/35024105. [DOI] [PubMed] [Google Scholar]
- 8.Klausen M, Heydorn A, Ragas P, Lambertsen L, Aaes-Jorgensen A, et al. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol Microbiol. 2003;48:1511–1524. doi: 10.1046/j.1365-2958.2003.03525.x. [DOI] [PubMed] [Google Scholar]
- 9.O'Toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 10.Greiner LL, Edwards JL, Shao J, Rabinak C, Entz D, et al. Biofilm Formation by Neisseria gonorrhoeae. Infect Immun. 2005;73:1964–1970. doi: 10.1128/IAI.73.4.1964-1970.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton HL, Dillard JP. Natural transformation of Neisseria gonorrhoeae: from DNA donation to homologous recombination. Mol Microbiol. 2006;59:376–385. doi: 10.1111/j.1365-2958.2005.04964.x. [DOI] [PubMed] [Google Scholar]
- 12.Goodman SD, Scocca JJ. Identification and arrangement of the DNA sequence recognized in specific transformation of Neisseria gonorrhoeae. Proc Natl Acad Sci U S A. 1988;85:6982–6986. doi: 10.1073/pnas.85.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen I, Dubnau D. DNA transport during transformation. Front Biosci. 2003;8:s544–556. doi: 10.2741/1047. [DOI] [PubMed] [Google Scholar]
- 14.Parge HE, Forest KT, Hickey MJ, Christensen DA, Getzoff ED, et al. Structure of the fibre-forming protein pilin at 2.6 A resolution. Nature. 1995;378:32–38. doi: 10.1038/378032a0. [DOI] [PubMed] [Google Scholar]
- 15.Chen I, Christie PJ, Dubnau D. The ins and outs of DNA transfer in bacteria. Science. 2005;310:1456–1460. doi: 10.1126/science.1114021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peabody CR, Chung YJ, Yen MR, Vidal-Ingigliardi D, Pugsley AP, et al. Type II protein secretion and its relationship to bacterial type IV pili and archaeal flagella. Microbiology. 2003;149:3051–3072. doi: 10.1099/mic.0.26364-0. [DOI] [PubMed] [Google Scholar]
- 17.Hazes B, Frost L. Towards a systems biology approach to study type II/IV secretion systems. Biochim Biophys Acta. 2008;1778:1839–1850. doi: 10.1016/j.bbamem.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Strom MS, Nunn DN, Lory S. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc Natl Acad Sci USA. 1993;90:2404–2408. doi: 10.1073/pnas.90.6.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fluhrer R, Steiner H, Haass C. Intramembrane proteolysis by signal peptide peptidases: a comparative discussion of GXGD-type aspartyl proteases. J Biol Chem. 2009;284:13975–13979. doi: 10.1074/jbc.R800040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morand PC, Bille E, Morelle S, Eugene E, Beretti JL, et al. Type IV pilus retraction in pathogenic Neisseria is regulated by the PilC proteins. EMBO J. 2004;23:2009–2017. doi: 10.1038/sj.emboj.7600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Biais N, Ladoux B, Higashi D, So M, Sheetz M. Cooperative retraction of bundled type IV pili enables nanonewton force generation. PLoS Biol. 2008;6:e87. doi: 10.1371/journal.pbio.0060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maier B, Potter L, So M, Long CD, Seifert HS, et al. Single pilus motor forces exceed 100 pN. Proc Natl Acad Sci U S A. 2002;99:16012–16017. doi: 10.1073/pnas.242523299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winther-Larsen HC, Wolfgang M, Dunham S, van Putten JP, Dorward D, et al. A conserved set of pilin-like molecules controls type IV pilus dynamics and organelle-associated functions in Neisseria gonorrhoeae. Mol Microbiol. 2005;56:903–917. doi: 10.1111/j.1365-2958.2005.04591.x. [DOI] [PubMed] [Google Scholar]
- 24.Collins RF, Davidsen L, Derrick JP, Ford RC, Tonjum T. Analysis of the PilQ secretin from Neisseria meningitidis by transmission electron microscopy reveals a dodecameric quaternary structure. J Bacteriol. 2001;183:3825–3832. doi: 10.1128/JB.183.13.3825-3832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins RF, Frye SA, Balasingham S, Ford RC, Tonjum T, et al. Interaction with type IV pili induces structural changes in the bacterial outer membrane secretin PilQ. J Biol Chem. 2005;280:18923–18930. doi: 10.1074/jbc.M411603200. [DOI] [PubMed] [Google Scholar]
- 26.Newhall WJ, Wilde CE, 3rd, Sawyer WD, Haak RA. High-molecular-weight antigenic protein complex in the outer membrane of Neisseria gonorrhoeae. Infect Immun. 1980;27:475–482. doi: 10.1128/iai.27.2.475-482.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Genin S, Boucher CA. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol Gen Genet. 1994;243:112–118. doi: 10.1007/BF00283883. [DOI] [PubMed] [Google Scholar]
- 28.Bitter W, Koster M, Latijnhouwers M, de CH, Tommassen J. Formation of oligomeric rings by XcpQ and PilQ, which are involved in protein transport across the outer membrane of Pseudomonas aeruginosa. Mol Microbiol. 1998;27:209–219. doi: 10.1046/j.1365-2958.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 29.Chami M, Guilvout I, Gregorini M, Remigy HW, Muller SA, et al. Structural insights into the secretin PulD and its trypsin-resistant core. J Biol Chem. 2005;280:37732–37741. doi: 10.1074/jbc.M504463200. [DOI] [PubMed] [Google Scholar]
- 30.Nouwen N, Stahlberg H, Pugsley AP, Engel A. Domain structure of secretin PulD revealed by limited proteolysis and electron microscopy. Embo J. 2000;19:2229–2236. doi: 10.1093/emboj/19.10.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nouwen N, Ranson N, Saibil H, Wolpensinger B, Engel A, et al. Secretin PulD: association with pilot PulS, structure, and ion-conducting channel formation. Proc Natl Acad Sci U S A. 1999;96:8173–8177. doi: 10.1073/pnas.96.14.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Opalka N, Beckmann R, Boisset N, Simon MN, Russel M, et al. Structure of the filamentous phage pIV multimer by cryo-electron microscopy. J Mol Biol. 2003;325:461–470. doi: 10.1016/s0022-2836(02)01246-9. [DOI] [PubMed] [Google Scholar]
- 33.Reichow SL, Korotkov KV, Hol WG, Gonen T. Structure of the cholera toxin secretion channel in its closed state. Nat Struct Mol Biol. 2010 doi: 10.1038/nsmb.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins RF, Ford RC, Kitmitto A, Olsen RO, Tonjum T, et al. Three-dimensional structure of the Neisseria meningitidis secretin PilQ determined from negative-stain transmission electron microscopy. J Bacteriol. 2003;185:2611–2617. doi: 10.1128/JB.185.8.2611-2617.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins RF, Frye SA, Kitmitto A, Ford RC, Tonjum T, et al. Structure of the Neisseria meningitidis outer membrane PilQ secretin complex at 12 A resolution. J Biol Chem. 2004;279:39750–39756. doi: 10.1074/jbc.M405971200. [DOI] [PubMed] [Google Scholar]
- 36.Balasingham SV, Collins RF, Assalkhou R, Homberset H, Frye SA, et al. Interactions between the lipoprotein PilP and the secretin PilQ in Neisseria meningitidis. JBacteriol. 2007;189:5716–5727. doi: 10.1128/JB.00060-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guilvout I, Chami M, Engel A, Pugsley AP, Bayan N. Bacterial outer membrane secretin PulD assembles and inserts into the inner membrane in the absence of its pilotin. Embo J. 2006;25:5241–5249. doi: 10.1038/sj.emboj.7601402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burghout P, Beckers F, de Wit E, van Boxtel R, Cornelis GR, et al. Role of the pilot protein YscW in the biogenesis of the YscC secretin in Yersinia enterocolitica. J Bacteriol. 2004;186:5366–5375. doi: 10.1128/JB.186.16.5366-5375.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schuch R, Maurelli AT. MxiM and MxiJ, base elements of the Mxi-Spa type III secretion system of Shigella, interact with and stabilize the MxiD secretin in the cell envelope. J Bacteriol. 2001;183:6991–6998. doi: 10.1128/JB.183.24.6991-6998.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okon M, Moraes TF, Lario PI, Creagh AL, Haynes CA, et al. Structural characterization of the type-III pilot-secretin complex from Shigella flexneri. Structure. 2008;16:1544–1554. doi: 10.1016/j.str.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Carbonnelle E, Helaine S, Prouvensier L, Nassif X, Pelicic V. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function. Mol Microbiol. 2005;55:54–64. doi: 10.1111/j.1365-2958.2004.04364.x. [DOI] [PubMed] [Google Scholar]
- 42.Jonsson AB, Nyberg G, Normark S. Phase variation of gonococcal pili by frameshift mutation in pilC, a novel gene for pilus assembly. Embo J. 1991;10:477–488. doi: 10.1002/j.1460-2075.1991.tb07970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rahman M, Kallstrom H, Normark S, Jonsson AB. PilC of pathogenic Neisseria is associated with the bacterial cell surface. MolMicrobiol. 1997;25:11–25. doi: 10.1046/j.1365-2958.1997.4601823.x. [DOI] [PubMed] [Google Scholar]
- 44.Folea IM, Zhang P, Nowaczyk MM, Ogawa T, Aro EM, et al. Single particle analysis of thylakoid proteins from Thermosynechococcus elongatus and Synechocystis 6803: localization of the CupA subunit of NDH-1. FEBS Lett. 2008;582:249–254. doi: 10.1016/j.febslet.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 45.Collin S, Guilvout I, Chami M, Pugsley AP. YaeT-independent multimerization and outer membrane association of secretin PulD. Mol Microbiol. 2007;64:1350–1357. doi: 10.1111/j.1365-2958.2007.05743.x. [DOI] [PubMed] [Google Scholar]
- 46.Voulhoux R, Bos MP, Geurtsen J, Mols M, Tommassen J. Role of a highly conserved bacterial protein in outer membrane protein assembly. Science. 2003;299:262–265. doi: 10.1126/science.1078973. [DOI] [PubMed] [Google Scholar]
- 47.Bentley SD, Vernikos GS, Snyder LA, Churcher C, Arrowsmith C, et al. Meningococcal genetic variation mechanisms viewed through comparative analysis of serogroup C strain FAM18. PLoS Genet. 2007;3:e23. doi: 10.1371/journal.pgen.0030023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tonjum T, Caugant DA, Dunham SA, Koomey M. Structure and function of repetitive sequence elements associated with a highly polymorphic domain of the Neisseria meningitidis PilQ protein. Mol Microbiol. 1998;29:111–124. doi: 10.1046/j.1365-2958.1998.00910.x. [DOI] [PubMed] [Google Scholar]
- 49.Rudel T, Boxberger HJ, Meyer TF. Pilus biogenesis and epithelial cell adherence of Neisseria gonorrhoeae pilC double knock-out mutants. Mol Microbiol. 1995;17:1057–1071. doi: 10.1111/j.1365-2958.1995.mmi_17061057.x. [DOI] [PubMed] [Google Scholar]
- 50.Koo J, Tammam S, Ku SY, Sampaleanu LM, Burrows LL, et al. PilF is an outer membrane lipoprotein required for multimerization and localization of the Pseudomonas aeruginosa Type IV pilus secretin. J Bacteriol. 2008;190:6961–6969. doi: 10.1128/JB.00996-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trindade MB, Job V, Contreras-Martel C, Pelicic V, Dessen A. Structure of a widely conserved type IV pilus biogenesis factor that affects the stability of secretin multimers. J Mol Biol. 2008;378:1031–1039. doi: 10.1016/j.jmb.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 52.Drake SL, Sandstedt SA, Koomey M. PilP, a pilus biogenesis lipoprotein in Neisseria gonorrhoeae, affects expression of PilQ as a high-molecular-mass multimer. Mol Microbiol. 1997;23:657–668. doi: 10.1046/j.1365-2958.1997.2511618.x. [DOI] [PubMed] [Google Scholar]
- 53.Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M, et al. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol Cell. 2006;23:651–662. doi: 10.1016/j.molcel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Nicolson IJ, Perry AC, Heckels JE, Saunders JR. Genetic analysis of variant pilin genes from Neisseria gonorrhoeae P9 cloned in Escherichia coli: physical and immunological properties of encoded pilins. J Gen Microbiol. 1987;133:553–561. doi: 10.1099/00221287-133-3-553. [DOI] [PubMed] [Google Scholar]
- 55.Aho EL, Botten JW, Hall RJ, Larson MK, Ness JK. Characterization of a class II pilin expression locus from Neisseria meningitidis: evidence for increased diversity among pilin genes in pathogenic Neisseria species. Infect Immun. 1997;65:2613–2620. doi: 10.1128/iai.65.7.2613-2620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carbonnelle E, Helaine S, Prouvensier L, Nassif X, Pelicic V. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function. Mol Microbiol. 2005;55:54–64. doi: 10.1111/j.1365-2958.2004.04364.x. [DOI] [PubMed] [Google Scholar]
- 57.Kouril R, Arteni AA, Lax J, Yeremenko N, D'Haene S, et al. Structure and functional role of supercomplexes of IsiA and Photosystem I in cyanobacterial photosynthesis. FEBS Lett. 2005;579:3253–3257. doi: 10.1016/j.febslet.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 58.Yeremenko N, Kouril R, Ihalainen JA, D'Haene S, van ON, et al. Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry. 2004;43:10308–10313. doi: 10.1021/bi048772l. [DOI] [PubMed] [Google Scholar]
- 59.Nudleman E, Wall D, Kaiser D. Polar assembly of the type IV pilus secretin in Myxococcus xanthus. Mol Microbiol. 2006;60:16–29. doi: 10.1111/j.1365-2958.2006.05095.x. [DOI] [PubMed] [Google Scholar]
- 60.Kellogg DS, Jr, Peacock WL, Jr, Deacon WE, Brown L, Pirkle DI. Neisseria gonorrhoeae.I. Virulence genetically linked to clonal variation. J Bacteriol. 1963;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton HL, Dominguez NM, Schwartz KJ, Hackett KT, Dillard JP. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol. 2005;55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 62.Hamilton HL, Schwartz KJ, Dillard JP. Insertion-duplication mutagenesis of neisseria: use in characterization of DNA transfer genes in the gonococcal genetic island. J Bacteriol. 2001;183:4718–4726. doi: 10.1128/JB.183.16.4718-4726.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Horton RM, Cai ZL, Ho SN, Pease LR. Gene splicing by overlap extension: tailor-made genes using the polymerase chain reaction. Biotechniques. 1990;8:528–535. [PubMed] [Google Scholar]
- 64.Oostergetel GT, Keegstra W, Brisson A. Automation of specimen selection and data acquisition for protein electron crystallography. Ultramicroscopy. 1998;74:47–59. [Google Scholar]
- 65.van Heel M, Gowen B, Matadeen R, Orlova EV, Finn R, et al. Single-particle electron cryo-microscopy: towards atomic resolution. Q Rev Biophys. 2000;33:307–369. doi: 10.1017/s0033583500003644. [DOI] [PubMed] [Google Scholar]
- 66.Kereiche S, Bourinet L, Keegstra W, Arteni AA, Verbavatz JM, et al. The peripheral light-harvesting complexes from purple sulfur bacteria have different ‘ring’ sizes. FEBS Lett. 2008;582:3650–3656. doi: 10.1016/j.febslet.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 67.Hancock RE, Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978;136:381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bos MP, Tommassen J. Viability of a capsule- and lipopolysaccharide-deficient mutant of Neisseria meningitidis. Infect Immun. 2005;73:6194–6197. doi: 10.1128/IAI.73.9.6194-6197.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai CM, Frasch CE. Chemical analysis of major outer membrane proteins of Neisseria meningitidis: comparison of serotypes 2 and 11. J Bacteriol. 1980;141:169–176. doi: 10.1128/jb.141.1.169-176.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Black WJ, Schwalbe RS, Nachamkin I, Cannon JG. Characterization of Neisseria gonorrhoeae protein II phase variation by use of monoclonal antibodies. Infect Immun. 1984;45:453–457. doi: 10.1128/iai.45.2.453-457.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PilQ is a major outer membrane protein in Neisseria species. (A) Coomassie stained PAGE gel and (B) Western blot using the monoclonal antibody raised against N.meningitidis PilQ [1] of phenol treated outer membrane enriched samples from N.meningitidis (lane 1 and 4), N.gonorrhoeae MS11 (lane 2 and 5) and the N.gonorrhoeae pilQ mutant (lane 3 and 6).
(DOCX)
Nanogold labeling of isolated N.gonorrhoeae membranes with PilQ monoclonal antibody. Membranes are labeled with PilQ antibody-gold conjugate. Besides uncoated pores (red boxes), some pores are covered with gold clusters; the fact that some of the clusters are right on top of the pores is visible from the bright circular circumference or “halo” around the gold clusters (green boxes). The scale bar is 100 nm.
(DOCX)
The PilQ monoclonal antibody specifically labels PilQ. (A) Labeling of membranes of N.gonorrhoeae MS11 (WT) with secondary antibody-gold conjugate in the absence of the primary PilQ antibody shows no gold-conjugates. (B) Labeling of mixed membranes of the N.gonorrhoeae pilQ mutant (left) and N.gonorrhoeae MS11 (WT, right) on the same electron microscopy grid with PilQ antibody, followed by detection with a secondary antibody-gold conjugate directed against the PilQ antibody labels only membranes containing PilQ (right) Both pictures are representative selections of the same grid.
(DOCX)
Immunoblots on Neisseria membranes with PilE and PilQ antibody. (A) Western blot using a monoclonal antibody raised against N.meningitidis PilE-SM1. Lanes show outer membrane enriched samples from N.gonorrhoeae MS11 (lane 1), and the pilQ (Lane 2), pilP (Lane 3) and pilE mutants (Lane 4), and N.meningitidis strains M986 (lane 5), H44/76 (lane 6) and HB1 (lane 7). (B) Western blot using the monoclonal antibody raised against N.meningitidis PilQ on phenol treated outer membrane enriched samples from N.gonorrhoeae MS11 (lane 1), and pilQ (Lane 2), pilP (Lane 3), and pilE (Lane 4) mutants.
(DOCX)