Abstract
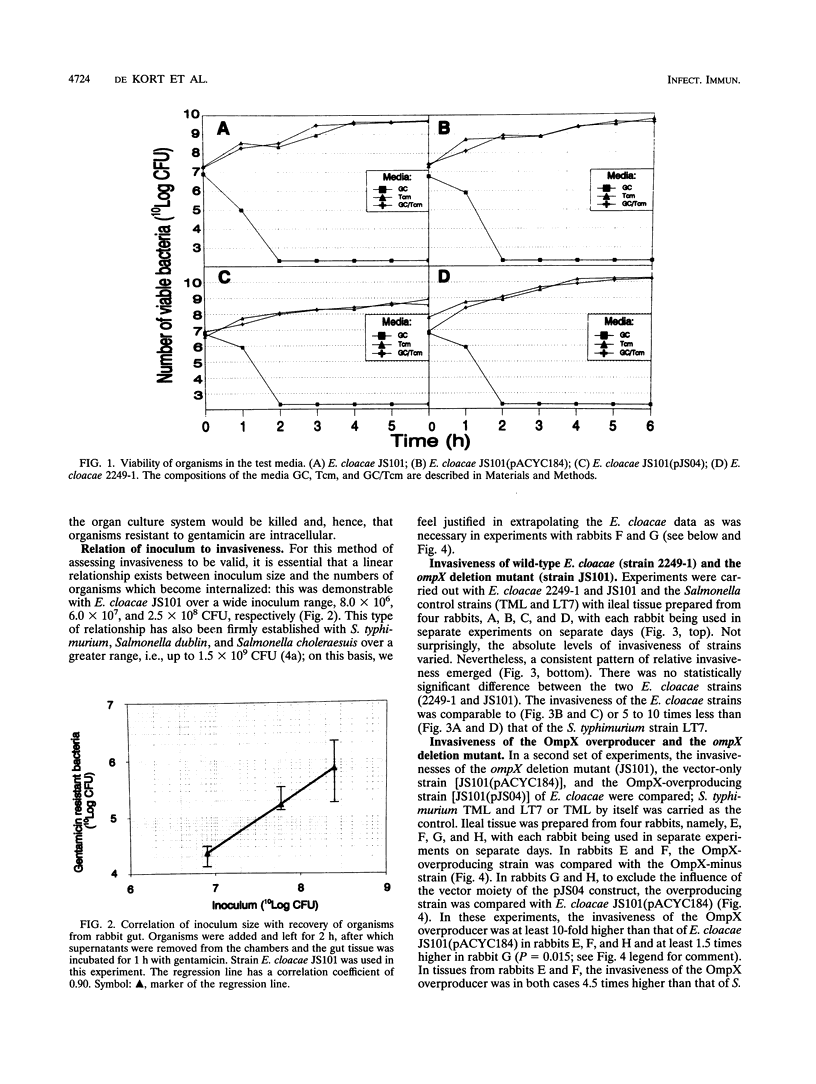
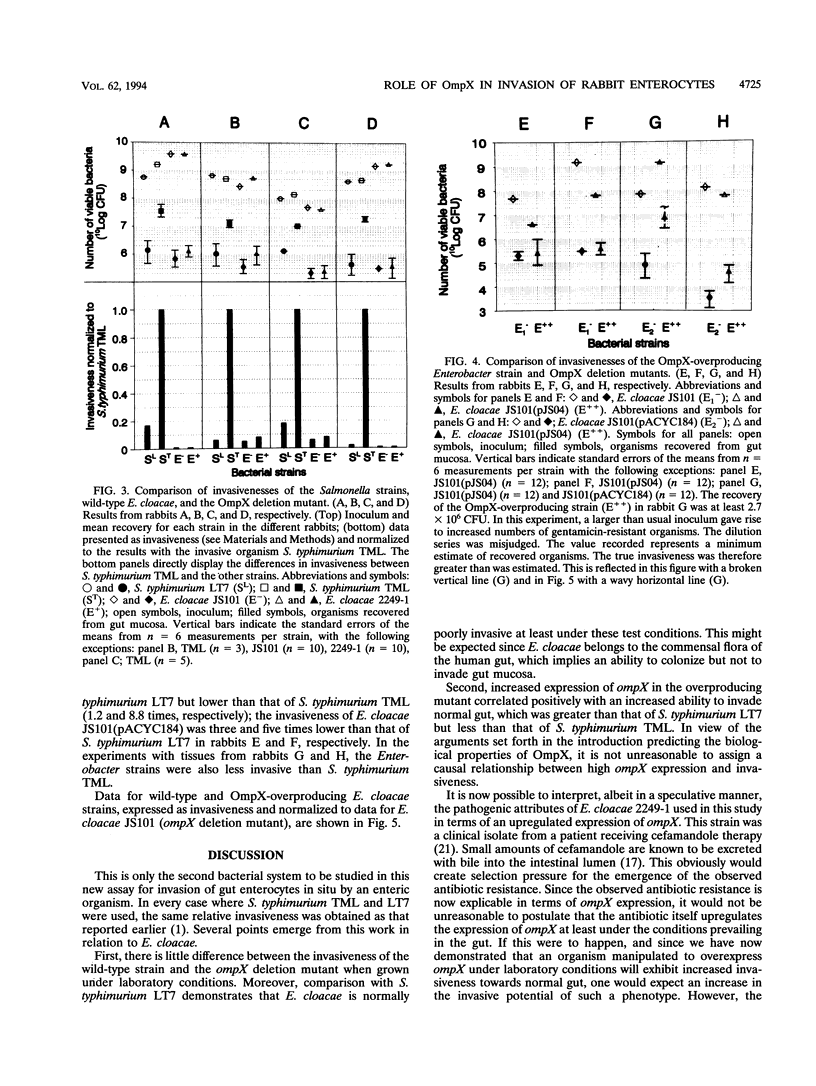
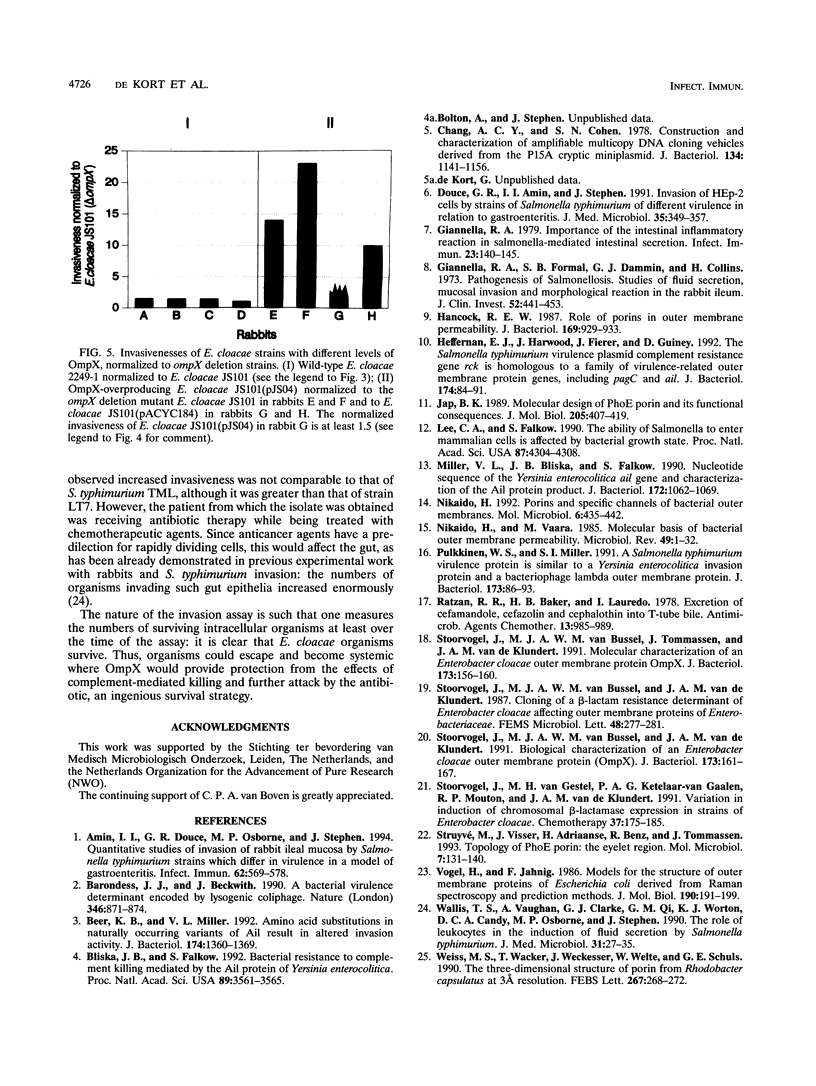
The outer membrane protein OmpX of Enterobacter cloacae shows high amino acid homology with virulence proteins PagC and Rck from Salmonella typhimurium and with Ail from Yersinia enterocolitica. Here we demonstrate a role for OmpX in the invasion of rabbit ileal tissue by E. cloacae. An organ culture system was used for maintenance of rabbit gut tissue during the experiments. The invasiveness of three E. cloacae strains, which differed in OmpX content, were compared with each other and with that of Salmonella typhimurium TML (a highly invasive strain) and S. typhimurium LT7 (a noninvasive strain). There was no significant difference between the invasiveness of the wild type and that of an ompX deletion mutant strain of E. cloacae; they were equally as invasive or less invasive than S. typhimurium LT7. The invasiveness of an OmpX overproducer strain of E. cloacae was 10-fold higher than that of its immediate parent carrying only the multicopy plasmid, higher than that of S. typhimurium LT7, but lower than that of S. typhimurium TML. The invasiveness of E. cloacae thus varied directly with the level of OmpX in the outer membrane in rabbit ileal enterocytes challenged in situ.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amin I. I., Douce G. R., Osborne M. P., Stephen J. Quantitative studies of invasion of rabbit ileal mucosa by Salmonella typhimurium strains which differ in virulence in a model of gastroenteritis. Infect Immun. 1994 Feb;62(2):569–578. doi: 10.1128/iai.62.2.569-578.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barondess J. J., Beckwith J. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature. 1990 Aug 30;346(6287):871–874. doi: 10.1038/346871a0. [DOI] [PubMed] [Google Scholar]
- Beer K. B., Miller V. L. Amino acid substitutions in naturally occurring variants of ail result in altered invasion activity. J Bacteriol. 1992 Feb;174(4):1360–1369. doi: 10.1128/jb.174.4.1360-1369.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Falkow S. Bacterial resistance to complement killing mediated by the Ail protein of Yersinia enterocolitica. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3561–3565. doi: 10.1073/pnas.89.8.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douce G. R., Amin I. I., Stephen J. Invasion of HEp-2 cells by strains of Salmonella typhimurium of different virulence in relation to gastroenteritis. J Med Microbiol. 1991 Dec;35(6):349–357. doi: 10.1099/00222615-35-6-349. [DOI] [PubMed] [Google Scholar]
- Giannella R. A., Formal S. B., Dammin G. J., Collins H. Pathogenesis of salmonellosis. Studies of fluid secretion, mucosal invasion, and morphologic reaction in the rabbit ileum. J Clin Invest. 1973 Feb;52(2):441–453. doi: 10.1172/JCI107201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A. Importance of the intestinal inflammatory reaction in salmonella-mediated intestinal secretion. Infect Immun. 1979 Jan;23(1):140–145. doi: 10.1128/iai.23.1.140-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Role of porins in outer membrane permeability. J Bacteriol. 1987 Mar;169(3):929–933. doi: 10.1128/jb.169.3.929-933.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan E. J., Harwood J., Fierer J., Guiney D. The Salmonella typhimurium virulence plasmid complement resistance gene rck is homologous to a family of virulence-related outer membrane protein genes, including pagC and ail. J Bacteriol. 1992 Jan;174(1):84–91. doi: 10.1128/jb.174.1.84-91.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jap B. K. Molecular design of PhoE porin and its functional consequences. J Mol Biol. 1989 Jan 20;205(2):407–419. doi: 10.1016/0022-2836(89)90351-3. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Bliska J. B., Falkow S. Nucleotide sequence of the Yersinia enterocolitica ail gene and characterization of the Ail protein product. J Bacteriol. 1990 Feb;172(2):1062–1069. doi: 10.1128/jb.172.2.1062-1069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992 Feb;6(4):435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulkkinen W. S., Miller S. I. A Salmonella typhimurium virulence protein is similar to a Yersinia enterocolitica invasion protein and a bacteriophage lambda outer membrane protein. J Bacteriol. 1991 Jan;173(1):86–93. doi: 10.1128/jb.173.1.86-93.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratzan K. R., Baker H. B., Lauredo I. Excretion of cefamandole, cefazolin, and cephalothin into T-tube bile. Antimicrob Agents Chemother. 1978 Jun;13(6):985–987. doi: 10.1128/aac.13.6.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel J., van Bussel M. J., Tommassen J., van de Klundert J. A. Molecular characterization of an Enterobacter cloacae outer membrane protein (OmpX). J Bacteriol. 1991 Jan;173(1):156–160. doi: 10.1128/jb.173.1.156-160.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel J., van Bussel M. J., van de Klundert J. A. Biological characterization of an Enterobacter cloacae outer membrane protein (OmpX). J Bacteriol. 1991 Jan;173(1):161–167. doi: 10.1128/jb.173.1.161-167.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel J., van Gestel M. H., Ketelaar-van Gaalen P. A., Mouton R. P., van de Klundert J. A. Variation in induction of chromosomal beta-lactamase expression in strains of Enterobacter cloacae. Chemotherapy. 1991;37(3):175–185. doi: 10.1159/000238851. [DOI] [PubMed] [Google Scholar]
- Struyvé M., Visser J., Adriaanse H., Benz R., Tommassen J. Topology of PhoE porin: the 'eyelet' region. Mol Microbiol. 1993 Jan;7(1):131–140. doi: 10.1111/j.1365-2958.1993.tb01104.x. [DOI] [PubMed] [Google Scholar]
- Vogel H., Jähnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J Mol Biol. 1986 Jul 20;190(2):191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- Wallis T. S., Vaughan A. T., Clarke G. J., Qi G. M., Worton K. J., Candy D. C., Osborne M. P., Stephen J. The role of leucocytes in the induction of fluid secretion by Salmonella typhimurium. J Med Microbiol. 1990 Jan;31(1):27–35. doi: 10.1099/00222615-31-1-27. [DOI] [PubMed] [Google Scholar]
- Weiss M. S., Wacker T., Weckesser J., Welte W., Schulz G. E. The three-dimensional structure of porin from Rhodobacter capsulatus at 3 A resolution. FEBS Lett. 1990 Jul 16;267(2):268–272. doi: 10.1016/0014-5793(90)80942-c. [DOI] [PubMed] [Google Scholar]


