Abstract
With No lysine (K) kinase 4 (WNK4) is a protein serine/threonine kinase associated with a Mendelian form of hypertension. WNK4 is an integrative regulator of renal transport of Na+, K+, and Cl− as shown in Xenopus oocyte system. In addition, WNK4 enhances the surface expression of epithelial Ca2+ channel TRPV5, which plays a key role in the fine tuning of renal Ca2+ reabsorption. Variations in the magnitude of WNK4-mediated regulation on TRPV5 in Xenopus oocytes suggest additional cellular components with limited expression are required for the regulation. In this study, we identified the Na+/H+ exchanger regulating factor 2 (NHERF2) as a critical component for the positive regulation of TRPV5 by WNK4. NHERF2 augmented the positive effects of WNK4 on TRPV5, whereas its homolog NHERF1 had no effect when tested in the Xenopus oocyte system. The C-terminal PDZ binding motif of TRPV5 was required for the regulation by NHERF2. While NHERF2 interacted with TRPV5, no association between NHERF2 and WNK4 was detected using a GST pull-down assay. WNK4 increased the forward trafficking of TRPV5; however, it also caused an accelerated decline of the functional TRPV5 channels at later stage of co-expression. NHERF2 stabilized TRPV5 at the plasma membrane without interrupting the forward trafficking of TRPV5, thus prevented the decline of functional TRPV5 channel caused by WNK4 at later stage. The complementary and orderly regulations of WNK4 and NHERF2 allow TRPV5 functions at higher level for a longer period to maximize Ca2+ influx.
Keywords: NHERF2, NHERF1, WNK4, TRPV5, TRPV6, PDZ binding motif
1. Introduction
Mutations in With No lysine (K) kinase 4 (WNK4), a protein serine/threonine kinase, are associated with psuedohypoaldosteronism type II (PHA II), a genetic form of hypertension featuring hyperkalemia, hypertension, and metabolic acidosis [21]. Hypercalciuria and low bone mineral density are additional manifestations in patients carrying the Q565E mutation in WNK4 [12]. WNK4 regulates several key ion transport proteins, such as the thiazide-sensitive Na+-Cl− cotransporter NCC, the amiloride-sensitive epithelial Na+ channel ENaC, and the renal outer medullary K+ channel ROMK when co-expressed in Xenopus oocyte system (reviewed in ref 16). WNK4 also enhances the forward trafficking of epithelial Ca2+ channel TRPV5 via the secretory pathway [6], resulting in an increase in the complexly N-glycosylated mature forms of TRPV5 in the plasma membrane [7]. This regulation is partially blocked by the co-expression of NCC and this blocking effect is increased in the presence of the Q565E mutant [7]. Because TRPV5 plays a key role in regulating Ca2+ excretion [13], these results suggest that the regulation of TRPV5 by WNK4 is likely involved in the pathogenesis of hypercalciuria in PHA II.
Like WNK4, the serum- and glucocorticoid-induced kinase SGK1 also regulates various electrolyte transporters and ion channels [10]. Interestingly, a scaffolding PDZ (PSD-95/Discs-large/ZO-1) containing protein NHERF2 is often required for the SGK1-mediated regulation of ion transporters such as Na+/H+ exchanger NHE3 [22], ROMK [14;23], and TRPV5 [4]. Similar to TRPV5 and WNK4, NHERF2 is also distributed to the distal tubule [20]. Therefore, NHERF2 could be a component of WNK4 signaling that is not readily expressed in Xenopus oocytes. We therefore evaluated the role of NHERF2 in the regulation of TRPV5 by WNK4 in this study.
2. Materials and methods
2.1. cDNA constructs
cDNAs for human TRPV5, TRPV6, WNK4 [7], and the N358Q mutant of TRPV5 [6] were described previously. Human NHERF1 and NHERF2 cDNAs were amplified by PCR using cDNA templates from human colon carcinoma T84 cells and the cDNAs were subcloned into the Xenopus oocytes expression vector pIN [7]. FLAG tagged NHERF2 and HA tagged WNK4 constructs were generated using pIN-FLAG and pIN-HA vector, respectively. The construct with the TRPV5 C-terminal 2 amino-acids substituted with those of TRPV6 (TRPV5-C6) was generated by PCR. The PCR products were subcloned into pIN vector using Bgl II and Sal I for TRPV5-C6 and Mlu I and Sal I for TRPV6-C5. pIN-GST and pIN-GST-NHERF2 were constructed by subcloning of PCR-generated GST into pIN and pIN-NHERF2 using Bgl II and Xho I, and Bgl II and Mlu I restriction sites, respectively. All cDNAs generated by PCR were sequenced to confirm that no errors were incorporated.
2.2. Ca2+ uptake in Xenopus oocytes
The Ca2+ uptake assay using Xenopus oocytes has been described previously [7;24]. The use of Xenopus frogs was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Alabama at Birmingham.
2.3. Trafficking and plasma membrane stability of TRPV5
For evaluation of the forward trafficking of TRPV5, oocytes were injected with water (as control) or cRNA(s) for NHERF2, WNK4, or NHERF2 and WNK4 at 12.5 ng/oocyte for each cRNA. Twelve hours later, TRPV5 cRNA (12.5 ng/oocyte) was injected into the pre-injected oocytes. Ca2+ uptake experiments were carried out 3 hours later. For assessing the plasma membrane stability of TRPV5, oocytes were injected with the same respective cRNA(s) and were incubated for 36 hours at 18 °C. Then oocytes were incubated with Brefeldin A (Tocris Bioscience, Ellisville, MO) at 20 μg/ml for 0, 4, and 8 hours before Ca2+ uptake assay was carried out. Ca2+ uptake values were subtracted by those of oocytes injected with water and were expressed as percentage of the Ca2+ uptake value at 0 hour of incubation with BFA.
2.4. Western blot analysis
Western blot analysis experiments were carried out as previously described [24]. Antibodies used in this study were rabbit anti-TRPV5 (1:3,000 dilution, CAT21-A, Alpha Diagnostic, San Antonio, TX), customer-made rabbit anti-TRPV6 antiserum [24], and HRP-conjugated goat anti-rabbit secondary antibody (1:5,000, SC-2004, Santa Cruz Biotechnology, Santa Cruz, CA).
2.5. Immunofluorescent staining
Immunofluorescent staining experiments with oocyte sections were performed as described previously [24]. The primary antibodies used include anti-TRPV5 (1:100 dilution, Alpha Diagnostic) and anti-FLAG (1:100 dilution, Sigma-Aldrich, St. Louis, MO). The goat anti-rabbit IgG-FITC antibody (sc-2012, 1:500, Santa Cruz) or Alexa Fluor 594 goat anti-rabbit IgG (H+L) (A11012, 1:1,000, Invitrogen, Carlsbad, CA) was used as secondary antibody.
2.6. GST pull-down assay
Xenopus oocytes were injected with cRNAs encoding GST (as control) or GST–NHERF2 together with FLAG-TRPV5 and HA-WNK4 and were cultured in 0.5 × L-15 medium at 18 °C. Two days after injection, 50 oocytes were lysed with lysis buffer (NaCl 100 mM, Tris·Cl 20 mM, Triton X100 1%, plus protease inhibitor cocktail, pH 7.5) at 20 μl/oocyte. After vigorous vortex, the oocytes were centrifuged at 2,500 g for 10 min at 4 °C to remove the cellular debris and yolk proteins. The supernatants were incubated with 50 μl of 1:1 slurry glutathione Sepharose (GE Healthcare, Piscataway, NJ). After rocking at 4°C for 2 hours, the Sepharose beads were washed 3 times with 500 μl lysis buffer supplemented with protease inhibitor cocktail. Then GST or GST-NHERF2 proteins were eluted from Sepharose beads by incubation with 10 mM L-glutathione reduced for 30 min at 4 °C. Both the supernatants and the proteins bound to the Sepharose beads were respectively subjected to SDS–PAGE. The proteins were transferred from the SDS-PAGE to PVDF membrane and immunoblotting experiments were carried out with anti-GST (27-4577-01, GE healthcare, 1:2000), anti-HA (H9658, Sigma-Aldrich, 1: 5000) and anti-FLAG (F7425, Sigma-Aldrich, 1: 5000) antibodies to determine proteins associated with GST-fusion protein.
3. Results
3.1. The positive effect of WNK4 on TRPV5 is augmented specifically by NHERF2
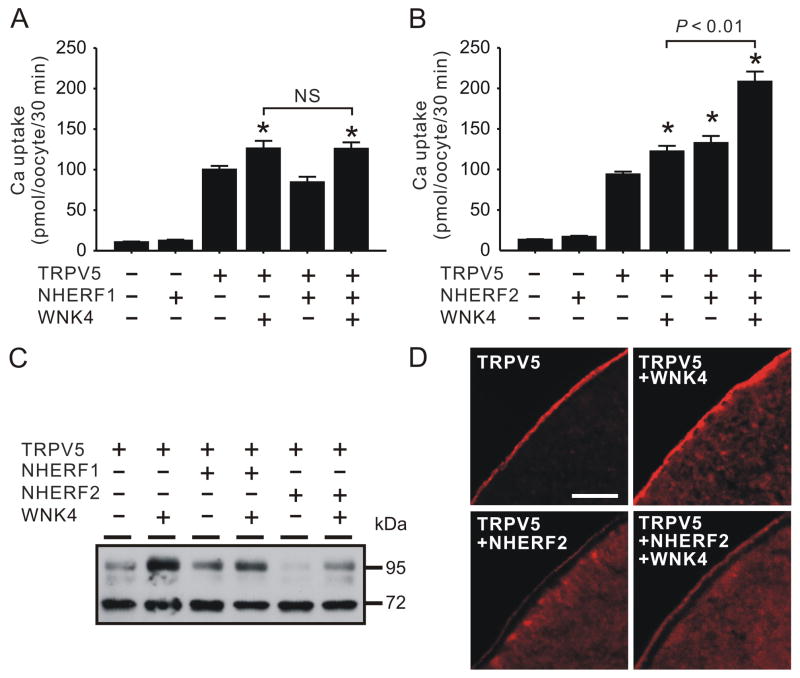
When NHERF1 was co-expressed with TRPV5 in Xenopus oocytes, TRPV5-meidated Ca2+ uptake was unaltered, so was the effect of WNK4 on TRPV5 (Fig. 1A). There was no significant difference in the Ca2+ uptake value of the oocytes expressing TRPV5 and WNK4 in absence or presence of NHERF1 (122.1 ± 9.4 vs. 125.7 ± 7.9 pmol/oocyte/30 min). In contrast, TRPV5-mediated Ca2+ uptake was significantly increased by NHERF2 (41.3 ± 9.3% increase, Fig 1B). Furthermore, the enhancing effect of WNK4 on TRPV5 was also significantly augmented by NHERF2 (Fig. 1B). A moderate increase (30.2 ± 7.3%) in TRPV5-mediated Ca2+ uptake in the presence of WNK4 was observed using recent batches of oocytes without NHERF2 co-expression. In contrast, the effect of WNK4 on TRPV5 was almost doubled (57.1 ± 9.4% increase) when NHERF2 was co-expressed (Fig. 1B). As a result of the concerted action of NHERF2 and WNK4, TRPV5-mediated uptake was increased by 121.8 ± 13.3% over the TRPV5 alone group. Western blot analyses indicated that the intensity complexly N-glycosylated band of TRPV5 (~95 kDa) was reduced by NHERF2 but this didn’t disrupt the enhancing effect of WNK4 on this band (Fig. 1C). TRPV5 proteins appeared to be accumulated underneath the plasma membrane when NHERF2 was co-expressed (Fig. 1D). In the presence of WNK4, the TRPV5 staining underneath the plasma membrane decreased while a concomitant increase in TRPV5 staining at the plasma membrane was observed (Fig. 1D). Despite a significant increase in TRPV5 activity in the presence of NHERF2, the level of TRPV5 staining was decreased compared to those without NHERF2 co-expression (Fig. 1D).
Fig. 1.
NHERF2 but not NHERF1 augmented the enhancing effect of WNK4 on TRPV5. A. NHERF1 exhibited no effects on TRPV5-mediated Ca2+ uptake in the presence and absence of WNK4 (n = 21). B. NHERF2 increased TRPV5-mediated Ca2+ uptake and significantly augmented the enhancing effects of WNK4 on TRPV5 (n = 56). C. A representative Western blot analysis using antibody against TRPV5. D. Immunofluorecent staining of TRPV5 in sections of oocytes expressing TRPV5, TRPV5 and WNK4, TRPV5 and NHERF2, and TRPV5, NHERF2 and WNK4, respectively. Bar, 25 μm. * indicates P < 0.05 compared to TRPV5 alone group. NS, not statistically significant (P > 0.05).
3.2. The C-terminal PDZ binding motif of TRPV5 is critical for the effects of NHERF2
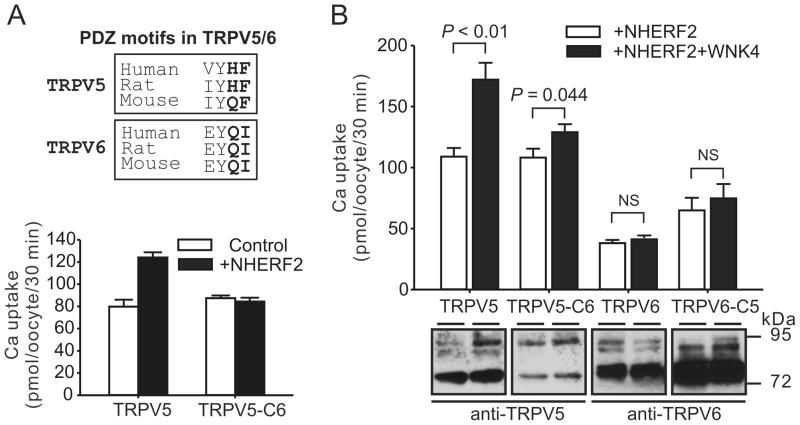
Both TRPV5 and TRPV6 possess a class II PDZ binding motif at its C-terminus with the -X-Φ-X-Φ consensus sequence where Φ represents a hydrophobic amino acid and X represents an unspecified amino acid (Fig. 2A, upper panel) [5]. The PDZ binding motif at the C-terminus of TRPV6 is necessary for its interaction with a PDZ protein PDZK2 (also known as NHERF4) [8]. To determine whether the putative PDZ binding motif at the C-terminus of TRPV5 is necessary for NHERF2-mediated effects on TRPV5, we swapped the last 2 amino-acids at the C-terminus between TRPV5 and TRPV6. The up-regulation of TRPV5 by NHERF2 was abolished when its C-terminus was substituted by that of TRPV6 (TRPV5-C6, Fig. 2A, lower panel). Consistent with this observation, in the presence of NHERF2, the effect of WNK4 on TRPV5-C6 was significantly reduced compared to that of the wild-type TRPV5 (Fig. 2B). However, the last 2 amino-acids of TRPV5 were not sufficient to mediate the action of NHERF2 on TRPV6, as neither TRPV6 nor TRPV6 with the last 2 amino-acids replaced by those of TRPV5 (TRPV6-C5) was significantly increased by WNK4 in the presence of NHERF2 (Fig. 2B). The protein abundance of TRPV5-C6 was decreased whereas that of TRPV6-C5 was increased compared to their wild-type protein, respectively, suggesting the PDZ binding motif of TRPV5/6 affect protein stability (Fig. 2B, lower panel).
Fig. 2.
The C-terminal PDZ binding motif of TRPV5 is required for the effects of NHERF2 on TRPV5 and the regulation of WNK4 on TRPV5. A. The stimulation effect of NHERF2 on TRPV5 was abolished in the TRPV5-C6 construct which has the sequence of TRPV5 with the last 2 amino-acids substituted by those of TRPV6 (n = 14). The PDZ binding motifs of TRPV5 and TRPV6 are shown in the upper panel. B. The effect of NHERF2 on the regulation of TRPV5 by WNK4 was abolished in the TRPV5-C6 construct; however, substituting the last 2 amino-acids of TRPV6 with those of TRPV5 (TRPV6-C5) was not sufficient to make it responsive to WNK4 (n = 14). NHERF2 was co-expressed in all groups. NS, not statistically significant (P > 0.05). Representative Western blot analyses using anti-TRPV5 or anti-TRPV6 antibody are shown in the lower panel.
3.3. No physical association between NHERF2 and WNK4 was observed
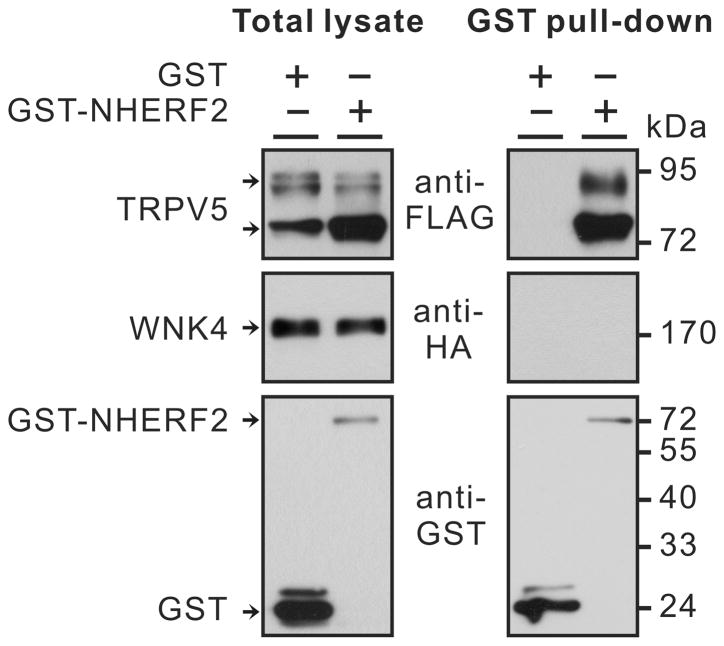
Since NHERF2 often interacts with both transporters/channels and the protein kinases that regulate them [3;22], we next assessed whether NHERF2 interacts with WNK4. TRPV5 interacts with NHERF2 through the second PDZ domain [15]. We confirmed this interaction with GST pull-down assay (Fig. 3). However, HA-tagged WNK4 was not detected among the proteins associated with GST-NHERF2 (Fig. 3). Because SGK1 interacts with NHERF2 and WNK4 [18;22], we also co-expressed the active S422DSGK1 with GST-NHERF2, FLAG-TRPV5 and HA-WNK4. Yet, no HA-WNK4 protein was detected in the proteins pulled-down by GST-NHERF2 (data not shown).
Fig. 3.
FLAG-TRPV5 but not HA-WNK4 could be pulled down by GST-NHERF2 when they were co-expressed in Xenopus oocytes. For details see Materials and methods section.
3.4. Unlike WNK4, NHERF2 does not affect the forward trafficking of TRPV5
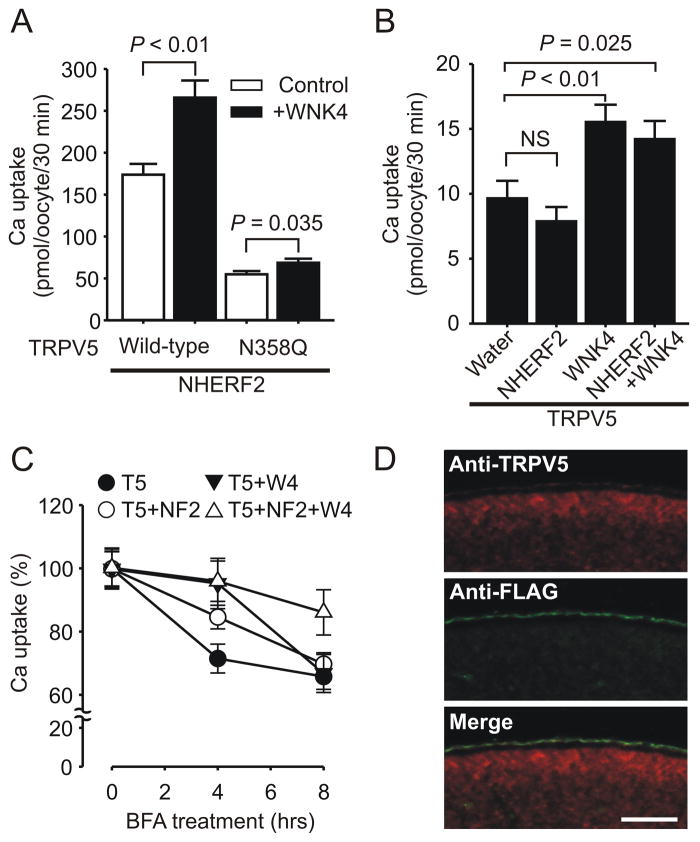
To understand the mechanism how NHERF2 enhances the positive effect of WNK4 on TRPV5, we first evaluated the effects of NHERF2 on the TRPV5 N358Q mutant that is defect in N-linked glycosylation and forward trafficking via the secretory pathway [6]. In the presence of NHERF2, the enhancing effect of WNK4 on the N358Q mutant remained limited (Fig. 4A), similar to the unresponsiveness of N358Q mutant to WNK4 in the absence of NHERF2 shown previously [6]. This indicated that the defect of the N358Q mutant in being regulated by WNK4 could not be mended by NHERF2. We next tested to what extent the forward trafficking of wild-type TRPV5 is affected by NHERF2 and WNK4 (Fig. 4B). In order to evaluate the forward trafficking of TRPV5 by NHERF2 or WNK4, oocytes were first injected with water (control) or cRNA(s) for NHERF2, WNK4, or NHERF2 and WNK4. Twelve hours later, TRPV5 cRNA was injected into these oocytes; and 3 hours later their Ca2+ influx activity was assessed. The forward trafficking is likely a major event for TRPV5 within the 3-hour time frame. By this assay, NHERF2 exhibited little effect on TRPV5 forward trafficking in the absence or presence of WNK4; whereas WNK4 had similar enhancing effect on TRPV5 forward trafficking in the absence or presence of NHERF2 (Fig. 4B).
Fig. 4.
NHERF2 did not alter the forward trafficking of TRPV5 but rendered TRPV5 more stable at the plasma membrane in the presence or absence of WNK4. A. NHERF2 didn’t improve the lack of robust response of N-glycosylation defective N358Q mutant of TRPV5 to the regulation by WNK4 (n = 14). B. Assessment of WNK4 and NHERF2 on TRPV5 forward trafficking. Oocytes injected with water, or cRNA(s) for NHERF2, WNK4, or NHERF2 and WNK4 (12.5 ng/oocyte each), were injected with TRPV5 (12.5 ng/oocyte) 12 hours later. Ca2+ uptake were performed 3 hours later (n =21). C. Assessment of stability of functional TRPV5 at the cell surface. Oocytes expressing TRPV5 (T5), TRPV5 and NHERF2 (T5+NF2), TRPV5 and WNK4 (T5+W4), and all the 3 proteins (T5+NF2+W4) for 36 hours were treated with Brefeldin A (BFA) at 20 μg/ml for indicated time period before Ca2+ uptake experiments were performed. Ca2+ uptake values (with the water injected control groups subtracted) are expressed as percentage of the value in the group with 0 hour BFA treatment (n=21). D. Plasma membrane distribution of FLAG-NHERF2 (green) and TRPV5 (red) as detected by co-immunostaining with anti-FLAG and anti-TRPV5 antibodies in oocytes co-expressing TRPV5 and FLAG-NHERF2. Bar, 50 μm.
3.5. NHERF2 increases the stability of functional TRPV5 in the presence of WNK4
To evaluate the effect of NHERF2 on the stability of functional TRPV5 at the plasma membrane, oocytes were treated with Brefeldin A (BFA), which disrupts the Golgi complex, to block the forward trafficking (Fig. 4C). NHERF2 significantly slowed down the decline of functional TRPV5 in the presence of BFA (Fig. 4C). TRPV5 activity was stabilized at the first 4 hours of BFA treatment in the presence of WNK4 compared to the group without WNK4; however, an increased decline of functional TRPV5 in the presence of WNK4 was evident during the second 4-hour treatment with BFA (Fig. 4C). NHERF2 had no effect on the stabilizing effect of WNK4 on TRPV5 at the initial 4-hour period; however, it prevented a rapid decline of functional TRPV5 by WNK4 at the second 4-hour treatment with BFA (Fig. 4C). By immunostaining of sections of oocytes expressing TRPV5 and FLAG-NHERF2, the two proteins exhibited an overlapping distribution pattern along the plasma membrane (Fig. 4D). This distribution pattern allows NHERF2 to stabilize TRPV5 at the plasma membrane.
4. Discussion
Xenopus oocyte system has been used to evaluate the effects of WNK kinases on a variety of electrolyte transporters and ion channels [16]; however, oocytes may express cellular components necessary for the signaling pathways at variable levels due to genetic variations of Xenopus frogs and seasonal variations in gene expression. This likely contributes to the fluctuations of the effects of WNK4 on ion transporters/channels such as TRPV5 [7]. In this study, we identify NHERF2 as a candidate cellular component in mediating the regulation of WNK4 on TRPV5. We found that WNK4 have two opposite effects on TRPV5: WNK4 increases the forward trafficking of TRPV5 but also renders the functional TRPV5 at plasma membrane unstable at later stage likely due to the excessive Ca2+ influx. NHERF2 acts synergistically with WNK4 by stabilizing TRPV5 at the plasma membrane to maintain the positive regulation of WNK4 on TRPV5.
NHERF2 is a scaffolding protein involved in mediating the action of kinases on ion transporters and channels. NHERF2 is required for the cGMP-mediated inhibition of NHE3 by a cyclic GMP kinase II-dependent mechanism [3]. NHERF2 also plays a role in the inhibition of NHE3 by Ca2+ [9], a process involving binding of PKCα and NHERF2 [11]. Interestingly, NHERF2 also interacts with SGK1 to regulate NHE3 activity [22]. In addition, NHERF2 is also required for SGK1-mediated regulation of the Na+-dicarboxylate cotransporter NaDC-1[1], ROMK1 [14;23], the proton coupled peptide transporter PEPT2 [2], and TRPV5 [4;15]. In these regulations, NHERF2 serves as a scaffolding protein linking kinases and transporters /channels through the interactions using its PDZ domains. Consistent with these studies, we found that the C-terminal PDZ binding motif of TRPV5 is essential for the regulation of TRPV5 by NHERF2; however, we were unable to demonstrate an association between the NHERF2 with WNK4, although under the same condition NHERF2 interacted with TRPV5 (Fig. 3). This suggests either the association between NHERF2 and WNK4 is too weak to be detected or WNK4 acts on NHERF2 via another unidentified cellular component. The interaction between NHERF2 and TRPV5 appears to be specific, as NHERF1 had no effect on either TRPV5 alone or the positive regulation of TRPV5 by WNK4. NHERF2 appeared to have no effects on TRPV6 and substituting the last 2 amino-acids of TRPV5 with those of TRPV6 abolished the regulation (Fig. 2).
There is a synergistic action between WNK4 and NHERF2 because they regulate 2 separate steps of TRPV5 trafficking. WNK4 increases the forward trafficking of TRPV5 whereas NHERF2 stabilizes it at the plasma membrane, similar to its effect on PEPT2 [2]. The synergy is lost when one of the two processes does not function well, such as in the cases of TRPV6 and N358QTRPV5, which could not be effectively mobilized by WNK4 to be delivered to the plasma membrane through the secretory pathway (Fig. 2B, Fig. 4A)[6]. The stabilizing effect of NHERF2 on TRPV5 could be due to an altered endocytosis or endocytic recycling, the later has been shown to be the case for CFTR [19]. This is consistent with the observation that NHERF2 localizes closely with TRPV5 at the plasma membrane (Fig. 4D), but the detailed mechanism is yet to be illustrated.
It is unclear how the co-expression of NHERF2 resulted in the accumulation of TRPV5 protein underneath the plasma membrane as most NHERF2 exhibited plasma membrane distribution (Fig. 1D). It is possible those TRPV5 proteins underneath the plasma membrane might be the reserved TRPV5 in the secretory pathway ready to be delivered to the plasma membrane. If this is the case, it is expected that the effect of WNK4 will be augmented by NHERF2 as the secretory pathway is a target of WNK4 [6]. Indeed, we observed a significant reduction of the accumulation of TRPV5 underneath the plasma membrane and an increase in the TRPV5 at the plasma membrane in the presence of WNK4 (Fig. 1D). We also observed a decreased plasma membrane staining of TRPV5 in the presence of NHERF2 (Fig. 1D); this apparently contradicts to the increased TRPV5-mediated Ca2+ uptake in the presence of NHERF2 (Fig. 1B). An explanation is that binding with NHERF2 may alter the confirmation of the C-tail of TRPV5 or limited the accessibility of the C-tail to the anti-TRPV5 antibody, which recognize the C-tail of TRPV5. Another possibility is that NHERF2 may increase channel activity of TRPV5 since an increased single channel open probability of CFTR by NHERF1 was documented [17].
In summary, WNK4 and NHERF2 synergistically regulate TRPV5 by enhancing its forward trafficking and increasing its stability at plasma membrane, respectively. NHERF2 prevents the negative effect of WNK4 and sustains the positive aspect of WNK4-mediated regulation on TRPV5. The biphasic effects of WNK4 and the variable expression of NHERF2 and WNK related kinases in Xenopus oocytes likely contribute to the fluctuation of WNK4 effects on TRPV5 and other ion transporters and channels.
Research highlights.
NHERF2 but not NHERF1 enhances the positive effect of WNK4 on TRPV5.
The C-terminal PDZ binding motif of TRPV5 is required for the regulation by NHERF2.
WNK4 enhances forward trafficking and decreases stability of functional TRPV5.
NHERF2 stabilizes functional TRPV5 and prevents the negative effect of WNK4.
The synergistic regulation of WNK4 and NHERF2 maximizes overall activity of TRPV5.
Acknowledgments
We thank Drs. Xavier Jeunemaitre and Juliette Hadchouel for the WNK4 cDNA. Haiyan Jing is a recipient of a scholarship from China Scholarship Council. Wei Zhang is a recipient of postdoctoral fellowship award from the American Heart Association. This work was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072154) and from the American Heart Association Greater Southeast Affiliate (09GRNT2160024).
Abbreviations
- TRPV5/6
Transient Receptor Potential cation channel, Vanilloid subfamily, subtype 5/6
- NHERF1/2
Na+/H+ exchanger regulating factor 1/2
- WNK
With No Lysine (K)
- PDZ
PSD-95/Discs-large/ZO-1
- ENaC
Epithelial Na+ Channel
- ROMK
Renal Outer Medullary K+ channel
- SGK
Serum- and Glucocorticoid-induced Kinase
- HA tag
hemagglutinin epitope
- GST
Glutathione S-Transferase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boehmer C, Embark HM, Bauer A, Palmada M, Yun CH, Weinman EJ, Endou H, Cohen P, Lahme S, Bichler KH, Lang F. Stimulation of renal Na+ dicarboxylate cotransporter 1 by Na+/H+ exchanger regulating factor 2, serum and glucocorticoid inducible kinase isoforms, and protein kinase B. Biochem.Biophys. Res Commun. 2004;313:998–1003. doi: 10.1016/j.bbrc.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Boehmer C, Palmada M, Klaus F, Jeyaraj S, Lindner R, Laufer J, Daniel H, Lang F. The peptide transporter PEPT2 is targeted by the protein kinase SGK1 and the scaffolding protein NHERF2. Cell Physiol Biochem. 2008;22:705–714. doi: 10.1159/000185554. [DOI] [PubMed] [Google Scholar]
- 3.Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, Tse CM, Yun C, de Jonge HR, Donowitz M. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem. 2005;280:16642–16650. doi: 10.1074/jbc.M500505200. [DOI] [PubMed] [Google Scholar]
- 4.Embark HM, Setiawan I, Poppendieck S, van de Graaf SF, Boehmer C, Palmada M, Wieder T, Gerstberger R, Cohen P, Yun CC, Bindels RJ, Lang F. Regulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase isoforms SGK1 and SGK3 expressed in Xenopus oocytes. Cell Physiol Biochem. 2004;14:203–212. doi: 10.1159/000080329. [DOI] [PubMed] [Google Scholar]
- 5.Hung AY, Sheng M. PDZ Domains: Structural Modules for Protein Complex Assembly. Journal of Biological Chemistry. 2002;277:5699–5702. doi: 10.1074/jbc.R100065200. [DOI] [PubMed] [Google Scholar]
- 6.Jiang Y, Cong P, Williams SR, Zhang W, Na T, Ma HP, Peng JB. WNK4 regulates the secretory pathway via which TRPV5 is targeted to the plasma membrane. Biochem Biophys Res Commun. 2008;375:225–229. doi: 10.1016/j.bbrc.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, Ferguson WB, Peng JB. WNK4 enhances TRPV5-mediated calcium transport: potential role in hypercalciuria of familial hyperkalemic hypertension caused by gene mutation of WNK4. Am J Physiol Renal Physiol. 2007;292:F545–F554. doi: 10.1152/ajprenal.00187.2006. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Yang DK, So I. PDZ domain-containing protein as a physiological modulator of TRPV6. Biochem Biophys Res Commun. 2007;361:433–438. doi: 10.1016/j.bbrc.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Lee-Kwon W, Park JB, Ryu SH, Yun CH, Donowitz M. Ca2+-dependent inhibition of Na+/H+ exchanger 3 (NHE3) requires an NHE3-E3KARP-alpha-actinin-4 complex for oligomerization and endocytosis. J Biol Chem. 2002;277:23714–23724. doi: 10.1074/jbc.M200835200. [DOI] [PubMed] [Google Scholar]
- 10.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- 11.Lee-Kwon W, Kim JH, Choi JW, Kawano K, Cha B, Dartt DA, Zoukhri D, Donowitz M. Ca2+-dependent inhibition of NHE3 requires PKC alpha which binds to E3KARP to decrease surface NHE3 containing plasma membrane complexes. Am J Physiol Cell Physiol. 2003;285:C1527–C1536. doi: 10.1152/ajpcell.00017.2003. [DOI] [PubMed] [Google Scholar]
- 12.Mayan H, Vered I, Mouallem M, Tzadok-Witkon M, Pauzner R, Farfel Z. Pseudohypoaldosteronism type II: marked sensitivity to thiazides, hypercalciuria, normomagnesemia, and low bone mineral density. J Clin Endocrinol Metab. 2002;87:3248–3254. doi: 10.1210/jcem.87.7.8449. [DOI] [PubMed] [Google Scholar]
- 13.Mensenkamp AR, Hoenderop JG, Bindels RJ. TRPV5, the gateway to Ca2+ homeostasis. Handb Exp Pharmacol. 2007:207–220. doi: 10.1007/978-3-540-34891-7_12. [DOI] [PubMed] [Google Scholar]
- 14.Palmada M, Embark HM, Yun C, Bohmer C, Lang F. Molecular requirements for the regulation of the renal outer medullary K+ channel ROMK1 by the serum- and glucocorticoid-inducible kinase SGK1. Biochem Biophys Res Commun. 2003;311:629–634. doi: 10.1016/j.bbrc.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 15.Palmada M, Poppendieck S, Embark HM, van de Graaf SF, Boehmer C, Bindels RJ, Lang F. Requirement of PDZ domains for the stimulation of the epithelial Ca2+ channel TRPV5 by the NHE regulating factor NHERF2 and the serum and glucocorticoid inducible kinase SGK1. Cell Physiol Biochem. 2005;15:175–182. doi: 10.1159/000083650. [DOI] [PubMed] [Google Scholar]
- 16.Peng JB, Warnock DG. WNK4-mediated regulation of renal ion transport proteins. Am J Physiol Renal Physiol. 2007;293:F961–F973. doi: 10.1152/ajprenal.00192.2007. [DOI] [PubMed] [Google Scholar]
- 17.Raghuram V, Mak DO, Foskett JK. Regulation of cystic fibrosis transmembrane conductance regulator single-channel gating by bivalent PDZ-domain-mediated interaction. Proc Natl Acad Sci USA. 2001;98:1300–1305. doi: 10.1073/pnas.031538898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA. 2007;104:4025–4029. doi: 10.1073/pnas.0611728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Swiatecka-Urban A, Duhaime M, Coutermarsh B, Karlson KH, Collawn J, Milewski M, Cutting GR, Guggino WB, Langford G, Stanton BA. PDZ domain interaction controls the endocytic recycling of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:40099–40105. doi: 10.1074/jbc.M206964200. [DOI] [PubMed] [Google Scholar]
- 20.Wade JB, Welling PA, Donowitz M, Shenolikar S, Weinman EJ. Differential renal distribution of NHERF isoforms and their colocalization with NHE3, ezrin, and ROMK. Am J Physiol Cell Physiol. 2001;280:C192–C198. doi: 10.1152/ajpcell.2001.280.1.C192. [DOI] [PubMed] [Google Scholar]
- 21.Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science. 2001;293:1107–1112. doi: 10.1126/science.1062844. [DOI] [PubMed] [Google Scholar]
- 22.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na+/H+ exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem. 2002;277:7676–7683. doi: 10.1074/jbc.M107768200. [DOI] [PubMed] [Google Scholar]
- 23.Yun CC, Palmada M, Embark HM, Fedorenko O, Feng Y, Henke G, Setiawan I, Boehmer C, Weinman EJ, Sandrasagra S, Korbmacher C, Cohen P, Pearce D, Lang F. The serum and glucocorticoid-inducible kinase SGK1 and the Na+/H+ exchange regulating factor NHERF2 synergize to stimulate the renal outer medullary K+ channel ROMK1. J Am Soc Nephrol. 2002;13:2823–2830. doi: 10.1097/01.asn.0000035085.54451.81. [DOI] [PubMed] [Google Scholar]
- 24.Zhang W, Na T, Wu G, Jing H, Peng JB. Down-regulation of intestinal apical calcium entry channel TRPV6 by ubiquitin E3 ligase Nedd4-2. J Biol Chem. 2010;285:36586–36596. doi: 10.1074/jbc.M110.175968. [DOI] [PMC free article] [PubMed] [Google Scholar]