Abstract
Ehrlichia are obligately intracellular bacteria that reside in a vacuole in the cytoplasm of phagocytes. We determined by confocal microscopy the interaction between Ehrlichia and mitochondria in DH82 cells to investigate the mechanism of Ehrlichia survival inside the phagocyte. The most remarkable finding of our study was that Ehrlichia morulae interacted with mitochondria and inhibited mitochondrial metabolism,. We showed that in E. chaffeensis-infected DH82 cells, mitochondria did not incorporate BrdU and transcriptional level of the mitochondrial gene NADPH2 was significantly reduced, indicating the inhibition of mitochondrial metabolism. This study demonstrates that Ehrlichia are able to inhibit mitochondrial activities, and it opens up a new avenue for the study of Ehrlichia pathogenesis.
Keywords: Ehrlichia, mitochondria, ehrlichiosis
1. Introduction
Ehrlichia chaffeensis causes a life-threatening disease, human monocytic ehrlichiosis, which is an emerging infectious disease prevalent in the southern, eastern, and south-central United States[1]. E. chaffeensis is an obligatory intracellular bacterium with a remarkable tropism for monocytes and macrophages. The primary function of the monocyte and macrophage is to destroy engulfed bacteria. However, not only are Ehrlichia organisms not destroyed by phagocytes, but they actually thrive inside macrophages [1]. The key to their success in survival inside the host cell is to prevent fusion of the phagosome and lysosome. Another factor that contributes to the successful intracellular survival of Ehrlichia is inhibition of host cell apoptosis[2]. Ehrlichia has a developmental cycle that takes at least 72 h to complete [3]. Therefore, Ehrlichia must prevent host cell apoptosis in response to Ehrlichia infection. However, the mechanism of Ehrlichia organisms preventing fusion of the phagosome and lysosome and the mechanisms of Ehrlichia inhibiting apoptosis are not known. Understanding the structure of the Ehrlichia vacuole and its interaction with host cell organelles is essential for revealing the mechanisms of Ehrlichia surviving inside phagocytes. In this study we analyzed the interaction of the E. chaffeensis vacuole with mitochondria. The most remarkable finding of our study is that Ehrlichia interact with mitochondria and inhibit mitochondrial activities.
2. Materials and methods
2.1. Cultivation of Ehrlichia and preparation of antigen slides for confocal microscopy
E. chaffeensis (Arkansas strain) was cultivated in DH82 cells, a canine histiocyte cell line. To prepare cell-free Ehrlichia inocula, Ehrlichia-infected cells in a T25 flask were harvested by centrifugation at 600 xg for 10 min. The pellet was resuspended in 5 ml of MEM without bovine serum, and the cells were broken with glass beads by vortexing. The cell debris was removed by centrifugation at 200 xg for 10 min. The supernatant containing cell-free Ehrlichia (0.4 ml) was inoculated into each well of Labtech II 8-well chamber slides (Nalgene Nunc, Naperville, Illinois), which contained a cell monolayer of DH82 cells. The 8-well chamber slides were incubated at 37 °C for 3 days in a 5% CO2 atmosphere. Ehrlichia-infected cells were permeabilized with acetone-methanol (1:1) for 10 minutes at −20°C. The slides were kept at −20°C until use and were rehydrated for 1 h at room te mperature with PBS before use. The slides were blocked with 1% BSA for 1 h at room temperature and then incubated with mouse monoclonal antibodies to mitochondria (Abcam, Cambridge, MA) and subsequently with corresponding secondary antibodies for 1 hour at 37 °C each.
2.2. BrdU and aphidicolin treatment of DH82 cells
Aphidicolin inhibits DNA polymerase-α, and is a potent inhibitor of nuclear DNA synthesis, but has no effect on mitochondrial DNA synthesis because the replication of mitochondrial DNA requires DNA polymerase-γ not -α [4]. To observe mitochondrial DNA synthesis, two days after E. chaffeensis infection, infected DH82 cells or mock-infected DH82 cells were treated with aphidicolin (20 μM) (Sigma) for 1 hour, and then BrdU (Sigma) was added at a final concentration of 15 μM. After incubation at 37°C for 24 h, the cells were stained with MitoTracker Orange. Cellular DNA was denatured by incubation in 2 N HCl at 37°C for 1 h, and acid was neutralized with 100 mM borate buffer, pH 8.5. The slides were blocked with 1% BSA and incubated with a mouse anti-BrdU monoclonal antibody (Sigma) and FITC-labeled anti-mouse IgG. The cells were stained with DAPI before observation by confocal microscopy. To observe nuclear DNA synthesis, the cells were labeled with BrdU without aphidicolin treatment, and the nuclear DNA was stained with anti-BrdU antibody without DNA denaturation.
2.3. Confocal microscopy
The slides were examined with a Olympus DSU-IX81 Spinning Disk Confocal Microscope in the Center for Biomedical Engineering at the University of Texas Medical Branch. The images were analyzed using the Olympus Fluoview Ver. 2.0b Viewer.
2.4. JC-1 staining
DH82 cells cultivated in 8-chamber slides were stained with JC-1 following the instructions of the manufacturer (Invitrogen). Fluorescence of stained cells was observed using an Olympus IX70 Inverted Microscope.
2.5. Transmission electron microscopy (TEM)
T25 flasks containing monolayers of DH82 cells were inoculated with E. chaffeensis at an average number of 50 bacteria per host cell. The cells were incubated in Minimum Essential Medium (MEM) with 5% bovine calf serum at 37 °C with 5% CO2 for 72 hours. The cells were fixed for TEM with 2.5% formaldehyde prepared from paraformaldehyde, and 0.1% glutaraldehyde in 0.05 M cacodylate buffer pH 7.3 to which 0.03% trinitrophenol and 0.03% CaCl2 were added. After fixation the cells were washed with 0.1 M cacodylate buffer, scraped off the flasks and pelleted. The pellets were post-fixed in 1% OsO4 in 0.1 M cacodylate buffer, en bloc stained with 2% aqueous uranyl acetate, dehydrated in ethanol and embedded in Poly/Bed 812 (Polysciences, Warrington, PA). Ultrathin sections were cut on a Reichert-Leica Ultracut S ultramicrotome, stained with lead citrate and examined in a Philips 201 or CM-100 electron microscope at 60 kV.
Quantitative real time PCR. RNA was extracted from E. chaffeensis-infected and mock-infected DH82 cells using the RNeasy Mini kit (Qiagen). One step of reverse quantitative PCR was performed using iScript One Step RT-PCR Kit with SYBR Green (Bio-Rad). PCR primers for the NADPH2 gene (GCCCACATAGGCTGAATAGC and ATAGGGTCGTGGTGGATGAG) were designed from the canine mitochondrial genome sequence (EU789780.1). Primers for the canine GAPDH gene (TGTCCCCACCCCCAATGTATC and CTCCGATGCCTGCTTCACTACCTT ) were described previously [5].
3. Results
3.1. E. chaffeensis morulae recruited mitochondria
In uninfected DH82 cells mitochondria are evenly distributed in the perinuclear region (Fig 1). In E. chaffeensis-infected DH82 cells, E. chaffeensis morulae were surrounded by mitochondria. Actually, most of the mitochondria in the host cells concentrated around E. chaffeensis morulae rather than in the cytoplasm around the cell nucleus (Fig.1). Next we addressed the question whether mitochondria are specifically recruited by E. chaffeensis. We infected DH82 cells with E. chaffeensis and fluorescence-labeled 1 μm latex beads at the same time (Figure 1) Confocal microscopy showed that E. chaffeensis and mitochondria were colocalized, but phagocytosed beads did not colocalize with mitochondria (Fig.1). A 3D reconstruction by confocal microscopy further showed that host cell mitochondria concentrate around Ehrlichia morulae (Fig 2). TEM results also confirmed that most of mitochondria were closely associated with E. chaffeensis morulae and free mitochondria were scarce in the cytoplasm in the infected DH82 cells (Fig 3). Thus, mitochondria are specifically recruited by E. chaffeensis.
Fig. 1.
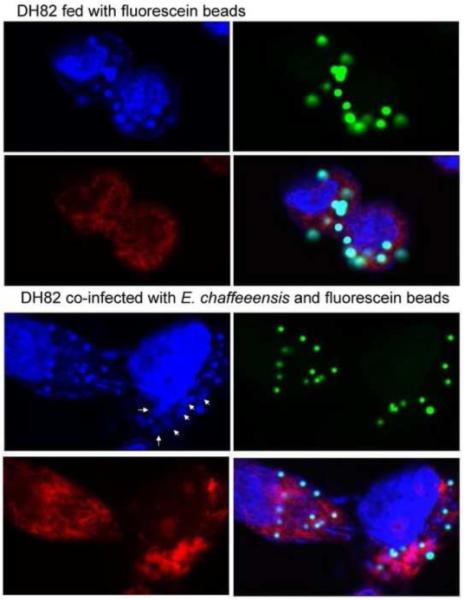
Confocal microscopy of E. chaffeensis-infected DH82 cells. DH82 cells were co-infected with E. chaffeensis and fluorescein-labeled 1 μm plastic beads. At 72 hours after infection, cells were stained with DAPI and antibody to mitochondria. The confocal images were (A) DAPI-stained nuclei and ehrlichial morulae (Blue); (B) fluorescein-labeled beads (green); (C) Antibody stained mitochondria (Red); , and (D) merged image of A, B, and C. Mitochondria in the cells were colocalized with E. chaffeensis morulae (arrow), but did colocalize with plastic beads.
Fig. 2.
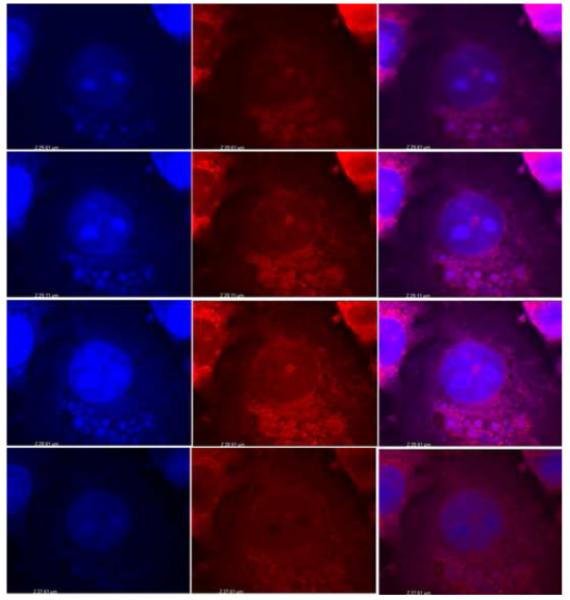
The 3D reconstruction E. chaffeensis infected DH82 cells. Nuclei and Ehrlichia were stained by DAPI (Blue) and mitochondria were stained with antibody specific to mitochondria (Red). Three sections (29.61 μM, 29.11 μM, and 28.11 μM) were shown to indicate that E. chaffeensis are associated with mitochondria.
Fig 3.
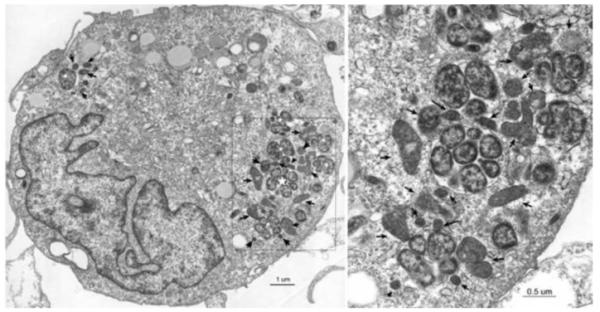
Electron micrograph of E. chaffeensis infected DH82 cells. Arrows indicate mitochondria, and white stars indicate ehrlichial morulae. The area in the box in the picture on the left was enlarged in the picture on the right.
3.2. E. chaffeensis inhibit mitochondrial metabolism
We used BrdU incorporation to determine whether mitochondria surrounding E. chaffeensis morulae are metabolically active. The mitochondria incorporating BrdU actively synthesize DNA, and the ones that do not incorporate BrdU are not synthesizing DNA. We first labeled the nuclei of DH82 cells with BrdU without inhibition of DNA synthesis by aphidicolin. The nuclei of E. chaffeensis-infected and mock-infected DH82 were labeled intensely by BrdU, suggesting that E. chaffeensis did not inhibit DNA synthesis of host cell nuclei. Compared to the nuclei, the mitochondria contained little DNA, and mitochondria were invisible compared with the intense green color of nuclei (Figure 4A). Therefore, we used aphidicolin to inhibit DNA synthesis in host cell nuclei, which did not affect mitochondrial DNA synthesis. After aphidicolin treatment, nuclei were not stained by BrdU, but mitochondria in mock-infected DH82 cells were labeled brightly by anti-BrdU antibody, suggesting that mitochondria actively incorporated BrdU to synthesize mitochondrial DNA in uninfected DH82 cells (Fig.4 B). Conversely, in E. chaffeensis-infected DH82 cells, very few mitochondria were labeled with anti-BrdU antibody, suggesting that most mitochondria in infected DH82 cells were not synthesizing DNA (Fig. 4C).
Fig.4.
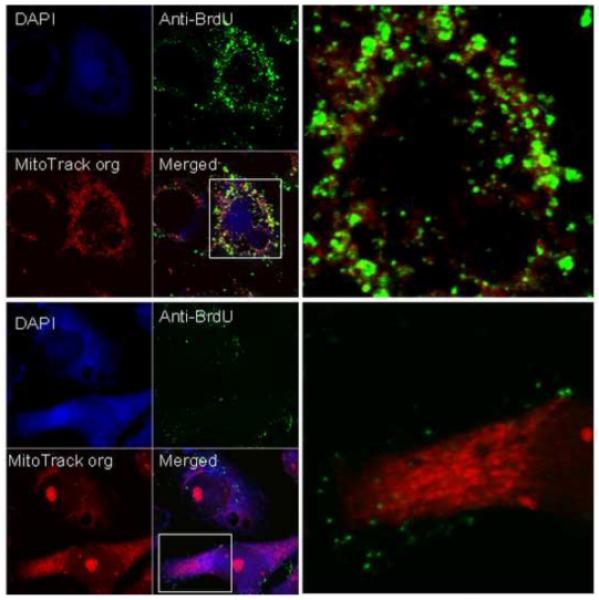
Confocal microscopy of BrdU-labeled DH82 cell nuclei and mitochondria. In all pictures, blue color is DAPI-stained nuclei or E. chaffeensis (arrows), red is MitoTrack orange stained-mitochondria and green is BrdU-labeled nuclei or mitochondria. A, E. chaffeensis-infected (left) and mock-infected DH82 cells without aphidicolin treatment. B and C, E. chaffeensis-infected and mock-infected DH82 cells were treated with aphidicolin. The areas in the boxes are enlarged on the right.
3.3. Analysis of mitochondrial metabolism by real time PCR
To determine the inhibition by E. chaffeensis of nuclear gene transcription and mitochondrial gene transcription, we determined the transcription levels of the housekeeping gene GAPDH (a nuclear gene) and the mitochondrial genes nadph2 and cytB. Real time PCR demonstrated that the transcriptional levels of the nuclear GAPDH gene and mitochondrial genes nadph2 and cytochrome b (cyt b) were decreased from the second day to the third day after infection. However, the transcriptional level of mitochondrial genes was significant (p<0.01) at all time points from the second day after infection compared to the nuclear GAPDH gene. (Fig. 5).
Fig 5.
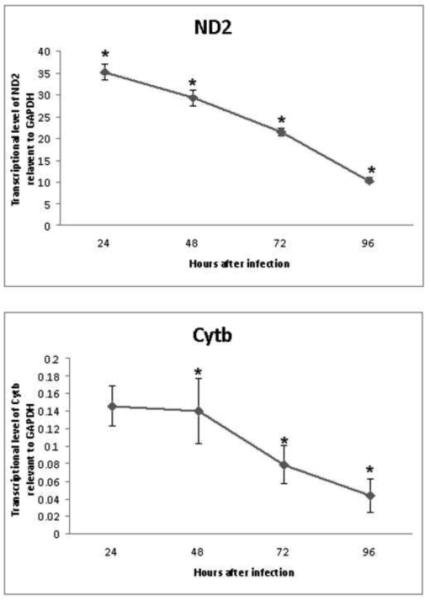
Real time PCR analysis of E. chaffeensis inhibition of nuclear and mitochondrial gene transcription. RNA was extracted from DH82 cells harvested at 24, 48, 72, and 96 hours after E. chaffeensis infection and used as template for reverse transcription. The cDNA was amplified by real time PCR using primers from the nuclear GAPDH gene and mitochondrial genes nadph2 (ND) and cyt b. The relevant transcriptional level of mitochondrial gene were calculated by the transcriptional level of mitochondrial gene/ the transcriptional level of nuclear gene GAPDH. * indicating significant difference of transcriptional levels between mitochondrial NADPH or Cyt b gene and nuclear GAPDH gene.
3.4. Mitochondrial membrane permeability in E. chaffeensis-infected DH82 cells
We further determined whether E. chaffeensis affected the mitochondrial membrane permeability. Mitochondrial membrane potential, Δψm, is an important parameter of mitochondrial function used as an indicator of cell health. In healthy cells with high mitochondrial Δψm, JC-1 spontaneously forms complexes known as J-aggregates with intense red fluorescence. On the other hand, in apoptotic or unhealthy cells with low Δψm, JC-1 remains in the monomeric form, which shows only green fluorescence [6-8]. We stained E. chaffeensis infected DH82 cells with JC-1 at 24, 48, 72 and 96 hours after infection. Mock-infected DH82 cells were stained at the same time points as controls. All E. chaffeensis-infected DH82 showed intense red fluorescence at all time points from 24 to 120 hours after infection despite the fact that all cells were heavily infected (Fig. 6). The intensity of red fluorescence was even stronger in E. chaffeensis-infected DH82 cells than in mock-infected DH82 cells at 120 hours. After 48 hours of infection, E. chaffeensis-infected DH82 monocytes were differentiated into macrophages, which firmly attached to the flask by elongated pseudopodia. Most of E. chaffeensis-infected cells contained multiple nuclei at 120 hours after infection (Fig. 6). At all time points uninfected DH82 cells remained as monocytes and became condensed after 72 hours (Fig. 6). These results suggest that in E. chaffeensis-infected DH82 cells mitochondria maintained a normal Δψm and E. chaffeensis inhibited apoptosis of host cells.
Figure 6.
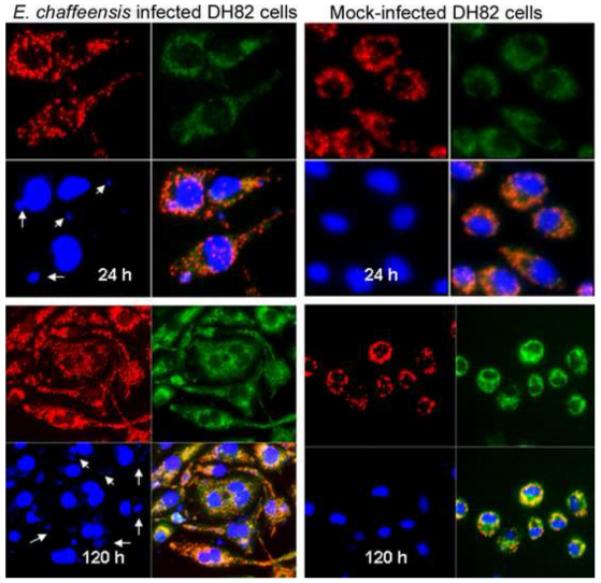
JC-1 stained E. chaffeensis infected DH82 cells. The images are representatives of E. chaffeensis-infected or mock-infected DH82 at 24 and 120 hours after infection. Red fluorescence shows JC-1 stained mitochondria; green florescence shows JC-1 stained cytoplasm; and blue color is DAPI-stained nuclei and E. chaffeensis morulae (arrows). The image on the bottom-right of each panel is the merged picture. Mock-infected DH82 cells remain as monocytes and became condensed at day 5, but E. chaffeensis-infected DH82 cells became elongated macrophages with multiple nuclei in each cell, suggesting that the infected cells were still multiplying after 5 days of infection in spite of heavy infection with E. chaffeensis. The panels on top are at the same magnification, and the panels on bottom are at the same magnification.
4. Discussion
Ehrlichia morulae had been found to be surrounded by mitochondria previously by electron microscopy [9]. Here we further investigated the association of Ehrlichia chaffeensis with mitochondria and whether Ehrlichia inhibited mitochondrial activity. We demonstrated that in Ehrlichia infected cells mitochondria redistributed around Ehrlichia morulae and mitochondrial metabolism was inhibited by Ehrlichia infection. In Ehrlichia infected cells mitochondria maintained membrane permeability. Stabilizing mitochondrial membrane permeability may be related to inhibition of apoptosis as been demonstrated in E. ewingii [10]. The mechanism of Ehrlichia inhibition of the host mitochondrial metabolism is not known currently and needs to be further investigated in vivo. Ehrlichia might secrete proteins that interact with mitochondria to stabilize the mitochondrial membrane permeability, which results in mitochondrial hibernation. A dormant mitochondrion may not trigger apoptosis in the infected cell, or it will not compete for nutrients with Ehrlichia. Although mitochondria are essential for producing energy, cells can survive without mitochondria by anaerobic respiration [11,12]. We hypothesize that in E. chaffeensis-infected cells, the cells may survive by anaerobic respiration because of inhibition of mitochondrial activities by E. chaffeensis.
Mitochondria have been reported to accumulate around the parasitophorous vacuole of obligate intracellular protozoa including Toxoplasma gondii and the intracellular bacteria Chlamydia psittaci and Legionella pneumophila [13-15]. The observation that mitochondria accumulate around the parasitophorous vacuole conveyed the impression that C. psittaci acquires ATP from mitochondria [16]. However, transfer of ATP from mitochondria to the parasitophorous vacuole is not required by either bacterium associated with mitochondria. Neither Legionella pneumophila nor Ehrlichia apparently needs ATP from the host cells because their genomes encode all the enzymes involved in ATP generation in the TCA cycle and these bacteria do not possess an ATP/ADP translocase for transporting ATP [17,18]. Therefore, these observations suggest that the association of bacteria with mitochondria is not for acquisition of ATP. Our study demonstrated that Ehrlichia recruit mitochondria to manipulate mitochondrial metabolism, which may results in inhibition of host cell apoptosis [19]. One mechanism utilized by Chlamydia to prevent apoptosis is the use of a secreted product to prevent cytochrome c release [19]. We hypothesize that the close apposition of mitochondria and intracellular bacteria such as Ehrlichia, Chlamydia and Legionella is required for bacteria to effectively deliver proteins to mitochondria to inhibit the mitochondrial apoptosis pathway.
5. Acknowledgement
This study was supported in part by a grant from National Institute of Allergy and Infectious Diseases (AI31431). The authors are grateful to Professor Thomas Albrecht (Department of Microbiology and Immunology, University of Texas Medical Branch, Galveston Texas) for technical assistance with confocal microscopic imaging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Referwnces
- [1].Paddock CD, Childs JE. Ehrlichia chaffeensis: a prototypical emerging pathogen. Clin. Microbiol. Rev. 2003;16:37–64. doi: 10.1128/CMR.16.1.37-64.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang JZ, Sinha M, Luxon BA, Yu XJ. Survival strategy of obligately intracellular Ehrlichia chaffeensis: novel modulation of immune response and host cell cycles. Infect. Immun. 2004;72:498–507. doi: 10.1128/IAI.72.1.498-507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Zhang JZ, Popov VL, Gao S, Walker DH, Yu XJ. The developmental cycle of Ehrlichia chaffeensis in vertebrate cells. Cell Microbiol. 2007;9:610–618. doi: 10.1111/j.1462-5822.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- [4].Zimmermann W, Chen SM, Bolden A, Weissbach A. Mitochondrial DNA replication does not involve DNA polymerase alpha. J. Biol. Chem. 1980;255:11847–11852. [PubMed] [Google Scholar]
- [5].Schlotter YM, Veenhof EZ, Brinkhof B, Rutten VP, Spee B, Willemse T, Penning LC. A GeNorm algorithm-based selection of reference genes for quantitative real-time PCR in skin biopsies of healthy dogs and dogs with atopic dermatitis. Vet. Immunol. Immunopathol. 2009;129:115–118. doi: 10.1016/j.vetimm.2008.12.004. [DOI] [PubMed] [Google Scholar]
- [6].Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1) Biochem. Biophys. Res. Commun. 1993;197:40–45. doi: 10.1006/bbrc.1993.2438. [DOI] [PubMed] [Google Scholar]
- [7].Reers M, Smith TW, Chen LB. J-aggregate formation of a carbocyanine as a quantitative fluorescent indicator of membrane potential. Biochemistry. 1991;30:4480–4486. doi: 10.1021/bi00232a015. [DOI] [PubMed] [Google Scholar]
- [8].Smiley ST, Reers M, Mottola-Hartshorn C, Lin M, Chen A, Smith TW, Steele GD, Jr., Chen LB. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. U. S. A. 1991;88:3671–3675. doi: 10.1073/pnas.88.9.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Popov VL, Chen SM, Feng HM, Walker DH. Ultrastructural variation of cultured Ehrlichia chaffeensis. J. Med. Microbiol. 1995;43:411–421. doi: 10.1099/00222615-43-6-411. [DOI] [PubMed] [Google Scholar]
- [10].Xiong Q, Bao W, Ge Y, Rikihisa Y. Ehrlichia ewingii infection delays spontaneous neutrophil apoptosis through stabilization of mitochondria. J Infect Dis. 2008;197:1110–1118. doi: 10.1086/533457. [DOI] [PubMed] [Google Scholar]
- [11].Desjardins P, Frost E, Morais R. Ethidium bromide-induced loss of mitochondrial DNA from primary chicken embryo fibroblasts. Mol. Cell Biol. 1985;5:1163–1169. doi: 10.1128/mcb.5.5.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vergani L, Prescott AR, Holt IJ. Rhabdomyosarcoma rho(0) cells: isolation and characterization of a mitochondrial DNA depleted cell line with ‘muscle-like’ properties. Neuromuscul. Disord. 2000;10:454–459. doi: 10.1016/s0960-8966(00)00096-1. [DOI] [PubMed] [Google Scholar]
- [13].Matsumoto A, Bessho H, Uehira K, Suda T. Morphological studies of the association of mitochondria with chlamydial inclusions and the fusion of chlamydial inclusions. J. Electron Microsc. (Tokyo) 1991;40:356–363. [PubMed] [Google Scholar]
- [14].Tanabe K. Visualization of the mitochondria of Toxoplasma gondii-infected mouse fibroblasts by the cationic permeant fluorescent dye rhodamine 123. Experientia. 1985;41:101–102. doi: 10.1007/BF02005897. [DOI] [PubMed] [Google Scholar]
- [15].Chong A, Lima CA, Allan DS, Nasrallah GK, Garduno RA. The purified and recombinant Legionella pneumophila chaperonin alters mitochondrial trafficking and microfilament organization. Infect Immun. 2009;77:4724–4739. doi: 10.1128/IAI.00150-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peterson EM, de la Maza LM. Chlamydia parasitism: ultrastructural characterization of the interaction between the chlamydial cell envelope and the host cell. J. Bacteriol. 1988;170:1389–1392. doi: 10.1128/jb.170.3.1389-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mavromatis K, Doyle CK, Lykidis A, Ivanova N, Francino MP, Chain P, Shin M, Malfatti S, Larimer F, Copeland A, Detter JC, Land M, Richardson PM, Yu XJ, Walker DH, McBride JW, Kyrpides NC. The genome of the obligately intracellular bacterium Ehrlichia canis reveals themes of complex membrane structure and immune evasion strategies. J. Bacteriol. 2006;188:4015–4023. doi: 10.1128/JB.01837-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chien M, Morozova I, Shi S, Sheng H, Chen J, Gomez SM, Asamani G, Hill K, Nuara J, Feder M, Rineer J, Greenberg JJ, Steshenko V, Park SH, Zhao B, Teplitskaya E, Edwards JR, Pampou S, Georghiou A, Chou IC, Iannuccilli W, Ulz ME, Kim DH, Geringer-Sameth A, Goldsberry C, Morozov P, Fischer SG, Segal G, Qu X, Rzhetsky A, Zhang P, Cayanis E, De Jong PJ, Ju J, Kalachikov S, Shuman HA, Russo JJ. The genomic sequence of the accidental pathogen Legionella pneumophila. Science. 2004;305:1966–1968. doi: 10.1126/science.1099776. [DOI] [PubMed] [Google Scholar]
- [19].Faherty CS, Maurelli AT. Staying alive: bacterial inhibition of apoptosis during infection. Trends Microbiol. 2008;16:173–180. doi: 10.1016/j.tim.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


