Abstract
The receptor protein tyrosine phosphatase PTPmu has a cell-adhesion molecule-like extracellular segment and a catalytically active intracellular segment. This structure gives PTPmu the ability to transduce signals in response to cell-cell adhesion. Full-length PTPmu is down-regulated in glioma cells by proteolysis which is linked to increased migration of these cells in the brain. To gain insight into the substrates PTPmu may be dephosphorylating to suppress glioma cell migration, we used a substrate trapping method to identify PTPmu substrates in tumor cell lines. We identified both PKCdelta and PLCgamma1 as PTPmu substrates. As PLCgamma1 activation is linked to increased invasion of cancer cells, we set out to determine whether PTPmu may be upstream of PLCgamma1 in regulating glioma cell migration. We conducted brain slice assays using U87-MG human glioma cells in which PTPmu expression was reduced by shRNA to induce migration. Treatment of the same cells with PTPmu shRNA and a PLCgamma1 inhibitor prevented migration of the cells within the brain slice. These data suggest that PLCgamma1 is downstream of PTPmu and that dephosphorylation of PLCgamma1 is likely to be a major pathway through which PTPmu suppresses glioma cell migration.
Keywords: Receptor protein tyrosine phosphatase, Phospholipase C, Protein kinase C delta, RACK1, Cell migration, Glioblastoma, Tyrosine phosphorylation
INTRODUCTION
Glioblastoma multiforme, also referred to as grade IV astrocytoma, is the most common primary brain tumor in adults. Glioblastomas are particularly devastating due to their highly dispersive nature, which makes them resistant to the most advanced current therapies [Furnari et al., 2007]. Therefore, understanding the mechanism of glioma dispersal is key to designing effective therapeutics that limit the spread of disease throughout the brain.
Dispersal of glioma cells into the brain parenchyma involves a series of interactions with both the extracellular matrix (ECM) and neighboring cells. These interactions and the biochemical events that follow, result in changes to tumor cell surface receptors and remodeling of the ECM by proteolysis [Furnari et al., 2007; Nakada et al., 2007; Rao, 2003; Sala et al., 2008; Teodorczyk and Martin-Villalba, 2010]. Gliomas overexpress growth factor receptor tyrosine kinases (RTKs) and their ligands, creating a situation for autocrine and paracrine stimulation of cell survival and migration [Furnari et al., 2007; Kanu et al., 2009; Nakada et al., 2007]. The pathways initiated in response to RTK activation have been well described and the role these signaling pathways play in tumor progression is established. In addition to the role of amplified and mutated RTKs in human cancers, receptor protein tyrosine phosphatases (RPTPs) are also implicated in regulating tumor progression [Ostman et al., 2006]. However, in contrast to the signaling downstream of RTKs, the signaling pathways and substrates downstream of most receptor tyrosine phosphatases remain largely unknown.
RPTPs are transmembrane PTPs. The type IIb RPTP subfamily member, PTPμ, has cell adhesion molecule-like domains in its extracellular segment and two phosphatase domains in its intracellular segment. Only the membrane proximal (first) phosphatase domain has been demonstrated to have catalytic activity as there is a naturally occurring mutation in the second phosphatase domain which renders it catalytically inactive. PTPμ is capable of mediating cell-cell adhesion [Brady-Kalnay et al., 1993; Gebbink et al., 1993] and neurite outgrowth [Burden-Gulley and Brady-Kalnay, 1999]. In addition, PTPμ regulates cadherin-dependent adhesion [Hellberg et al., 2002] and neurite outgrowth [Burden-Gulley and Brady-Kalnay, 1999; Oblander et al., 2007; Oblander and Brady-Kalnay, 2010]. Recently, we found that full-length PTPμ is downregulated in primary human glioblastomas by proteolytic cleavage generating both and extracellular and an intracellular fragment [Burden-Gulley et al., 2010; Burgoyne et al., 2009a; Burgoyne et al., 2009b]. While over-expression of full-length PTPμ protein reduces glioma cell migration [Burgoyne et al., 2009b], the presence of a proteolytically cleaved intracellular fragment of PTPμ, capable of translocating to the nucleus, increases glioma cell migration [Burgoyne et al., 2009b]. These results imply that the presence of full-length PTPμ suppresses migration.
Studies from our laboratory demonstrated that catalytic activity of PTPμ is essential for regulating PTPμ-mediated processes [Burden-Gulley and Brady-Kalnay, 1999; Ensslen-Craig and Brady-Kalnay, 2005; Oblander et al., 2007]. To gain a better understanding of PTPμ function, a number of direct PTPμ binding proteins and potential substrates have been identified including, RACK1, p120, IQGAP1 and BCCIP [Mourton et al., 2001; Phillips-Mason et al., 2006; Phillips-Mason et al., 2008; Zondag et al., 2000]. RACK1, receptor for activated C kinase, is a WD-40 repeat protein that binds to protein kinase C (PKC), src family kinases, phospholipase C γ1 (PLCγ1) and cyclic AMP-specific phosphodiesterase PDE4 family members, among other proteins [McCahill et al., 2002]. RACK1 is thought to function as a scaffolding protein to bring signaling proteins in close proximity to one another. Both the association of RACK1 with PKCδ and PKCδ activity are required for the PTPμ-dependent regulation of axonal migration [Ensslen and Brady-Kalnay, 2004; Rosdahl et al., 2002]. BCCIP and IQGAP1 are required for PTPμ-dependent axonal migration [Phillips-Mason et al., 2006; Phillips-Mason et al., 2008] and BCCIP has been identified as a substrate for PTPμ in vitro [Phillips-Mason et al., 2008].
The mechanism by which PTPμ is able to suppress glioma cell migration and dispersal is not known. In this study we performed “substrate trapping” experiments aimed at identifying PTPμ substrates involved in the regulation of cell migration. This experimental approach has proven to be successful in identifying substrates for other protein tyrosine phosphatases (methods reviewed in [Blanchetot et al., 2005]). In this manuscript, we determined that both PKCδ and PLCγ1 are substrates of PTPμ in vitro.
PLCγ is a key regulator of cell migration downstream of RTK signaling [Piccolo et al., 2002; Wells and Grandis, 2003]. Phosphorylation on tyrosine residue 783 of PLCγ1 is critical to its activation [Poulin et al., 2005; Sekiya et al., 2004; Yu et al., 1998]. To ascertain whether PLCγ1 is a downstream target of PTPμ involved in suppression of glioma cell invasion, we conducted brain slice assays using U-87 MG glioma cells in which PTPμ protein had been knocked-down using shRNA. Reduced PTPμ expression in U-87 MG cells results in an increase in cell migration and dispersal [Burgoyne et al., 2009a]. In this manuscript, we demonstrate that addition of a PLCγ1 inhibitor reverses this phenotype, and reduces glioma cell migration. These data imply that PLCγ1 is functionally downstream of PTPμ. We hypothesize that PTPμ dephosphorylation of PLCγ1 on residue Y783 prevents PLCγ activation thus blocking PLCγ1-activated remodeling of the cytoskeleton and therefore, migration of glioma cells.
MATERIALS AND METHODS
CELL CULTURE
A549 non-small cell lung carcinoma cells were obtained from American Type Culture Collection (ATCC, Manassas, VA) and maintained in F12 media (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT), and 2mM L-glutamine (Invitrogen) at 37°C and 5% CO2. U-87 MG and LN-229 human glioma cell lines were obtained from ATCC and maintained in DMEM (Invitrogen) supplemented with 10% fetal bovine serum at 37°C and 5% CO2. Sf9 insect cells (CRL 1711; ATCC) were maintained at 27°C in Grace's insect media (Invitrogen) supplemented with 10% fetal bovine serum and 1μg/ml gentamicin.
REAGENTS
The SK18 monoclonal antibody directed against the intracellular domain of PTPμ has been described previously [Brady-Kalnay et al., 1993; Brady-Kalnay and Tonks, 1994]. A polyclonal antibody to PKCδ and a monoclonal anti-phosphotyrosine antibody (PY99) were obtained from Santa Cruz (Santa Cruz, CA). The polyclonal, phospho-specific antibody to Y311 of PKCδ was obtained from BioSource (Camarillo, CA). Monoclonal antibodies to PLCγ1 and RACK1 as well as polyclonal, phospho-specific antibodies to Y783 and Y771 of PLCγ1 were obtained from BD Transduction Laboratories (San Diego, CA). A monoclonal antibody to vinculin was purchased from Sigma (St. Louis, MO). U-73122, a specific inhibitor of PLCγ1 activity, was purchased from Calbiochem (La Jolla, CA). Purified, active His-tagged PLCγ1 was purchased from Calbiochem. Purified, active His-PKCδ, active Src and active GST-epidermal growth factor receptor (EGFR) were purchased from Upstate Biosciences (Lake Placid, NY).
EXPRESSION OF GST FUSION PROTEINS IN ESCHERICHIA COLI
The intracellular segment of PTPμ contains two phosphatase domains. Only the membrane proximal (first) phosphatase domain has been demonstrated to have catalytic activity. For our studies we used intracellular constructs of PTPμ containing the juxtamembrane domain and the first phosphatase domain. iPTPμWT-ΔD2 and iPTPμDA-ΔD2 GST constructs have been described previously [Phillips-Mason et al., 2006]. iPTPμDA-ΔD2 contains a D1063A mutation in the first phosphatase domain. Plasmids containing the intracellular PTPμ GST fusion proteins (iPTPμWT-ΔD2 and iPTPμDA-ΔD2) or GST alone were expressed in E. coli under the regulation of the lac promoter. GST and GST fusion proteins were isolated from E.Coli using glutathione Sepharose 4B beads (Amersham Biosciences). Catalytically active iPTPμ GST fusion proteins used in substrate trapping experiments were isolated as previously described [Phillips-Mason et al., 2006]. Briefly, bacteria were resuspended in 10ml of buffer (0.1 M NaCl, 10 mM Tris-HCl at pH 8.0, and 1 mM EDTA) and incubated on ice for 15 minutes. To lyse bacterial cells, 1 ml of 0.5 M EDTA, 1.1 ml of 20% Triton X-100, 55 μl of 1M dithiothreitol, 10 μl of ß-mercaptoethanol, 100 μl of 100 mM phenylmethylsulfonylfluoride, and 30 μl of protease inhibitor cocktail (Sigma) was added to 10 ml of resuspended cells. Cells were sonicated and spun at 15,000 rpm for 25 minutes. GST fusion proteins were isolated from the cleared supernatant using glutathione Sepharose beads. GST and GST-iPTPμWT used for in vitro binding assays with PLCγ1 and RACK1 (described below) were isolated in PBST (PBS, 1% Triton X-100, 1 mM benzamidine, 5 μg/ml aprotinin and leupeptin and 1 μg/ml pepstatin). Protein expression and concentration of GST proteins was determined by Coomassie stain using BSA as a protein standard.
SUBSTRATE TRAPPING EXPERIMENTS
A549, U-87 MG and LN-229 cells were grown to 85–95% confluence and treated with or without pervanadate (100 μM) for 20 minutes (Sodium orthovanadate is activated with hydrogen peroxide to make the cell-permeable, tyrosine phosphatase inhibitor, pervanadate). Cells were collected by scraping into lysis buffer containing 20 mM Hepes at pH 7.5, 1% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, 1 mM benzamidine, 5 μg/ml aprotinin and leupeptin, 1 μg/ml pepstatin and 5 mM iodoaceteic acid (IAA) to inhibit any endogenous phosphatases. Cell lysates were vortexed and incubated on ice for 15 minutes. Dithiothreitol was added to a final concentration of 10 mM and cell lysates were incubated on ice for an additional 15 minutes and then centrifuged at 3000 rpm for 3 minutes. Protein concentration of the cell lysates was determined using the BCA™ Protein Assay Kit (Pierce, Rockford, IL) and equal amounts of protein (800 μg-1 mg) were added to equal amounts of GST alone or GST fusion proteins adsorbed on glutathione Sepharose. Samples were rocked for two hours at 4°C, washed four times with lysis buffer without IAA and incubated with 2× SDS sample buffer. One-third of the sample was resolved by SDS-PAGE and transferred to nitrocellulose for immunoblotting as described previously [Phillips-Mason et al., 2006]. The substrate trapping pull down assays were repeated a minimum of two times from each of the three cell lines used. Therefore, each protein described has been identified as a PTPμ interacting protein a minimum of six times.
IN VITRO KINASE AND PHOSPHATASE ASSAYS
Purified PKCδ was phosphorylated in vitro using Src tyrosine kinase as described below. PKCδ (2 μg) was incubated with 15U of active Src kinase for 1.5 hours at room temperature in Src kinase buffer (50 mM Hepes at pH 7.4, 50 mM NaCl, 5 mM MgCl2, 5 mM MnCl2 and 1 mM ATP). After the kinase reaction was complete, the entire reaction volume (40μl) was diluted 1:20 with phosphatase buffer (25 mM Hepes at pH 7.4, 50 mM NaCl and 5 mM DTT). Then, 250 ng of tyrosine phosphorylated PKCδ in 100 μl of phosphatase buffer was incubated with 7, 15, or 30 μg of active, GST-iPTPμWT or GST-iPTPμDA, on glutathione Sepharose, for 15 minutes at 30°C. The phosphatase assay was stopped by adding 100 μl 2× SDS sample buffer and incubating the samples at 95°C for five minutes. Approximately 6 ng of PKCδ from each sample was resolved by SDS-PAGE and transferred to nitrocellulose for immunoblot. Purified PLCγ1 was phosphorylated in vitro by purified, active GST-tagged epidermal growth factor receptor (EGFR) as follows. PLCγ1 (2 μg) was incubated with 400 ng (101 U/mg) EGFR for 10 minutes at 30°C in EGFR kinase buffer (25 mM Hepes at pH 7.4, 10 mM MgCl2, 2.5 mM MnCl2, 0.1 mM DTT, 0.2 % Triton X-100 and 25 μM ATP.) After the EGFR kinase reaction was complete, the entire reaction volume (40μl) was diluted 1:20 with phosphatase buffer (25 mM Hepes at pH 7.4, 50 mM NaCl and 5 mM DTT). The EGFR was removed from the reaction by a brief incubation (15 minutes) with 20 μl packed glutathione Sepharose. Then, 250 ng of tyrosine phosphorylated PLCγ1 in 100 μl of phosphatase buffer was used in a phosphatase assay as described above for PKCδ.
IN VITRO BINDING ASSAY
Equal amounts of GST or GST-iPTPμWT fusion protein adsorbed on glutathione Sepharose were incubated with purified His-tagged proteins (PLCγ1 and RACK1) individually or in combination. Incubations were performed in PBS, 1% Triton X-100, 1 mM benzamidine, 5 μg/ml aprotinin and leupeptin and 1 μg/ml pepstatin. Samples were rocked at 4°C for two hours, washed 4 times with buffer and incubated at 95°C for five minutes in 2× SDS sample buffer. One fourth of the sample was resolved by SDS-PAGE and transferred to nitrocellulose for immunoblot analysis. His-RACK1 was purfied as follows. Full length RACK1 cloned into the pAcHTL-C baculovirus expression vector (BD Pharmingen, San Diego, CA) has been described previously [Mourton et al., 2001]. Baculovirus was produced using the RACK1-pAcHTL-C plasmid and the BaculoGold™ system (BD Pharmingen) and used to infect Sf9 insect cells. Forty-eight hours post infection, the cells were lysed and His-RACK1 was purified using the PrepEase™ His-Tagged Protein Purification Kit (USB, Cleveland, OH), following the manufacturer's protocol with one modification, 1% Triton X-100 was added to the lysis and wash buffers. Purity of the His-RACK1 protein was determined by Coomassie stain and verified by immunoblotting with a monoclonal antibody to RACK1.
CO-IMMUNOPRECIPITATIONS
A549 cells were grown to 90% confluency. Cells were washed twice with PBS and treated with the crosslinking reagent DSP (Pierce) at a final concentration of 1 mM in PBS for 10 minutes at room temperature. Tris-HCl, pH 7.5 was added to a final concentration of 50 mM for 15 minutes at room temperature. Cells were then washed once with PBS and lysed in 50 mM Tris-HCl pH 7.5, 1% Triton X-100, 150 mM NaCl, 1 mM benzamidine and protease inhibitor cocktail. Samples were sonicated and centrifuged at 10,000 rpm for 5 minutes. Supernatants were saved and protein concentrations determined using the Bradford method. Immunoprecipitations were performed with equal amounts of supernatant protein (400μg) using protein A Sepharose (Pharmacia) pre-loaded with a rabbit polyclonal antibody directed to the extracellular MAM domain of PTPμ (494), or non-immune rabbit serum (NIRS). Samples were rocked at 4°C for 3–4 hours. Beads were washed four times with lysis buffer and heated to 37°C for 15 minutes and 95°C for 5 minutes in 2× SDS sample buffer. Immunoprecipitated proteins were resolved by SDS-PAGE and detected by immunoblotting.
LENTIVIRUS PRODUCTION AND INFECTION
The lentiviral shRNA plasmid V2LHS_171008 targeting a region in the extracellular domain of human PTPμ was purchased from Open Biosystems (Huntsville, AL). A control lentiviral plasmid was a gift from Drs E. Johnson and R. Keri (Case Western Reserve University, Cleveland, OH). As described previously [Burgoyne et al., 2009a; Dull et al., 1998], VSV-G-pseudotyped lentiviral particles were produced by a triple transfection with shRNA constructs, pCMVΔR8.91 and pMD.G packaging plasmids into 293T cells. Viral particles were concentrated by ultracentrifugation and used to infect U-87 MG cells in the presence of 6 μg/ml polybrene. Three days post transfection, cells were used for brain slice assays described below. Knockdown of PTPμ was verified by immunoblotting with antibodies to PTPμ. Infection efficiency was determined by visualization of a green fluorescent protein (GFP) reporter by fluorescence microscopy.
BRAIN SLICE ASSAY
All brain slice experiments were performed in accordance with an approved protocol from the Case Western Reserve University Institutional Animal Care and Use Committee. Organotypic brain slice cultures were prepared according to previously described protocols with some modifications [Burgoyne et al., 2009a; Gogolla et al., 2006; Jacobsen and Miller, 2003]. For the brain slice assay, U-87 MG cells were infected with lentiviral constructs encoding either control or PTPμ shRNA containing a GFP reporter. Three days post infection, U-87 MG cells were treated with either 18 μM U-73122 (Calbiochem) or DMSO (vehicle) for 30 minutes at 37°C. Infected cells were then trypsinized and resuspended in DMEM containing 2 mg/ml Matrigel (BD Biosciences) at a concentration of 105 cells/μl. The cell suspension (0.5 μl) was injected into the cortex of rat brain slices using a 2 μl micropipettor as described previously [Burgoyne et al., 2009a]. Post injection, slices were incubated at 37°C for 48 hours and then fixed in 4 % formaldehyde overnight at 4°C. Fixed slices were washed with PBS, mounted on slides and viewed under a fluorescence microscope to assess cell migration into the brain tissue by GFP fluorescence. Images were captured with a 10× objective using a Leica DMI 6000B automated inverted microscope (Leica Microsystems GmbH, Wetzlar, Germany) attached to a Retiga EXi camera (QImaging, Surrey, BC, Canada). The extent of migration of injected tumor cells into brain slices was quantitated using MetaMorph software (Molecular Devices, Downington, PA) as previously described [Burgoyne et al., 2009a]. The data is shown as area of migration in μm2 and represents 3 separate experiments with a minimum of 22 replicates. Error bars indicate standard error. Statistical significance was determined using an unpaired student's t-test.
RESULTS
PKCδ is a substrate of PTPμ
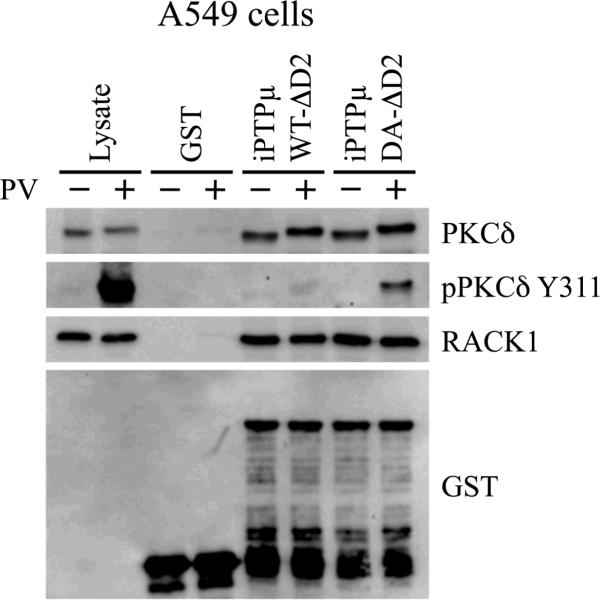
Substantial evidence from our laboratory suggests PKCδ is a substrate for PTPμ. PKCδ activity is required for both axonal migration of retinal ganglion cells on a PTPμ-coated surface and for PTPμ-mediated axon guidance of retinal ganglion cells [Ensslen and Brady-Kalnay, 2004; Rosdahl et al., 2002]. In addition, PTPμ regulation of E-cadherin-mediated adhesion involves PKCδ [Hellberg et al., 2002]. Therefore, to validate the “substrate trapping” procedure used in this manuscript, we chose to evaluate whether PKCδ could be identified as a PTPμ substrate using this method. We performed pull-down assays with two intracellular PTPμ-GST fusion proteins on glutathione Sepharose: wild-type (iPTPμWT-ΔD2) and a substrate trapping mutant, with the catalytically essential aspartate residue mutated to alanine (iPTPμDA-ΔD2). When the conserved aspartate residue of a PTP, which acts as a general acid during catalysis, is mutated to an alanine (DA), a “substrate trap” is created [Flint et al., 1997; Tiganis and Bennett, 2007]. The DA mutation generally only affects catalysis of the enzymatic reaction, rendering the mutant protein catalytically inactive, but still able to interact effectively with its substrates because the affinity for its substrate remains the same. We first used these intracellular PTPμ-GST fusion proteins in pull-down assays with lysates prepared from confluent A549 carcinoma cells. A549 cells were chosen for our initial experiments because they express high levels of endogenous PTPμ and have proved valuable for identifying other PTPμ interacting proteins [Phillips-Mason et al., 2006]. For this assay, A549 cells were treated with or without the membrane permeable tyrosine phosphatase inhibitor pervanadate. Pervanadate was used to increase the intracellular pool of tyrosine phosphorylated proteins, increasing the probability of identifying proteins that interact in a phosphorylation-dependent manner. During cell lysis iodoacetic acid, an irreversible phosphatase inhibitor, was used to irreversibly inhibit all the endogenous, cellular phosphatases. This inhibitor was then quenched with DTT, allowing the recombinant, PTPμ-GST fusion proteins to remain active during the pull-downs. Therefore, any dephosphorylation that occurred in vitro was a consequence of the PTPμ-GST fusion protein. Proteins associated with the PTPμ-GST fusion proteins were resolved by SDS-PAGE and immunoblotted with antibodies to PKCδ and RACK1. As shown in Figure 1, PKCδ associated with iPTPμWT-ΔD2 and iPTPμDA-ΔD2 independent of pervanadate treatment. RACK1, a known PTPμ binding partner, also associated with both PTPμ-GST fusion proteins in all samples. Neither PKCδ nor RACK1 associated with the GST control. These data are in agreement with our published data that PKCδ and RACK1 interact with PTPμ [Mourton et al., 2001; Rosdahl et al., 2002]. To determine whether PKCδ is a PTPμ substrate, the samples were immunoblotted with a commercially available antibody specific to phospho-PKCδ Y311. Phospho-PKCδ Y311 was observed only in the lysate treated with pervanadate. Furthermore, phospho-PKCδ Y311 was detected only in pull-downs performed with the substrate trapping mutant, iPTPμDA-ΔD2 and not the wild type construct, iPTPμWT-ΔD2. These data demonstrate that PKCδ is a PTPμ substrate since wild type PTPμ was able to dephosphorylate PKCδ, but the catalytically inactive construct was not. A mobility shift in PKCδ can be seen in those samples treated with pervanadate, suggesting the shift is due to tyrosine phosphorylation.
Figure 1.
PKCδ is a substrate for PTPμ. A549 cells were treated with (+) or without (−) 100 μM pervanadate (PV) for 20 minutes. Cells were lysed and equal amounts of protein were incubated with iPTPμWT-ΔD2-GST, iPTPμDA-ΔD2-GST or GST immobilized on glutathione Sepharose. Associated proteins were resolved by SDS-PAGE (8% for PKCδ and 10% for RACK1) and detected by immunoblot. PKCδ and RACK1 were detected in all the PTPμ pull downs. Phospho-PKCδ Y311 was detected only in the iPTPμDA-ΔD2-GST pull down, indicating PKCδ is a PTPμ substrate. The RACK1 immunoblot was stripped and probed with an antibody directed against GST to show the relative amounts of GST fusion proteins used.
It is known that, in addition to interaction via the catalytic pocket, substrates interact with their cognate enzymes using protein-protein interaction domains outside of the catalytic region. An increase of total protein binding to a DA mutant over a WT mutant is not necessarily expected. However, one only sees “trapping” of the tyrosine phosphorylated protein in the DA mutant pull down. This implies the WT protein has dephosphorylated the protein on tyrosine residue(s) but that the DA mutant is not capable of dephosphorylating the tyrosine residue(s).
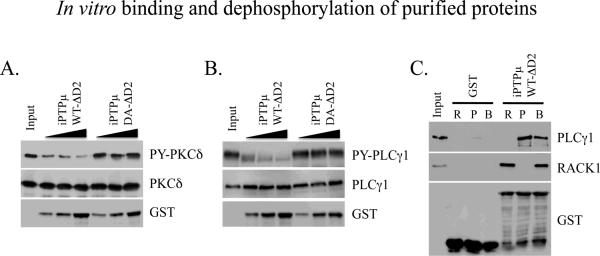
To verify PKCδ is a substrate of PTPμ we performed an in vitro phosphatase assay using purified PKCδ, phosphorylated by the non-receptor tyrosine kinase Src. Figure 3A shows that WT PTPμ was capable of dephosphorylating PKCδ in vitro, as measured by immunoblot with an anti-phospho-tyrosine antibody. The DA catalytic mutant of PTPμ was not able to dephosphorylate PKCδ confirming the requirement of PTPμ catalytic activity. The in vitro data, along with the substrate trapping results and our previously published functional data implying PKCδ is downstream of PTPμ, provide strong evidence that PKCδ is a substrate of PTPμ.
Figure 3.
In vitro binding and dephosphorylation of purified proteins. His-tagged PKCδ was phosphorylated by Src in vitro and used as a substrate for PTPμ. Dephosphorylation of PKCδ by PTPμ was assessed by incubating equal amounts of phosphorylated PKCδ with increasing amounts of iPTPµWT-ΔD2-GST or iPTPµDA-ΔD2-GST immobilized on glutathione Sepharose. PKCδ was resolved on an 8% SDS-PAGE gel and its phosphorylation status determined by immunoblot with an anti-phosphotyrosine antibody. WT PTPμ was able to dephosphorylate PKCδ whereas the DA mutant of PTPμ was unable to dephosphorylate PKCδ. A duplicate gel was probed for total PKCδ and the phosphotyrosine immunoblot was stripped and re-probed for GST to show the relative amounts of the PTPμ GST fusion proteins (A). His-tagged PLCγ1 was phosphorylated by Epidermal Growth Factor Receptor in vitro and used as a substrate for PTPμ. The ability of PTPμ to dephosphorylate PLCγ1 was assessed as described above for PKCδ. PLCγ1 was resolved on a 6% SDS-PAGE gel. The dephosphorylation of PLCγ1 by WT PTPμ is indicated by the reduction in PLCγ1 tyrosine phosphorylation and the increase in mobility of the dephosphorylated protein. The phosphotyrosine immunoblot was stripped and re-probed to show total protein (PLCγ1) and a duplicate gel was probed for GST (B). PLCγ1 and PTPμ interact directly in vitro. iPTPμWT-ΔD2-GST or GST alone were immobilized on glutathione Sepharose and incubated with purified His-PLCγ1 and His-RACK1 singly (R, RACK1; P, PLCγ1) or in combination (B, both RACK1 and PLCγ1). Bound proteins were resolved by SDS-PAGE (6% for PLCγ1 and 8% for RACK1) and detected by immunoblot. Both PLCγ1 and RACK1 were demonstrated to bind iPTPμWT-ΔD2-GST independently. There was binding between PLCγ1 and PTPμ even in the presence of RACK1. The RACK1 immunoblot was stripped and reprobed for GST to show the relative amounts of GST fusion proteins used (C).
PLCγ1 is a PTPμ substrate
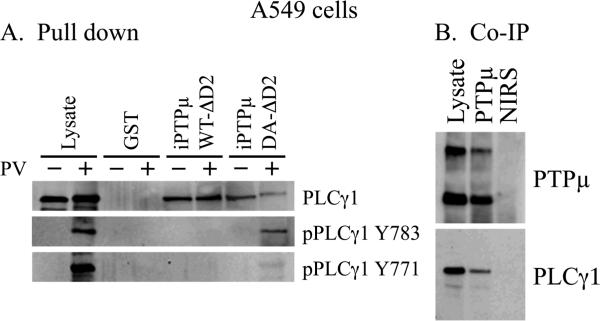
Identification of PKCδ as a substrate for PTPμ confirmed that the substrate trapping method was a valid tool to identify PTPμ substrates. Because RACK1 is an established PTPμ interacting protein [Mourton et al., 2001; Rosdahl et al., 2002], and the RACK1 binding protein PKCδ was found to be a PTPμ substrate, we investigated whether other RACK1 binding partners were also PTPμ substrates. PLCγ1 interacts with RACK1 [Disatnik et al., 1994] and is a good candidate to regulate cell migration downstream of PTPμ since PLCγ1 activation is commonly associated with the migratory and invasive activity of tumor cells [Kassis et al., 1999; Mouneimne et al., 2004; Peak et al., 2008; Piccolo et al., 2002; Teodorczyk and Martin-Villalba, 2010; Thomas et al., 2003; Turner et al., 1997]. As described above, lysates from confluent A549 cells treated with or without pervanadate were incubated with iPTPμWT-ΔD2 and iPTPμDA-ΔD2. We tested whether PLCγ1 was associated with the intracellular PTPμ-GST fusion proteins by immunoblotting with a PLCγ1 monoclonal antibody (Fig. 2A). PLCγ1 associated with iPTPμWT-ΔD2 and iPTPμDA-ΔD2, but not with the GST control (Fig. 2A). These data demonstrate PLCγ1 can associate with PTPμ in a complex. To determine if PLCγ1 is a substrate for PTPμ, we analyzed the pull-downs for the presence of phosphorylated PLCγ1 using the two commercially available phospho-specific antibodies, which recognize phosphorylated residues Y783 and Y771. We found that phospho-PLCγ1 Y783 was observed in the lysate treated with pervanadate, as expected. Phospho-PLCγ1 Y783 was detected only in the pull-down with the substrate trapping mutant and not the wild type PTPμ construct, demonstrating PTPμ is capable of dephosphorylating PLCγ1 on this residue (Fig. 2A). Because phosphorylation of PLCγ1 on Y783 has been shown to be critical to its activity [Poulin et al., 2005; Sekiya et al., 2004; Yu et al., 1998], these data imply that PTPμ phosphatase activity is able to modulate PLCγ1 activation. The Y771 phospho-specific antibody was able to detect a substantial pool of phosphorylated PLCγ1 in the lysate of pervanadate treated cells. However, this antibody was only able to detect trace amounts of the phosphorylated protein in the pull-downs suggesting that the Y771 phosphorylated protein does not interact with PTPμ. The role of Y771 in the regulation of PLCγ1 is not clear, but it has been shown to be dispensable for PLCγ1 activation [Serrano et al., 2005]. As with PKCδ, there is a slight shift in the mobility of PLCγ1 in the samples treated with pervanadate although the shift is not as apparent with PLCγ1 due to its larger size. The slight decrease in PLCγ1 protein associated with iPTPμDA-ΔD2 in the presence of pervanadate was not consistent.
Figure 2.
PLCγ1 is a substrate for PTPμ and interacts with PTPμ in cells. A549 cells were treated with (+) or without (−) 100 μM pervanadate (PV) for 20 minutes. Cells were lysed and equal amounts of protein were incubated with iPTPμWT-ΔD2-GST, iPTPμDA-ΔD2-GST or GST immobilized on glutathione Sepharose. Associated proteins were resolved by SDS-PAGE (6%) and detected by immunoblot. PLCγ1 was detected in all the PTPμ pull downs but phospho-PLCγ1 (Y783) was detected only in the iPTPμDA-ΔD2-GST pull down, and dephosphorylated by iPTPμWT-ΔD2 indicating that PLCγ1 is a PTPμ substrate (A). Confluent A549 cells were treated with the DSP cross-linking agent. Immunoprecipitations were performed using a polyclonal antibody to an extracellular epitope of PTPμ or non-immune rabbit serum (NIRS). Proteins were resolved by SDS-PAGE (6%) and immunoblotted for PTPμ and PLCγ1 (B).
We once again confirmed the substrate trapping data using an in vitro phosphatase assay. In this assay, PLCγ1 was phosphorylated by the epidermal growth factor receptor (EGFR) and used as a substrate for PTPμ. Figure 3B shows that with increasing amounts of WT PTPμ there is a clear decrease in the tyrosine phosphorylation of PLCγ1 as well as a dramatic shift downward in the mobility of the protein, which is consistent with its dephosphorylation. The PTPμ DA catalytic mutant was not able to dephosphorylate PLCγ1.
PTPμ and PLCγ1 interact in vitro and in cells
In order for PLCγ1 to be a bona fide substrate for PTPμ, PLCγ1 and PTPμ should interact in vitro and in cells [Tiganis and Bennett, 2007]. To determine whether PTPμ and PLCγ1 interact directly, we performed in vitro binding studies using purified proteins. We were also interested in determining whether RACK1 was required for the interaction, so we included purified RACK1 in the experiment. Figure 3C shows that the intracellular PTPμ-GST fusion protein (iPTPμWT-ΔD2) is able to bind purified PLCγ1 both in the absence and presence of RACK1. There is still binding of both RACK1 and PLCγ1 to PTPμ when both proteins are included in the assay. In these experiments it does not appear that PLCγ1 requires RACK1 to bind to PTPμ. However, RACK1 addition results in a complex of RACK1, PLCγ1 and PTPμ.
To test whether endogenous PLCγ1 interacts with endogenous PTPμ in cells, we conducted co-immunoprecipitations using lysates of confluent A549 cells that had been treated with the DSP cross-linking reagent. PTPμ was immunoprecipitated using an antibody that recognizes the extracellular domain of PTPμ (494). Full-length PTPμ migrates as a 200kDa band, while a proteolytically processed fragment of PTPμ migrates at approximately 100kDa. PLCγ1 interacts with PTPμ in co-immunoprecipitations from A549 cells (Fig. 2B), demonstrating that these two proteins interact in cells.
PKCδ and PLCγ1 from glioma cell lysates interact with PTPμ
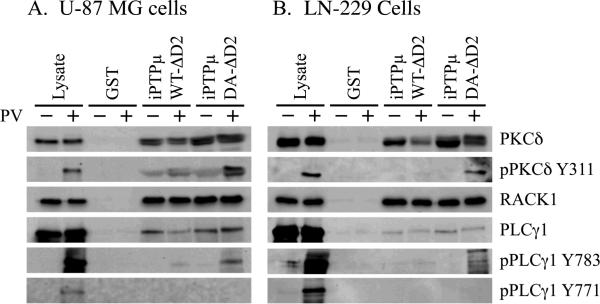
To verify that PKCδ and PLCγ1 are potential substrates of PTPμ in glioma cells, we conducted pulldown experiments using cell lysates from two different human glioma cell lines, U-87 MG and LN-229. PKCδ bound to iPTPμWTΔD2 and iPTPμDA-ΔD2 in both cell lines in the absence or presence of pervanadate (Fig. 4). Phospho-PKCδ was trapped by iPTPμDA-ΔD2 in both cell lines as determined by the phospho-specific PKCδ Y311 antibody (Fig. 4). Similar results were observed for PLCγ1. While PLCγ1 associated with both forms of PTPμ-GST fusion proteins in the presence or absence of pervanadate, only iPTPμDA-ΔD2 was able to trap pPLCγ1 Y783 (Fig. 4). Since phosphorylation of Y783 is essential for PLCγ1 activity, these results suggest that PTPμ may regulate PLCγ1 activity in glioma cells. As seen in the pull-downs with A549 cells, iPTPμDA-ΔD2 has little or no ability to bind the phospho-Y771 form of PLCγ1.
Figure 4.
PKCδ and PLCγ1 are PTPμ substrates in glioma cell lines. The human glioma cells lines U-87 MG and LN-229 were used in substrate trapping experiments. U-87 MG and LN-229 cells were treated with (+) or without (−) 100 μM pervanadate (PV) for 20 minutes. Cells were lysed and equal amounts of protein were incubated with iPTPμWT-ΔD2-GST, iPTPμDA-ΔD2-GST or GST immobilized on glutathione Sepharose. Associated proteins were resolved by SDS-PAGE (6% for PLCγ1, 8% for PKCδ and 10% for RACK1) and immunoblotted for the indicated proteins. PLCγ1, PKCδ and RACK1 were detected in all the PTPμ pull downs. Phospho-specific antibodies against PKCδ (Y311) and PLCγ1 (Y783) confirm PKCδ and PLCγ1 are PTPμ substrates in glioma cells.
PLCγ1 is downstream of PTPμ in the regulation of glioma cell migration
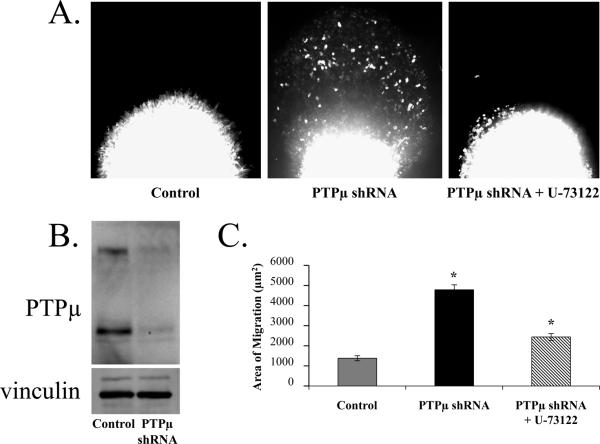
Using an ex vivo brain slice assay that simulates glioma cell dispersal in vivo, we previously determined that down regulating PTPμ protein in U-87 MG cells increased tumor cell migration [Burgoyne et al., 2009a]. Three days post-infection with either control or PTPμ shRNA containing a GFP reporter, U-87 MG cells were injected ex vivo into the cortex of adult rat brain slices. Cell migration through the 3-D matrix environment was measured by following migration using GFP fluorescence. The assay was quantitated by measuring the average area of fluorescent cells that migrated away from the injection site in a given slice after 48-hours. Control shRNA-infected U-87 MG cells were not dispersive and remained at the injection site (Fig. 5A). Knockdown of PTPμ by shRNA, however, induced a significant dispersal of cells away from the injection site into the adult brain tissue (Fig. 5A), suggesting that loss of PTPμ protein expression correlates with increased migration and dispersal of the glioma cells as previously demonstrated [Burgoyne et al., 2009a].
Figure 5.
PLCγ1 inhibition abrogates the glioma cell migration induced by PTPμ knockdown. U-87 MG cells were infected with lentivirus encoding either a control shRNA or PTPμ shRNA with a GFP reporter and injected into the cortex of adult rat brain slices. Control shRNA-infected cells remain in a tight cluster at the injection site whereas the PTPμ shRNA-infected cells disperse into the brain parenchyma. When PTPμ shRNA-infected cells are pre-incubated with U-73122 prior to injection, the cells no longer migrate. Images were taken 48 hours post injection (A). PTPμ shRNA is efficient at reducing PTPμ protein expression in U-87 MG cells. Cells were infected with either control shRNA or PTPμ shRNA containing lentivirus. Three days post-infection, cell lysates were prepared and resolved on a 6% SDS-PAGE gel followed by immunoblot with a monoclonal antibody to PTPμ. Infection with PTPμ shRNA reduced PTPμ protein expression by 64% based on densitometry (B). The PTPμ immunoblot was stripped and re-probed with an antibody to vinculin as a loading control. Quantitation of brain slice migration. Data from three separate experiments (with a minimum of 22 replicates) were quantitated and plotted as area of migration in μm2 according to the average threshold area of fluorescent cell that migrated away from the injection site (C). The asterisk represents a statistically significant difference (p<0.001).
To demonstrate that PLCγ1 is a substrate for PTPμ in vivo, and a potential target for migration regulation by PTPμ, we tested whether U-73122, a specific inhibitor of PLCγ1, had any effect on the PTPμ knockdown-induced dispersal of U-87 MG cells. Pre-treatment of U-87 MG cells expressing an shRNA directed against PTPμ with U-73122 blocked the cell migration induced by PTPμ knockdown in the adult brain (Fig. 5A). Quantitation of the migration data shows that knockdown of PTPμ induced migration nearly 4-fold over control. Pre-incubation with the PLCγ1 inhibitor reversed this phenotype, causing an approximate 2-fold reduction of the migration induced by knocking down PTPμ expression (Fig. 5C). These data suggest that PLCγ1 is downstream of PTPμ and that dephosphorylation of PLCγ1 is likely to be a key event in the pathway by which PTPμ suppresses glioma cell migration. It is important to note that the decrease in migration seen with the U-73122 pre-incubation was not due to altered cell viability. Excess cells from the brain slice injections were plated in tissue culture dishes and observed for 48 hours. There was no difference in cell viability between the control (DMSO treated) and U-73122 treated cells (data not shown). Figure 5B shows PTPμ protein expression was reduced by 64% following infection with lentivirus containing PTPμ shRNA.
DISCUSSION
PTPμ has been shown to regulate the migration of highly invasive glioma cells. In order to understand the molecular mechanism by which PTPμ influences glioma cell migration, we set out to identify substrates of PTPμ. In this manuscript, we identify PKCδ and PLCγ1 as PTPμ substrates. Following the guidelines outlined by Tiganis and Bennett to define a PTP substrate [Tiganis and Bennett, 2007], we determine that PTPμ directly interacts with PLCγ1 and that PTPμ is able to dephosphorylate PLCγ1 in vitro. Substrate trapping experiments show a catalytically inactive mutant of PTPμ is able to trap PLCγ1 from cell lysates as detected by a phospho-Y783 PLCγ1 antibody. We have not yet been able to demonstrate changes in the tyrosine phosphorylation status of PLCγ1 in intact cells when either wild type or catalytically inactive PTPμ is overexpressed in glioma cell lines followed by stimulation with various growth factors. However, we do provide functional data that PLCγ1 is required for PTPμ to suppress glioma cell migration. Together, the data presented here suggest PTPμ modulates migration by dephosphorylating PLCγ1 on Y783, rendering the enzyme inactive and unable to induce the cytoskeletal changes necessary for migration.
PTPμ expression is dramatically reduced in brain tissue excised from highly dispersive brain tumors compared to normal brain or low-grade astrocytomas [Burgoyne et al., 2009a]. The non-dispersive glioma cell line, U-87 MG, endogenously expresses PTPμ. In both an in vitro scratch wound assay and in a 3-dimensional ex vivo brain slice migration assay, we found that reduction of PTPμ expression in U-87 MG glioma cells increased cell migration [Burgoyne et al., 2009a]. These data suggest that PTPμ expression suppresses glioma cell dispersal and migration, perhaps by transducing signals in response to cell-cell adhesion. The mechanism whereby full-length PTPμ protein is reduced in glioma cells was determined to be via proteolytic cleavage of PTPμ into an extracellular and an intracellular fragment [Burden-Gulley et al., 2010; Burgoyne et al., 2009a; Burgoyne et al., 2009b]. The extracellular fragment of PTPμ retains its adhesive capabilities within the tumor microenvironment of gliomas. Its presence at the tumor edge has been exploited as a potential diagnostic marker for glioblastomas [Burden-Gulley et al., 2010]. The intracellular fragment of PTPμ is capable of translocating to the nucleus of glioma cells. Reduction of the intracellular fragment of PTPμ using shRNA and the use of a peptide inhibitor of PTPμ catalytic activity decrease glioma cell migration, demonstrating that the fragment remains catalytically active following cleavage [Burgoyne et al., 2009a; Burgoyne et al., 2009b]. Generation of an intracellular PTPμ fragment would preclude PTPμ from dephosphorylating PLCγ1, which is typically localized to the plasma membrane and the leading edge of migrating cells.
Tumor cell migration away from the central tumor mass occurs in response to growth factor gradients. Growth factors bind receptor tyrosine kinases that are often amplified or mutated in human gliomas, such as EGFR and PDGFR [Kanu et al., 2009; Teodorczyk and Martin-Villalba, 2010]. Upon ligand binding, RPTKs dimerize and undergo autophosphorylation to generate a series of phosphorylated tyrosine residues [Ullrich and Schlessinger, 1990]. Several of the resulting phospho-tyrosine residues serve as docking sites for adaptor proteins containing SH2 domains [Schlessinger, 2000]. Among the several proteins recruited to RPTKs upon ligand binding is PLCγ1 [Wells and Grandis, 2003], one of a family of enzymes that hydrolyses phosphatidylinositol (4,5) bisphosphate (PIP2) to its components, inositol-triphosphate (IP3) and diacylglycerol (DAG) [Choi et al., 2007]. In response to RPTK activation, PLCγ1 is recruited to the cell membrane and phosphorylated by the receptor's intrinsic tyrosine kinase activity. In response to growth factors, PLCγ1 can be phosphorylated on Y771, Y783, Y1253 [Sekiya et al., 2004] with the pattern and degree of phosphorylation depending on the growth factor. Of the tyrosine residues that can be phosphorylated, only tyrosine phosphorylation on residue 783 has been shown to be critical for PLCγ1 activity [Poulin et al., 2005; Sekiya et al., 2004; Yu et al., 1998]. However, it was shown recently that in response to antigen receptor activation, PLCγ1 is phosphorylated on Y775 [Serrano et al., 2005] and phosphorylation of this residue along with Y783 is required for PLCγ1-mediated responses in Jurkat cells. A role for PLCγ1 in the migration of carcinomas in response to growth factors has been established [Kassis et al., 1999; Mouneimne et al., 2004; Peak et al., 2008; Piccolo et al., 2002; Thomas et al., 2003; Turner et al., 1997] and there is evidence that PLCγ1 plays a role in mediating migration and dispersal in glioma cells as well [Bruce and Parsa, 1999; Sala et al., 2008; Teodorczyk and Martin-Villalba, 2010]. Yet, the mechanism by which PLCγ1 promotes migration is not completely understood. It is known that tumor cell invasion or dispersal requires rearrangement of the tumor cell cytoskeleton to form migratory structures such as lamellipodia and filopodia [Ridley et al., 2003; Yamaguchi and Condeelis, 2007]. Recent data suggests PLCγ1 contributes to the migratory phenotype by directly [Li et al., 2009] or indirectly activating Rac1 [Jones and Katan, 2007; Sala et al., 2008], inducing migratory structures such as membrane ruffles and lamellipodia.
Malignant glioblastomas are resistant to current therapies due to their dispersive nature. PTPμ has been shown to modulate glioma cell dispersal in both in vitro and ex vivo models. We have demonstrated that full length PTPμ protein expression at the cell surface suppresses migration. In highly dispersive tumors and glioma cell lines, PTPμ is constitutively cleaved to generate an intracellular fragment. When PTPμ is released from the cell membrane as a result of proteolysis, it changes the availability of its substrates. We hypothesize that the mechanism by which full length PTPμ suppresses glioma cell migration is by dephosphorylating and inactivating PLCγ1 at the cell surface, thereby suppressing PLCγ1's ability to initiate remodeling of the actin cytoskeleton. In keeping with this hypothesis, we have shown that the migratory phenotype of U-87 MG cells induced by PTPμ knockdown is blocked by a specific Rac1 inhibitor [Burgoyne et al., 2009a]. Our current data suggest PLCγ1 activates Rac1 to promote migration in glioma cells and PTPμ acts to suppress migration by dephosphorylating PLCγ1 on residue Y783. Therefore, proteolysis of PTPμ in gliomas would result in PLCγ1 Y783 phosphorylation leading to unchecked cell migration and subsequent dispersal. Future studies will test the hypothesis that PTPμ cleavage is a critical switch that alters the ability of PTPμ to dephosphorylate PLCγ1 thus leading to increased Rac1 activation and the promotion of cell migration.
ACKNOWLEDGEMENTS
We would like to thank Dr. Adam Burgoyne for help with the brain slice assay and Alyssa Ward along with other members of the Brady-Kalnay lab for their stimulating discussions on PTPμ function. We would like to thank Dr. Scott Howell for his help with imaging and quantitation and Sara Lou for her help in preparing the figures.
Grant sponsor: NIH; Grant numbers: RO1-NS051520, P30-EY11373.
REFERENCES
- Blanchetot C, Chagnon M, Dube N, Halle M, Tremblay ML. Substrate-trapping techniques in the identification of cellular PTP targets. Methods. 2005;35:44–53. doi: 10.1016/j.ymeth.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Flint AJ, Tonks NK. Homophilic binding of PTP mu, a receptor-type protein tyrosine phosphatase, can mediate cell-cell aggregation. J Cell Biol. 1993;122:961–72. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Tonks NK. Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTP mu. J Biol Chem. 1994;269:28472–7. [PubMed] [Google Scholar]
- Bruce JN, Parsa A. Inhibition of phospholipase C gamma 1 activation blocks glioma cell motility and invasion of fetal rat brain aggregates. Neurosurgery. 1999;44:577. doi: 10.1097/00006123-199903000-00073. [DOI] [PubMed] [Google Scholar]
- Burden-Gulley SM, Brady-Kalnay SM. PTPmu regulates N-cadherin-dependent neurite outgrowth. J Cell Biol. 1999;144:1323–36. doi: 10.1083/jcb.144.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden-Gulley SM, Gates TJ, Burgoyne AM, Cutter JL, Lodowski D, Robinson S, Sloan AE, Miller RH, Basilion JP, Brady-Kalnay SM. A novel molecular diagnostic of Glioblastomas: Detection of an extracellular fragment of PTPmu. Neoplasia. 2010;4:305–16. doi: 10.1593/neo.91940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne AM, Palomo JM, Phillips-Mason PJ, Burden-Gulley SM, Major DL, Zaremba A, Robinson S, Sloan AE, Vogelbaum MA, Miller RH, Brady-Kalnay SM. PTPmu suppresses glioma cell migration and dispersal. Neuro Oncol. 2009a;11:767–778. doi: 10.1215/15228517-2009-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne AM, Phillips-Mason PJ, Burden-Gulley SM, Robinson S, Sloan AE, Miller RH, Brady-Kalnay SM. Proteolytic cleavage of protein tyrosine phosphatase mu regulates glioblastoma cell migration. Cancer Res. 2009b;69:6960–8. doi: 10.1158/0008-5472.CAN-09-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JH, Ryu SH, Suh PG. On/off-regulation of phospholipase C-gamma 1-mediated signal transduction. Adv Enzyme Regul. 2007;47:104–16. doi: 10.1016/j.advenzreg.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Hernandez-Sotomayor SM, Jones G, Carpenter G, Mochly-Rosen D. Phospholipase C-gamma 1 binding to intracellular receptors for activated protein kinase C. Proc Natl Acad Sci U S A. 1994;91:559–63. doi: 10.1073/pnas.91.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dull T, Zufferey R, Kelly M, Mandel RJ, Nguyen M, Trono D, Naldini L. A third-generation lentivirus vector with a conditional packaging system. J Virol. 1998;72:8463–71. doi: 10.1128/jvi.72.11.8463-8471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensslen SE, Brady-Kalnay SM. PTPmu signaling via PKCdelta is instructive for retinal ganglion cell guidance. Mol Cell Neurosci. 2004;25:558–71. doi: 10.1016/j.mcn.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ensslen-Craig SE, Brady-Kalnay SM. PTP mu expression and catalytic activity are required for PTP mu-mediated neurite outgrowth and repulsion. Mol Cell Neurosci. 2005;28:177–88. doi: 10.1016/j.mcn.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Tiganis T, Barford D, Tonks NK. Development of “substrate-trapping” mutants to identify physiological substrates of protein tyrosine phosphatases. Proc Natl Acad Sci U S A. 1997;94:1680–5. doi: 10.1073/pnas.94.5.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Gebbink MF, Verheijen MH, Zondag GC, van Etten I, Moolenaar WH. Purification and characterization of the cytoplasmic domain of human receptor-like protein tyrosine phosphatase RPTP mu. Biochemistry. 1993;32:13516–22. doi: 10.1021/bi00212a017. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Galimberti I, DePaola V, Caroni P. Preparation of organotypic hippocampal slice cultures for long-term live imaging. Nat Protoc. 2006;1:1165–71. doi: 10.1038/nprot.2006.168. [DOI] [PubMed] [Google Scholar]
- Hellberg CB, Burden-Gulley SM, Pietz GE, Brady-Kalnay SM. Expression of the receptor protein-tyrosine phosphatase, PTPmu, restores E-cadherin-dependent adhesion in human prostate carcinoma cells. J Biol Chem. 2002;277:11165–73. doi: 10.1074/jbc.M112157200. [DOI] [PubMed] [Google Scholar]
- Jacobsen CT, Miller RH. Control of astrocyte migration in the developing cerebral cortex. Dev Neurosci. 2003;25:207–16. doi: 10.1159/000072269. [DOI] [PubMed] [Google Scholar]
- Jones NP, Katan M. Role of phospholipase C gamma1 in cell spreading requires association with a beta-Pix/GIT1-containing complex, leading to activation of Cdc42 and Rac1. Mol Cell Biol. 2007;27:5790–805. doi: 10.1128/MCB.00778-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanu OO, Hughes B, Di C, Lin N, Fu J, Bigner DD, Yan H, Adamson C. Glioblastoma Multiforme Oncogenomics and Signaling Pathways. Clin Med Oncol. 2009;3:39–52. doi: 10.4137/cmo.s1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassis J, Moellinger J, Lo H, Greenberg NM, Kim HG, Wells A. A role for phospholipase C-gamma-mediated signaling in tumor cell invasion. Clin Cancer Res. 1999;5:2251–60. [PubMed] [Google Scholar]
- Li S, Wang Q, Wang Y, Chen X, Wang Z. PLC-gamma1 and Rac1 coregulate EGF-induced cytoskeleton remodeling and cell migration. Mol Endocrinol. 2009;23:901–13. doi: 10.1210/me.2008-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahill A, Warwicker J, Bolger GB, Houslay MD, Yarwood SJ. The RACK1 scaffold protein: a dynamic cog in cell response mechanisms. Mol Pharmacol. 2002;62:1261–73. doi: 10.1124/mol.62.6.1261. [DOI] [PubMed] [Google Scholar]
- Mouneimne G, Soon L, DesMarais V, Sidani M, Song X, Yip SC, Ghosh M, Eddy R, Backer JM, Condeelis J. Phospholipase C and cofilin are required for carcinoma cell directionality in response to EGF stimulation. J Cell Biol. 2004;166:697–708. doi: 10.1083/jcb.200405156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourton T, Hellberg CB, Burden-Gulley SM, Hinman J, Rhee A, Brady-Kalnay SM. The PTPmu protein-tyrosine phosphatase binds and recruits the scaffolding protein RACK1 to cell-cell contacts. J Biol Chem. 2001;276:14896–901. doi: 10.1074/jbc.M010823200. [DOI] [PubMed] [Google Scholar]
- Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64:458–78. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblander SA, Ensslen-Craig SE, Longo FM, Brady-Kalnay SM. E-cadherin promotes retinal ganglion cell neurite outgrowth in a protein tyrosine phosphatase-mu-dependent manner. Mol Cell Neurosci. 2007;34:481–92. doi: 10.1016/j.mcn.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblander SA, Brady-Kalnay SM. Distinct PTPmu-associated signaling molecules differentially regulate neurite outgrowth on E-, N-, and R-cadherin. Mol Cell Neurosci. 2010;44:78–93. doi: 10.1016/j.mcn.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostman A, Hellberg C, Bohmer FD. Protein-tyrosine phosphatases and cancer. Nat Rev Cancer. 2006;6:307–20. doi: 10.1038/nrc1837. [DOI] [PubMed] [Google Scholar]
- Peak JC, Jones NP, Hobbs S, Katan M, Eccles SA. Phospholipase C gamma 1 regulates the Rap GEF1-Rap1 signalling axis in the control of human prostate carcinoma cell adhesion. Oncogene. 2008;27:2823–32. doi: 10.1038/sj.onc.1210954. [DOI] [PubMed] [Google Scholar]
- Phillips-Mason PJ, Gates TJ, Major DL, Sacks DB, Brady-Kalnay SM. The receptor protein-tyrosine phosphatase PTPμ interacts with IQGAP1. J Biol Chem. 2006;281:4903–10. doi: 10.1074/jbc.M506414200. [DOI] [PubMed] [Google Scholar]
- Phillips-Mason PJ, Mourton T, Major DL, Brady-Kalnay SM. BCCIP associates with the receptor protein tyrosine phosphatase PTPmu. J Cell Biochem. 2008;105:1059–72. doi: 10.1002/jcb.21907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo E, Innominato PF, Mariggio MA, Maffucci T, Iacobelli S, Falasca M. The mechanism involved in the regulation of phospholipase C gamma1 activity in cell migration. Oncogene. 2002;21:6520–9. doi: 10.1038/sj.onc.1205821. [DOI] [PubMed] [Google Scholar]
- Poulin B, Sekiya F, Rhee SG. Intramolecular interaction between phosphorylated tyrosine-783 and the C-terminal Src homology 2 domain activates phospholipase C-gamma1. Proc Natl Acad Sci U S A. 2005;102:4276–81. doi: 10.1073/pnas.0409590102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS. Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer. 2003;3:489–501. doi: 10.1038/nrc1121. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–9. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- Rosdahl JA, Mourton TL, Brady-Kalnay SM. Protein kinase C delta (PKCdelta) is required for protein tyrosine phosphatase mu (PTPmu)-dependent neurite outgrowth. Mol Cell Neurosci. 2002;19:292–306. doi: 10.1006/mcne.2001.1071. [DOI] [PubMed] [Google Scholar]
- Sala G, Dituri F, Raimondi C, Previdi S, Maffucci T, Mazzoletti M, Rossi C, Iezzi M, Lattanzio R, Piantelli M, Iacobelli S, Broggini M, Falasca M. Phospholipase C gamma1 is required for metastasis development and progression. Cancer Res. 2008;68:10187–96. doi: 10.1158/0008-5472.CAN-08-1181. [DOI] [PubMed] [Google Scholar]
- Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–25. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- Sekiya F, Poulin B, Kim YJ, Rhee SG. Mechanism of tyrosine phosphorylation and activation of phospholipase C-gamma 1. Tyrosine 783 phosphorylation is not sufficient for lipase activation. J Biol Chem. 2004;279:32181–90. doi: 10.1074/jbc.M405116200. [DOI] [PubMed] [Google Scholar]
- Serrano CJ, Graham L, DeBell K, Rawat R, Veri MC, Bonvini E, Rellahan BL, Reischl IG. A new tyrosine phosphorylation site in PLC gamma 1: the role of tyrosine 775 in immune receptor signaling. J Immunol. 2005;174:6233–7. doi: 10.4049/jimmunol.174.10.6233. [DOI] [PubMed] [Google Scholar]
- Teodorczyk M, Martin-Villalba A. Sensing invasion: Cell surface receptors driving spreading of glioblastoma. J Cell Physiol. 2010;222:1–10. doi: 10.1002/jcp.21901. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Coppelli FM, Wells A, Gooding WE, Song J, Kassis J, Drenning SD, Grandis JR. Epidermal growth factor receptor-stimulated activation of phospholipase C gamma-1 promotes invasion of head and neck squamous cell carcinoma. Cancer Res. 2003;63:5629–35. [PubMed] [Google Scholar]
- Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T, Epps-Fung MV, Kassis J, Wells A. Molecular inhibition of phospholipase c gamma signaling abrogates DU-145 prostate tumor cell invasion. Clin Cancer Res. 1997;3:2275–82. [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–12. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wells A, Grandis JR. Phospholipase C-gamma1 in tumor progression. Clin Exp Metastasis. 2003;20:285–90. doi: 10.1023/a:1024088922957. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Condeelis J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim Biophys Acta. 2007;1773:642–52. doi: 10.1016/j.bbamcr.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Fukami K, Itoh T, Takenawa T. Phosphorylation of phospholipase C gamma1 on tyrosine residue 783 by platelet-derived growth factor regulates reorganization of the cytoskeleton. Exp Cell Res. 1998;243:113–22. doi: 10.1006/excr.1998.4132. [DOI] [PubMed] [Google Scholar]
- Zondag GC, Reynolds AB, Moolenaar WH. Receptor protein-tyrosine phosphatase RPTPmu binds to and dephosphorylates the catenin p120(ctn) J Biol Chem. 2000;275:11264–9. doi: 10.1074/jbc.275.15.11264. [DOI] [PubMed] [Google Scholar]