Abstract
Clostridium plays an important role in commercial and medical use, for which targeted gene deletion is difficult. We proposed an intron-anchored gene deletion approach for Clostridium, which combines the advantage of the group II intron “ClosTron” system and homologous recombination. In this approach, an intron carrying a fragment homologous to upstream or downstream of the target site was first inserted into the genome by retrotransposition, followed by homologous recombination, resulting in gene deletion. A functional unknown operon CAC1493–1494 located in the chromosome, and an operon ctfAB located in the megaplasmid of C. acetobutylicum DSM1731 were successfully deleted by using this approach, without leaving antibiotic marker in the genome. We therefore propose this approach can be used for targeted gene deletion in Clostridium. This approach might also be applicable for gene deletion in other bacterial species if group II intron retrotransposition system is established.
Introduction
The genus Clostridium consists of over 100 species, ranking the second in size next to Streptomyces [1]. Many Clostridium species are closely related to human health. These include neurotoxigenic clostridia (C. botulinum and C. tetani) [2], clostridia involved in gas gangrene and necrotizing infections (C. perfringens and C. sordellii) [3], [4], [5], and the enteropathogenic C. difficile [6]. On the other hand, some Clostridium species are of great industrial importance. For example, C. thermocellum can produce ethanol from lignocellulosic waste at high temperature, while C. acetobutylicum and C. beijerinckii produce solvents (acetone, butanol, and ethanol) by utilizing a variety of substrates from monosaccharides to polysaccharides [7], [8], [9]. In view of the importance of Clostridium, it is desirable to understand both the virulence mechanism of pathogenic Clostridium and the industrial characteristics of Clostridium used in fermentation industry.
The virulence mechanism and the desirable industrial characteristics of clostridial strains are usually controlled by many genes [3], [4], [5], [10], [11]. Therefore, a systematic approach to understanding or engineering these strains often requires manipulating multiple genes [10]. Targeted inactivation of clostridial genes can be achieved by Campbell-like integration through homologous recombination of a replication-defective plasmid. Successful applications have been reported in C. acetobutylicum [12], [13], [14], [15], C. beijerinckii [16], C. perfringens [15], [17], [18], [19], and C. difficile [16], [20], but their transformation frequency was around 10−3, and usually one single-crossover integrant can be obtained from 1 mg plasmid DNA, suggesting the integration efficiency was very low. In addition, these single-crossover events are segregationally unstable [12], [13], [14], [15], [16], [17], [18], [19], [20]. Antisense RNA technology has also been applied in Clostridium, such as the downregulation of butyrate kinase and coenzyme A transferase in C. acetobutylicum [21], [22]. However, as the antisense RNA might affect the cell transcriptional program, the phenotypic changes might not be directly related to the downregulation of target genes [21], [23]. Recently, a gene knockout system that employees the Lactococcus lactis Ll.LtrB group II intron has been developed and adapted for directed insertional gene inactivation in Clostridium, which is termed as “ClosTron” [24]. To date, it has been applied in C. acetobutylicum, C. difficile, C. beijerinckii, C. botulinum, C. sporogenes and C. perfringens [1], [6], [24], [25], [26], [27], [28], [29], [30]. In particular, it should be noted that C. difficile was almost refractory to mutagenesis until the development of the ClosTron mutagenesis system [31]. This system involves homing of ribonucleoprotein (RNP) complex that consists of group II intron RNA molecule (Ll.LtrB) and the associated protein LtrA [32]. Base pairing with the DNA insertion site in the first place and then catalyzing insertion and reverse transcription of the intron RNA by RNP confer specificity upon the subsequent integration event [32]. To achieve rapid and efficient selection of positive integrants, a retrotransposition-activated selectable marker (RAM) was introduced into intron domain IV (DIV) [33]. However, this strategy cannot be used to isolate clones containing a second intron insertion in an already erythromycin-resistant mutant. To solve this problem, RAM was flanked by two repeated FLP (flippase) recognition target (FRT) sites and it can be removed from the chromosome in FLP recombinase-mediated step [30]. Therefore, the authors proposed that this system could be applied for insertional mutation of multiple gene in Clostridium [30]. Nevertheless, this approach would leave an intron residual fragment of over 0.9 kb in the genome [30]. The disruption of the other genes by this system in an already intron insertion mutant might lead to the instability of the previously mutated genes due to the presence of LtrA, through which the excision of a DNA sequence flanked by two intron fragments might occur via homologous recombination [1], [24], [34], [35]. So far, there has no report using the above described strategy for gene deletion in Clostridium yet.
To date, C. acetobutylicum and C. thermocellum are the only clostridial strains in which targeted gene deletion via homologous recombination have been established [36], [37]. In C. acetobutylicum, spo0A was deleted by using a replicative plasmid pETSPO capable of integrating into the chromosome through two rounds of crossover selection [36]. pETSPO contains a Gram-positive origin of replication and was methylated before introducing into C. acetobutylicum, which significantly increased the chance of homologous recombination [36]. Nevertheless, follow-up researchers suggested that this method is of low reproducibility and laborious to screen for double-crossover integration events [34], [38]. Moreover, this strategy would leave an erythromycin resistance marker in the genome for screening, which prevents it from manipulating multiple genes, since not many markers are available for Clostridia [21], [36], [39], [40], [41], [42], [43], [44]. In C. thermocellum, a replicating allelic exchange vector was also adopted to delete targeted genes because the current transformation efficiency does not meet the requirement for genetic manipulation; therefore this method should be laborious to screen for double-crossover integration events [37].
The aim of this study was to develop a more efficient targeted gene deletion strategy for Clostridium. By combining the principles of the “ClosTron” system and homologous recombination, we developed an accurate gene deletion procedure which enabled us efficiently delete DNA fragments without leaving any antibiotic resistance marker in the genome. This strategy might aid in the genetic dissection of clostridial virulence and engineering industrial clostridial strains for efficient production of biofuels and bio-based chemicals.
Results
The strategy to delete target gene in Clostridia
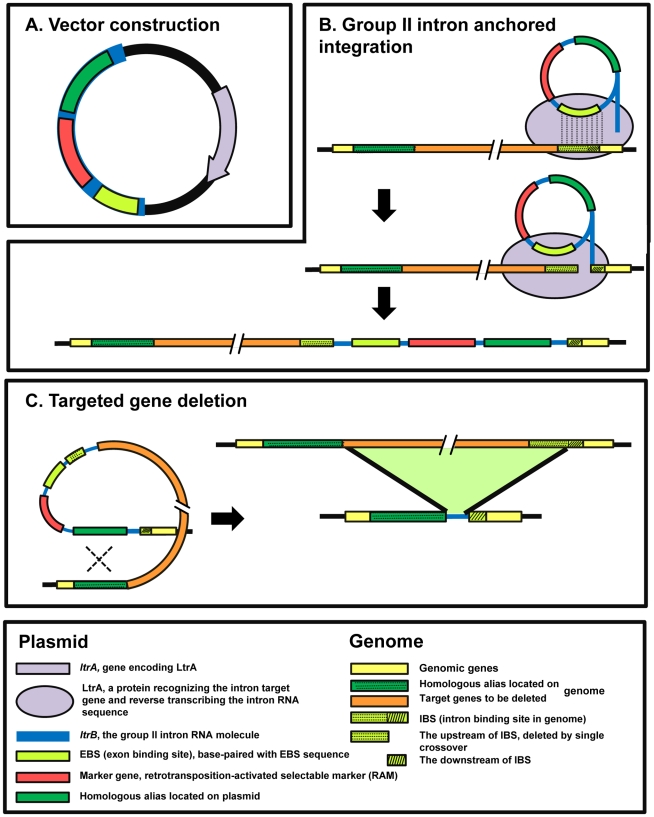
In microorganisms, gene deletion was usually conducted by using the classical homologous recombination strategy. Although single crossover integration can inactivate the target genes in Clostridium, the transformation and recombination frequencies were so low that double crossover events rarely occurred [45]. Targeted gene deletion via double crossover recombination remains a challenge for Clostridium [24], [36], [46]. ClosTron has been adopted for targeted gene disruption in Clostridium, with an integration frequency of nearly 100% in some clostridial species [24]. We therefore propose an intron-anchored gene deletion approach (Fig. 1). In this approach, an allele homologous to the upstream or downstream of the intron target site was constructed together with the intron. Upon introducing this construct into the target microorganism, an intron retrotransposition might occur in the first place, followed by homologous recombination which might result in the deletion of the target genes.
Figure 1. The strategy for target gene deletion in clostridial genome.
The functional annotation of operons in Clostridium is usually based on the comparison of C. acetobutylicum to Bacillus subtilis, whereas a significant number of predicted operons shared little homology [47]. To adequately understand the function of those unknown genes, targeted gene deletion is the first step towards identification of the function of an operon.
Construction of CAC1493-1494 Deleted Mutant C. acetobutylicum DDC14
In C. acetobutylicum, CAC1493 and CAC1494, annotated as a Zinc finger DNA-binding domain and a hypothetical protein, are located in a two-gene operon in the chromosome. Their expression must be co-transcribed because the stop codon of CAC1493 overlaps with the start codon of CAC1494. Therefore, their biological functions are expected to be related. Zinc finger structures are found in many microorganisms and known to perform important transcriptional regulation tasks [48], [49]. We are interested in characterizing the function of CAC1493–1494, but the prerequisite is to delete this operon.
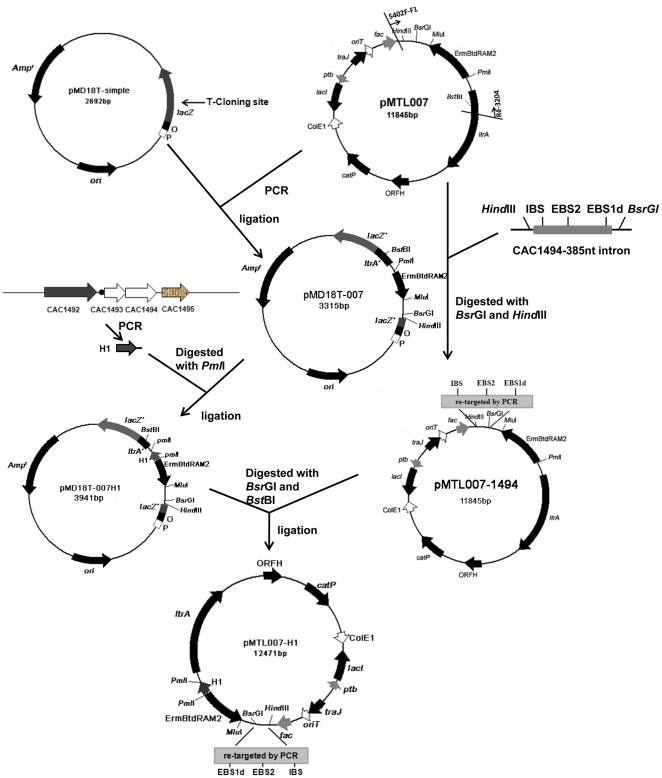
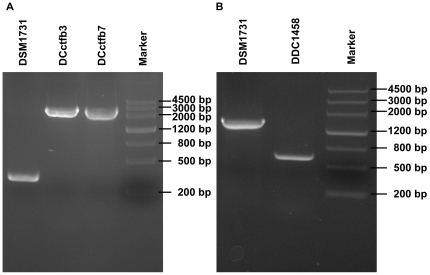
To achieve an intron retrotransposition and bring a homologous allele into the genome, an intron target site in the 3′ end of CAC1494, 385/386 nt in the sense strand, was selected. Subsequently, the intron re-targeting PCR primers 1494-385/386s-IBS, 1494-385/386s-EBS1d and 1494-385/386s-EBS2 (Table 1) were designed. The DNA sequence encoding the recognition part of the intron was altered via PCR to rationally re-program intron target specificity. Finally, we modified pMTL007 by introducing the upstream fragment of operon CAC1493-1494 (626 bp, named H1) as homologous allele into the downstream region of the eythromycin resistance gene (Erm r), the 3′-terminal sequence of intron (1907 to 2099), where multiple restriction sites including PmlI and SalI exist (Fig. 2). Intron insertions were verified by PCR using primers Cac1494B and Pex1494E (Table 1) flanking the target site and by sequencing the PCR results. A 2.8-kb fragment containing H1, intron sequence and erythromycin resistance gene was found to be inserted into CAC1494 (Fig. 3A and Fig. 4A) with an insertion frequency of 52.9% among 51 tested clones.
Table 1. Bacterial strains, plasmids and primers.
| Strains, plasmids or primers | Relevant characteristics | Reference or source |
| Strains | ||
| E. coli Top10 | mcrA Δ(mrr-hsdRMS-mcrBC) recA1 | Invitrogen |
| E. coli JM109 | recA1 mcrB+ hsdR17 | Lab storage |
| C. acetobutylicum DSM1731 | Contains operon CAC1493-1494, wild type | DSMZ |
| C. acetobutylicum DC1494 | CAC1494::intron with H1 fragment | This study |
| C. acetobutylicum DDC14 | ΔCAC1493-1494 | This study |
| C. acetobutylicum DDC245 | ΔCAC1493-1494 | This study |
| C. acetobutylicum DCctfb3 | ctfb::intron with H2 fragment | This study |
| C. acetobutylicum DCctfb7 | ctfB::intron with H2 fragment | This study |
| C. acetobutylicum DDC1458 | ΔctfAB | This study |
| Plasmids | ||
| pMTL007 | Cmr, ClosTron | [24] |
| pMTL007-1494 | Derived from pMTL007, targeting the CAC1494 in C. acetobutylicum | This study |
| pAN2 | Φ3t1, p15a ori, Tetr, methylating DNA prior to transformation to protect it against a C. acetobutylicum restriction system | [24] |
| pMD18T-simple | Ampr | Takara |
| pMD18T-007 | pMD18T-simple ligated with Ll.ltrB intron | This study |
| pMD18T-007H1 | pMD18T-007 ligated with H1 fragment | This study |
| pMTL007-H1 | pMTL007-1494 containing H1 fragment, CAC1493-1494 deletion vector | This study |
| pMTL007-ctfb | Derived from pMTL007, targeting the ctfB in C. acetobutylicum | This study |
| pMTL007-H2 | pMTL007-ctfb containing H2 fragment, ctfAB deletion vector | This study |
| Primer | ||
| 5402F-F1 | 5′-TTAAGGAGGTGTATTTCATATGACCATGATTACG-3′ | [24] |
| Re-3204 | 5′-TTCAGGTGTTATTCTTTCTGGACTTTCTCGGT-3′ | This study |
| 1494-385/386s-IBS | 5′-AAAAAAGCTTATAATTATCCTTAATGGTCTATATCGTGCGCCCAGATAGGGTG-3′ | This study |
| 1494-385/386s-EBS1d | 5′-CAGATTGTACAAATGTGGTGATAACAGATAAGTCTATATCATTAACTTACCTTTCTTTGT-3′ | This study |
| 1494-385/386s-EBS2 | 5′-TGAACGCAAGTTTCTAATTTCGGTTTCCTTTCGATAGAGGAAAGTGTCT-3′ | This study |
| Cac1494B | 5′-CGCGGATCCTTGTGTAAGCACATTTTAGG-3′ | This study |
| Pex1494E | 5′-CCGGAATTCTTATACACATATTGGCTCTC-3′ | This study |
| P1492-5s | 5′-ACGCGTCGACGCTGGTGCTTTACTTGAACT-3′ | This study |
| Clos-5 | 5′-AAAACACGTGATATGGCTAAACCTCCCAAG-3′ | This study |
| Clos-3 | 5′-ACGCCACGTGAAACTTGCCCTTTCCTATTC-3′ | This study |
| Sp5 | 5′-AATGGTGCTGCAACAAAATATATT-3′ | This study |
| Sp3 | 5′-CATCTTGATTAATAAATTCTACAT-3′ | This study |
| CTFB572/573s-IBS | AAAAAAGCTTATAATTATCCTTATTACTTCTCACTGTGCGCCCAGATAGGGTG | This study |
| CTFB572/573s -EBS1d | CAGATTGTACAAATGTGGTGATAACAGATAAGTCCTCACTGATAACTTACCTTTCTTTGT | This study |
| CTFB572/573s -EBS2 | TGAACGCAAGTTTCTAATTTCGGTTAGTAATCGATAGAGGAAAGTGTCT | This study |
| Dadhe-5 | AAAACACGTGTCAGAACACAATATTCCTAG | This study |
| Dad-3 | ACGCCACGTGCAATCATAATTGTCATCCCA | This study |
| Ct-5 | AGCCAATTGGATTGTTCCTG | This study |
| CTFB-3 | CAGCCATGGGTCTAAGTTCA | This study |
| Pro2-5 | ACTAGATGATCAATGCACAG | This study |
| Pr2-3 | GAGATTGTTTCTAGCTCTCA | This study |
Abbreviations: Ampr, ampicillin resistance; Cmr, chloramphenicol resistance; Tetr, tetracycline resistance; Φ3t1, Φ3t1 methyltransferase gene of Bacillus subtilis phage Φ3t1. DSMZ, German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany.
Figure 2. Schematic representation of the construction of pMTL007-H1 for deletion of CAC1493-1494.
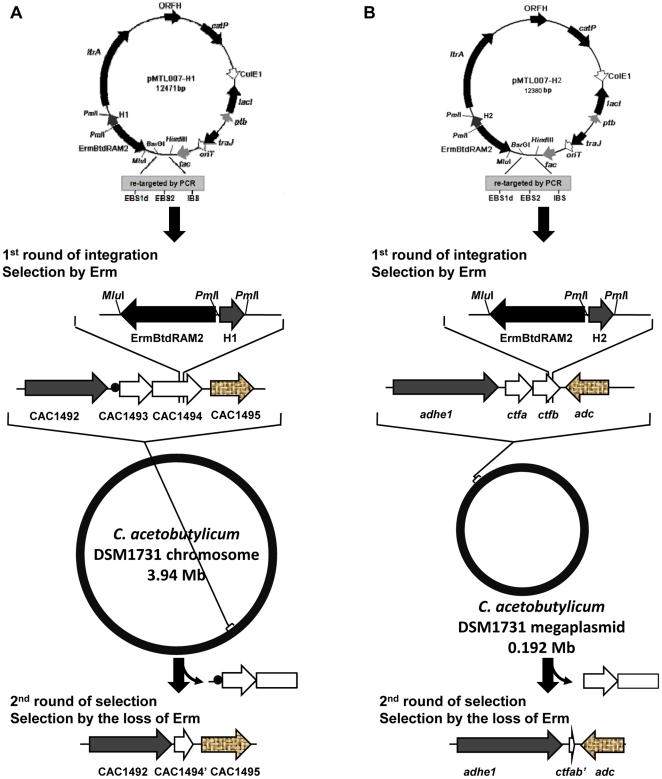
Figure 3. The procedure for deletion of CAC1493-1494 (A) and ctfAB (B) in the genome of C. acetobutylicum.
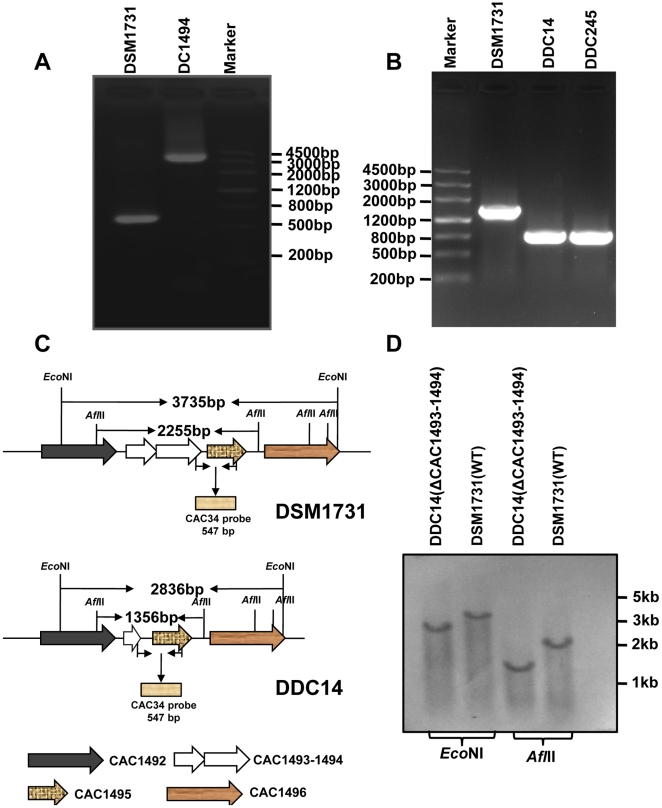
Figure 4. Construction of CAC1493-1494 deletion mutants.
A. Identification of an insertion mutant by PCR using primers Cac1494B and Pex1494E flanking the target site; B. Identification of the deletion mutants by PCR using primers P1492-5s and Pex1494E; C. Schematic show of operon CAC1493-1494 and the expected deleted operon CAC1493-1494 in the chromosome; D. Southern blot analysis of CAC1493-1494 deletion using CAC34 probe.
To delete CAC1493-1494 and the intron, secondary screening by successive transfer of the insertion mutant DC1494 into RCM medium was conducted in the absence of erythromycin. After 7 successive transfers in RCM medium (equivalent to 23 generations), two putative mutants with erythromycin sensitive phenotype were obtained from 648 colonies and designated as DDC14 and DDC245 (Fig. 3A). The genotype of the two mutants was confirmed by PCR and sequencing. Sequencing of the PCR products showed that about 0.9-kb fragment of the operon CAC1493-1494 was deleted as expected. This includes the upstream region of initiation codon (104 bp), CAC1493 and the majority part of CAC1494 (385 bp out of the total 615 bp) (Fig. 3A, 4B). Mutant DDC14 was selected for further analysis.
Southern analysis of the deletion of CAC1493-1494
Total genomic DNA of the mutant DDC14 and the parental strain DSM1731 were isolated for Southern hybridization. DNA was digested with either EcoNI or AflII and then probed with the labeled 547 bp CAC34 probe. Southern hybridization analysis showed that the size of the CAC34-hybridized DNA fragments of strain DSM1731 were about 0.9 kb larger than that of mutant DDC14 (Fig. 4C, 4D), suggesting that a single-copy of CAC1493-1494 exists in the genome of C. acetobutylicum and it had been deleted by this strategy.
The deletion of ctfAB in C. acetobutylicum DSM 1731
To further test the applicability of this new gene deletion strategy, we selected a function known operon ctfAB, which is involved in acetone formation, for deletion. The reason to select this operon is because it is located in the 192-kb megaplasmid of C. acetobutylicum. Deletion of the operon ctfAB is therefore a challenge, as it is generally known that gene deletion in plasmid is more difficult. An intron target site in the 3′ end of ctfB, 572/573 nt in the sense strand, was selected. Subsequently, pMTL007 was modified by altering the DNA sequence encoding the recognition part of the intron and introducing a homologous allele named H2 into the downstream region of erythromycin resistance gene (Table 1). Furthermore, the intron carrying H2 was inserted into the target site, the genotype of the insertional mutants was confirmed by PCR using primers flanking the targeted site and then sequencing. A 2.5-kb fragment containing H2, intron sequence and erythromycin resistance gene was found to be inserted into ctfB with an insertion frequency of 82.4% among 34 tested clones (Table 1, Fig. 3B, 5A). After 10 successive transfers in RCM medium in the absence of erythromycin (equivalent to 33 generations), a ctfAB-deleted mutant was screened from 1998 clones and designated as DDC1458. The genotype of DDC1458 was also verified by PCR using primers Pro2-5 and Pr2-3 and sequencing (Fig. 5B). PCR sequencing results showed that a DNA fragment of 1161 bp in the operon ctfAB (588 bp out of the total 657 bp in ctfA and 573 bp out of the total 666 bp in ctfB) was deleted.
Figure 5. Construction of ctfAB deletion mutants.
A. Identification of an insertion mutant by PCR using primers Ct-5 and CTFB-3 flanking the target site; B. Identification of the deletion mutant by PCR using primers Pro2-5 and Pr2-3.
Discussion
Gene deletion in microorganisms is usually conducted by homologous recombination. However, it is difficult to delete genes in Clostridium as the genomic integration remains a challenge [24], [36], [46]. Group II intron has been widely applied in directed insertional inactivation of a gene in many microorganisms. In Clostridium, after RAM was introduced into intron DIV, the intron insertion frequencies varied in the range of 1–10−7 integrants per cell, which is equivalent to hundreds or more ErmR integrants per experiment [24]. This indicated that ClosTron had overcome the difficulties in clostridial genomic integration. Since an additional DNA fragment of 1.0 kb besides a RAM can be inserted into the DIV and the activities of RNP remain high, the DNA fragments could be those homologous to upstream or downstream region of the intron target site, which may result in gene deletion in Clostridium via homologous recombination [24], [30], [46], [50]. Using this approach, we successfully deleted two operons CAC1493-1494 and ctfAB located in the chromosome and the megaplasmid, respectively. We found that once the intron carrying the homologous fragments was inserted into the genome, it is reliable to obtain the gene deletion mutants. This suggests that the first-step integration via the retrotransposition of intron is very important to increase the chance for the following homologous recombination.
Insertional mutation in target genes by the intron has been proved highly efficient and effective [24], [30]. Jiang et al reported that successively transferring the TargeTron-inactivated adc mutant in antibiotic-free CGM (Clostridium growth medium) for about 100 generations, the 1.5-kb intron fragment inserted into the adc gene was found to be lost in about 10% of the colonies, indicating the instability of intron insertion [51]. This problem does not exist in our strategy because the inserted intron was deleted together with the majority part of the target operons via homologous recombination. Previously, the deleted genes in Clostridia, namely spo0A in C. acetobutylicum, as well as pyrF and pta in C. thermocellum, are all chromosomally-encoded [36], [37]. Our practice of deleting ctfAB provides the first example of targeted gene deletion in the megaplasmid of C. acetobutylicum, without affecting the megaplasmid stability, further demonstrating the effectiveness of this gene deletion strategy.
In summary, the approach described above combines the advantage of the intron retrotransposition and homologous recombination. It eliminates the selection marker during the process of negative screening, which facilitates the genetic manipulation of multiple genes and operons in both chromosome and megaplasmid. To date, group II intron was used to inactivate genes in at least ten different bacterial species [52], for most of which targeted gene deletion is impossible. Therefore, the approach developed in this study has the potential to be applied for gene deletion in those species where first-step insertion via intron retrotransposition has been established.
Materials and Methods
Bacterial strains, plasmids and primers
The bacterial strains, plasmids and primers used in this study are listed in Table 1.
Growth conditions and maintenance of strains
E. coli strains were grown aerobically at 37°C in L broth. C. acetobutylicum were grown anaerobically at 37°C in reinforced clostridial medium (RCM) for routine growth and making competent cells [1], [53]. In all experiments, growth in liquid medium was monitored by measuring the absorbance at 600 nm (A 600) of appropriate dilutions with a UV/Vis 2802PC spectrophotometer (Unico, New Jersey, USA). For recombinant strains, antibiotics were added to the medium at the following final concentration: 100 µg/ml for ampicillin, 30 µg/ml for chloramphenicol, 40 µg/ml for tetracycline and 25 µg/ml for erythromycin. All C. acetobutylicum and E. coli strains were stored at −80°C in RCM and L broth supplemented with 15% glycerol, respectively.
DNA isolation and manipulation
Total genomic DNA of C. acetobutylicum and the plasmid DNA of E. coli were prepared using an E.Z.N.A Bacterial DNA Isolation Kit and E.Z.N.A Plasmid Extraction Kit (Omega Biotek Inc., Guangzhou, China). DNA restriction and cloning were performed according to standard procedure [54]. DNA sequencing were performed by Invitrogen Biotechnology Co., Ltd. Restriction enzymes, T4 DNA ligase, LongAmp Taq DNA polymerase, PrimerSTAR HS DNA polymerase and Taq DNA polymerase were purchased from New England BioLabs (Beijing, China) and TaKaRa Biotechology Co., Ltd (Dalian, China), respectively.
Modification of pMTL007
To improve CAC1493-1494 deletion rate, ClosTron gene knockout system was employed to insert a DNA fragment as homologous allele at the target site in CAC1494 for homologous recombination. To introduce this system into CAC1494, target site for insertion was predicted and the intron re-targeting PCR primers 1494-385/386s-IBS, 1494-385/386s-EBS1d and 1494-385/386s-EBS2 were designed in line with computer algorithm available at the Sigma-Aldrich website (www.sigmaaldrich.com/TargeTron Gene Knockout) [55]. 353 bp PCR product containing the modified IBS, EBS1d and EBS2 sequences was amplified and assembled by using one-tube SOEing PCRs and then cloned into the HindIII and BsrGI sites of pMTL007, generating pMTL007-1494 [1], [24]. Because two PmlI restriction sites exist in pMTL007 and H1 cannot be integrated into it directly, Ll.ltrB intron was amplified by PCR using pMTL007 as a template and 5402F-F1 and Re-3204 as primers, and then the PCR-generated fragment was TA cloned into pMD18T-simple, yielding plasmid pMD18T-007. With total genomic DNA of DSM1731 as template, primers clos-5 and clos-3 were used to amplify the homologous allele H1. Digested with PmlI, H1 was cloned into pMD18T-007 to generate plasmid pMD18T-007H1. Finally, a fragment containing Ll.ltrB intron and H1 was reintroduced into pMTL007-1494 through BsrGI and BstBI digestion, yielding plasmid pMTL007-H1 (Fig. 2).
Electrotransformation and Screening for CAC1493-1494 deletion strains
pMTL007-H1, together with pAN2, was first transformed into E. coli Top10. After overnight culture, pMTL007-H1 was isolated and then electroporated into C. acetobutylicum according to the protocol developed by Mermelstein[56]. The insertion mutants were screened based on the protocol reported by Heap [24], Intron insertions were verified by PCR using primers Cac1494B and Pex1494E flanking the target site and then PCR products were purified and sequenced, the sequence has been deposited in (Genbank number: HQ257448).
The insertion mutant DC1494 was inoculated into RCM without erythromycin resistance for successive transfer culture. For successive transfer, one milliliter fully grown culture was inoculated into 10 ml fresh RCM medium, grown anaerobically at 37°C for 12 h until full growth achieved. This transfer process was repeated for at least 7 times, each transfer is equivalent to three generations. The resulted culture was plated onto RCM plates containing 1.5% (w/v) agar. Isolated colonies were tested for the ability to resist erythromycin by streaking them onto solid RCM supplemented with 25 µg/ml erythromycin. The colonies without erythromycin resistance will be verified by Colony PCR using primers P1492-5s and Pex1494E (Fig. 3A). The PCR products were sequenced and the sequence has been deposited in (Genbank number: HQ257447).
Southern blot analysis of CAC1493-1494 deletion
For Southern blot analysis of CAC1493-1494 deletion, the genomic DNA from the wild-type strain DSM1731 and mutant DDC14 was extracted, digested with EcoNI and AflII, separated by electrophoresis overnight on a 0.8% agarose gel, and then transferred onto a hybond-N membrane in 0.4N NaOH. Hybridization was performed with digoxigenin-labeled DNA probe using DIG High Prime DNA Labelling and Detection Starter Kit I (Roche Diagnosis GmbH, Roche Applied Science, 68298 Mannhein Germany). DNA probe CAC34, amplified from the reserved region of CAC1494 and its downstream fragment with primers sp5 and sp3, were generated by random primed labeling technique according to the manual of the manufacturer.
The deletion of ctfAB in C. acetobutylicum DSM 1731
The deletion of ctfAB was based on the above mentioned strategy (Fig. 1). The DNA sequence encoding the recognition part of the intron in pMTL007 was altered by PCR using primers CTFB572/573s-IBS, CTFB572/573s -EBS1d, CTFB572/573s -EBS2, yielding plasmid pMTL007-ctfb. The homologous allele H2 of 535 bp was amplified by PCR using primers Dadhe-5 and Dad-3, cloned into pMD18T-007, and then ligated into vector pMTL007-ctfb after PmlI digestion, generating plasmid pMTL007-H2. The electroporation of pMTL007-H2, the retrotransposition of intron carrying the homologous allele at the site of 572/573 nt in ctfB and the deletion of ctfAB followed the above mentioned methods (Fig. 2 and Fig. 3B). The genotype of the insertion or deletion mutants was confirmed by PCR using primers flanking the targeted site or region and then by sequencing (Genbank number: HQ683763, 683762) (Fig. 5).
Acknowledgments
We thank Professor Nigel Peter Minton and Dr John Heap (University of Nottingham, UK) for gifting the pMTL007 and pAN2, and Hongjun Dong for helpful analysis of the results.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by National Basic Research Program of China (973 Project, #2007CB707803 and 2011CBA00807), National Science Foundation of China (31000024) and Hundreds Talents Program of the Chinese Academy of Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Dong H, Zhang Y, Dai Z, Li Y. Engineering clostridium strain to accept unmethylated DNA. PLoS One. 2010;5:e9038. doi: 10.1371/journal.pone.0009038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weingart OG, Schreiber T, Mascher C, Pauly D, Dorner MB, et al. The Case of Botulinum Toxin in Milk: Experimental Data. Appl Environ Microbiol. 2010;76:3293–3300. doi: 10.1128/AEM.02937-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hatheway CL. Toxigenic Clostridia. Clin Microbiol Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnston NC, Aygun-Sunar S, Guan Z, Ribeiro AA, Daldal F, et al. A phosphoethanolamine-modified glycosyl diradylglycerol in the polar lipids of Clostridium tetani. J Lipid Res. 71:1953–61. doi: 10.1194/jlr.M004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldape MJ, Bryant AE, Stevens DL. Clostridium sordellii infection: epidemiology, clinical findings, and current perspectives on diagnosis and treatment. Clin Infect Dis. 2006;43:1436–1446. doi: 10.1086/508866. [DOI] [PubMed] [Google Scholar]
- 6.Twine SM, Reid CW, Aubry A, McMullin DR, Fulton KM, et al. Motility and Flagellar Glycosylation in Clostridium difficile. J Bacteriol. 2009;191:7050–7062. doi: 10.1128/JB.00861-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ezeji T, Milne C, Price ND, Blaschek HP. Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol-producing microorganisms. Appl Microbiol Biotechnol. 2010;85:1697–1712. doi: 10.1007/s00253-009-2390-0. [DOI] [PubMed] [Google Scholar]
- 8.Otte B, Grunwaldt E, Mahmoud O, Jennewein S. Genome shuffling in Clostridium diolis DSM 15410 for improved 1,3-propanediol production. Appl Environ Microbiol. 2009;75:7610–7616. doi: 10.1128/AEM.01774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ezeji TC, Qureshi N, Blaschek HP. Bioproduction of butanol from biomass: from genes to bioreactors. Curr Opin Biotechnol. 2007;18:220–227. doi: 10.1016/j.copbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 10.Papoutsakis ET. Engineering solventogenic clostridia. Curr Opin Biotechnol. 2008;19:420–429. doi: 10.1016/j.copbio.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay A, Redding AM, Rutherford BJ, Keasling JD. Importance of systems biology in engineering microbes for biofuel production. Curr Opin Biotechnol. 2008;19:228–234. doi: 10.1016/j.copbio.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 12.Green EM, Boynton ZL, Harris LM, Rudolph FB, Papoutsakis ET, et al. Genetic manipulation of acid formation pathways by gene inactivation in Clostridium acetobutylicum ATCC 824. Microbiology. 1996;142(Pt 8):2079–2086. doi: 10.1099/13500872-142-8-2079. [DOI] [PubMed] [Google Scholar]
- 13.Wilkinson SR, Young M. Targeted Integration of Genes into the Clostridium acetobutylicum Chromosome. Microbiology-Uk. 1994;140:89–95. [Google Scholar]
- 14.Green EM, Bennett GN. Inactivation of an aldehyde/alcohol dehydrogenase gene from Clostridium acetobutylicum ATCC 824. Appl Biochem Biotechnol. 1996;57-58:213–221. doi: 10.1007/BF02941702. [DOI] [PubMed] [Google Scholar]
- 15.Raju D, Sarker MR. Comparison of the levels of heat resistance of wild-type, cpe knockout, and cpe plasmid-cured Clostridium perfringens type A strains. Appl Environ Microbiol. 2005;71:7618–7620. doi: 10.1128/AEM.71.11.7618-7620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liyanage H, Kashket S, Young M, Kashket ER. Clostridium beijerinckii and Clostridium difficile detoxify methylglyoxal by a novel mechanism involving glycerol dehydrogenase. Appl Environ Microbiol. 2001;67:2004–2010. doi: 10.1128/AEM.67.5.2004-2010.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendez M, Huang IH, Ohtani K, Grau R, Shimizu T, et al. Carbon catabolite repression of type IV pilus-dependent gliding motility in the anaerobic pathogen Clostridium perfringens. J Bacteriol. 2008;190:48–60. doi: 10.1128/JB.01407-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shimizu T, Ba-Thein W, Tamaki M, Hayashi H. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J Bacteriol. 1994;176:1616–1623. doi: 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiarezza M, Lyras D, Pidot SJ, Flores-Diaz M, Awad MM, et al. The NanI and NanJ sialidases of Clostridium perfringens are not essential for virulence. Infect Immun. 2009;77:4421–4428. doi: 10.1128/IAI.00548-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor JR, Lyras D, Farrow KA, Adams V, Powell DR, et al. Construction and analysis of chromosomal Clostridium difficile mutants. Mol Microbiol. 2006;61:1335–1351. doi: 10.1111/j.1365-2958.2006.05315.x. [DOI] [PubMed] [Google Scholar]
- 21.Sillers R, Al-Hinai MA, Papoutsakis ET. Aldehyde-alcohol dehydrogenase and/or thiolase overexpression coupled with CoA transferase downregulation lead to higher alcohol titers and selectivity in Clostridium acetobutylicum fermentations. Biotechnol Bioeng. 2009;102:38–49. doi: 10.1002/bit.22058. [DOI] [PubMed] [Google Scholar]
- 22.Desai RP, Papoutsakis ET. Antisense RNA strategies for metabolic engineering of Clostridium acetobutylicum. Appl Environ Microbiol. 1999;65:936–945. doi: 10.1128/aem.65.3.936-945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hebert CG, Valdes JJ, Bentley WE. Beyond silencing–engineering applications of RNA interference and antisense technology for altering cellular phenotype. Curr Opin Biotechnol. 2008;19:500–505. doi: 10.1016/j.copbio.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Heap JT, Pennington OJ, Cartman ST, Carter GP, Minton NP. The ClosTron: A universal gene knock-out system for the genus Clostridium. J Microbiol Meth. 2007;70:452–464. doi: 10.1016/j.mimet.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Underwood S, Guan S, Vijayasubhash V, Baines SD, Graham L, et al. Characterization of the Sporulation Initiation Pathway of Clostridium difficile and Its Role in Toxin Production. J Bacteriol. 2009;191:7296–7305. doi: 10.1128/JB.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Emerson JE, Reynolds CB, Fagan RP, Shaw HA, Goulding D, et al. A novel genetic switch controls phase variable expression of CwpV, a Clostridium difficile cell wall protein. Mol Microbiol. 2009;74:541–556. doi: 10.1111/j.1365-2958.2009.06812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirby JM, Ahern H, Roberts AK, Kumar V, Freeman Z, et al. Cwp84, a surface-associated cysteine protease, plays a role in the maturation of the surface layer of Clostridium difficile. J Biol Chem. 2009;284:34666–34673. doi: 10.1074/jbc.M109.051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camiade E, Peltier J, Bourgeois I, Couture-Tosi E, Courtin P, et al. Characterization of Acp, a peptidoglycan hydrolase of Clostridium perfringens with N-acetylglucosaminidase activity that is implicated in cell separation and stress-induced autolysis. J Bacteriol. 2010;192:2373–2384. doi: 10.1128/JB.01546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mullany P, Pallen M, Wilks M, Stephen JR, Tabaqchali S. A Group II intron in a conjugative transposon from the Gram-positive bacterium, Clostridium difficile. Gene. 1996;174:145–150. doi: 10.1016/0378-1119(96)00511-2. [DOI] [PubMed] [Google Scholar]
- 30.Heap JT, Kuehne SA, Ehsaan M, Cartman ST, Cooksley CM, et al. The ClosTron: Mutagenesis in Clostridium refined and streamlined. J Microbiol Methods. 2010;80:49–55. doi: 10.1016/j.mimet.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 31.Cartman ST, Heap JT, Kuehne SA, Cockayne A, Minton NP. The emergence of ‘hypervirulence’ in Clostridium difficile. Int J Med Microbiol. 2010;300:387–395. doi: 10.1016/j.ijmm.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Karberg M, Guo HT, Zhong J, Coon R, Perutka J, et al. Group II introns as controllable gene targeting vectors for genetic manipulation of bacteria. Nat Biotech. 2001;19:1162–1167. doi: 10.1038/nbt1201-1162. [DOI] [PubMed] [Google Scholar]
- 33.Zhong J, Karberg M, Lambowitz AM. Targeted and random bacterial gene disruption using a group II intron (targetron) vector containing a retrotransposition-activated selectable marker. Nucleic Acids Res. 2003;31:1656–1664. doi: 10.1093/nar/gkg248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shao L, Hu S, Yang Y, Gu Y, Chen J, et al. Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum. Cell Res. 2007;17:963–965. doi: 10.1038/cr.2007.91. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez SA, Yu JJ, Davis G, Arulanandam BP, Klose KE. Targeted inactivation of Francisella tularensis genes by group II introns. Appl Environ Microbiol. 2008;74:2619–2626. doi: 10.1128/AEM.02905-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris LM, Welker NE, Papoutsakis ET. Northern, morphological, and fermentation analysis of spo0A inactivation and overexpression in Clostridium acetobutylicum ATCC 824. J Bacteriol. 2002;184:3586–3597. doi: 10.1128/JB.184.13.3586-3597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tripathi SA, Olson DG, Argyros DA, Miller BB, Barrett TF, et al. Development of pyrF-based genetic system for targeted gene deletion in Clostridium thermocellum and creation of a pta mutant. Appl Environ Microbiol. 2010;76:6591–6599. doi: 10.1128/AEM.01484-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, et al. Fermentative butanol production by Clostridia. Biotechnol Bioeng. 2008;101:209–228. doi: 10.1002/bit.22003. [DOI] [PubMed] [Google Scholar]
- 39.Scotcher MC, Rudolph FB, Bennett GN. Expression of abrB310 and SinR, and effects of decreased abrB310 expression on the transition from acidogenesis to solventogenesis, in Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol. 2005;71:1987–1995. doi: 10.1128/AEM.71.4.1987-1995.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakayama S, Kosaka T, Hirakawa H, Matsuura K, Yoshino S, et al. Metabolic engineering for solvent productivity by downregulation of the hydrogenase gene cluster hupCBA in Clostridium saccharoperbutylacetonicum strain N1-4. Appl Microbiol Biotechnol. 2008;78:483–493. doi: 10.1007/s00253-007-1323-z. [DOI] [PubMed] [Google Scholar]
- 41.Borden JR, Jones SW, Indurthi D, Chen Y, Papoutsakis ET. A genomic-library based discovery of a novel, possibly synthetic, acid-tolerance mechanism in Clostridium acetobutylicum involving non-coding RNAs and ribosomal RNA processing. Metab Eng. 2010;12:268–281. doi: 10.1016/j.ymben.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raju D, Setlow P, Sarker MR. Antisense-RNA-mediated decreased synthesis of small, acid-soluble spore proteins leads to decreased resistance of Clostridium perfringens spores to moist heat and UV radiation. Appl Environ Microbiol. 2007;73:2048–2053. doi: 10.1128/AEM.02500-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scotcher MC, Bennett GN. SpoIIE regulates sporulation but does not directly affect solventogenesis in Clostridium acetobutylicum ATCC 824. J Bacteriol. 2005;187:1930–1936. doi: 10.1128/JB.187.6.1930-1936.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tummala SB, Welker NE, Papoutsakis ET. Design of antisense RNA constructs for downregulation of the acetone formation pathway of Clostridium acetobutylicum. J Bacteriol. 2003;185:1923–1934. doi: 10.1128/JB.185.6.1923-1934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakotte S, Schaffer S, Bohringer M, Durre P. Electroporation of, plasmid isolation from and plasmid conservation in Clostridium acetobutylicum DSM 792. Appl Microbiol Biotechnol. 1998;50:564–567. doi: 10.1007/s002530051335. [DOI] [PubMed] [Google Scholar]
- 46.Tummala SB, Junne SG, Papoutsakis ET. Antisense RNA downregulation of coenzyme A transferase combined with alcohol-aldehyde dehydrogenase overexpression leads to predominantly alcohologenic Clostridium acetobutylicum fermentations. J Bacteriol. 2003;185:3644–3653. doi: 10.1128/JB.185.12.3644-3653.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nolling J, Breton G, Omelchenko MV, Makarova KS, Zeng Q, et al. Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J Bacteriol. 2001;183:4823–4838. doi: 10.1128/JB.183.16.4823-4838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengupta S, Nagaraja V. YacG from Escherichia coli is a specific endogenous inhibitor of DNA gyrase. Nucleic Acids Res. 2008;36:4310–4316. doi: 10.1093/nar/gkn355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bouhouche N, Syvanen M, Kado CI. The origin of prokaryotic C2H2 zinc finger regulators. Trends Microbiol. 2000;8:77–81. doi: 10.1016/s0966-842x(99)01679-0. [DOI] [PubMed] [Google Scholar]
- 50.Matsuura M, Saldanha R, Ma H, Wank H, Yang J, et al. A bacterial group II intron encoding reverse transcriptase, maturase, and DNA endonuclease activities: biochemical demonstration of maturase activity and insertion of new genetic information within the intron. Genes Dev. 1997;11:2910–2924. doi: 10.1101/gad.11.21.2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang Y, Xu C, Dong F, Yang Y, Jiang W, et al. Disruption of the acetoacetate decarboxylase gene in solvent-producing Clostridium acetobutylicum increases the butanol ratio. Metab Eng. 2009;11:284–291. doi: 10.1016/j.ymben.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Rodriguez SA, Davis G, Klose KE. Targeted gene disruption in Francisella tularensis by group II introns. Methods. 2009;49:270–274. doi: 10.1016/j.ymeth.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tomas CA, Welker NE, Papoutsakis ET. Overexpression of groESL in Clostridium acetobutylicum results in increased solvent production and tolerance, prolonged metabolism, and changes in the cell's transcriptional program. Appl Environ Microbiol. 2003;69:4951–4965. doi: 10.1128/AEM.69.8.4951-4965.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sambrook JRD. CSHL Press; 2001. molecular cloning: a laboratory mannual. [Google Scholar]
- 55.Perutka J, Wang WJ, Goerlitz D, Lambowitz AM. Use of computer-designed group II introns to disrupt Escherichia coli DExH/D-box protein and DNA helicase genes. J Mol Biol. 2004;336:421–439. doi: 10.1016/j.jmb.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 56.Mermelstein LD, Welker NE, Bennett GN, Papoutsakis ET. Expression of cloned homologous fermentative genes in Clostridium acetobutylicum ATCC 824. Biotechnol (N Y) 1992;10:190–195. doi: 10.1038/nbt0292-190. [DOI] [PubMed] [Google Scholar]