Abstract
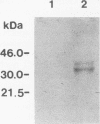
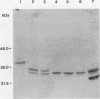
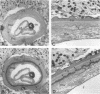
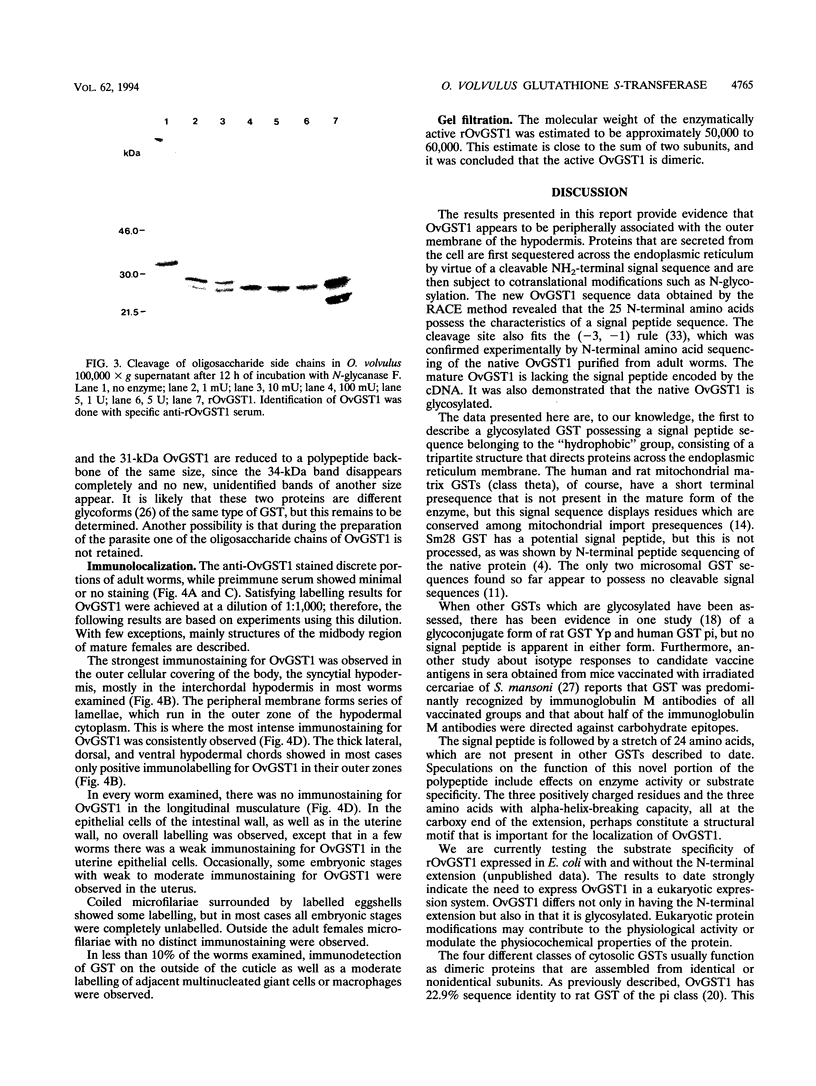
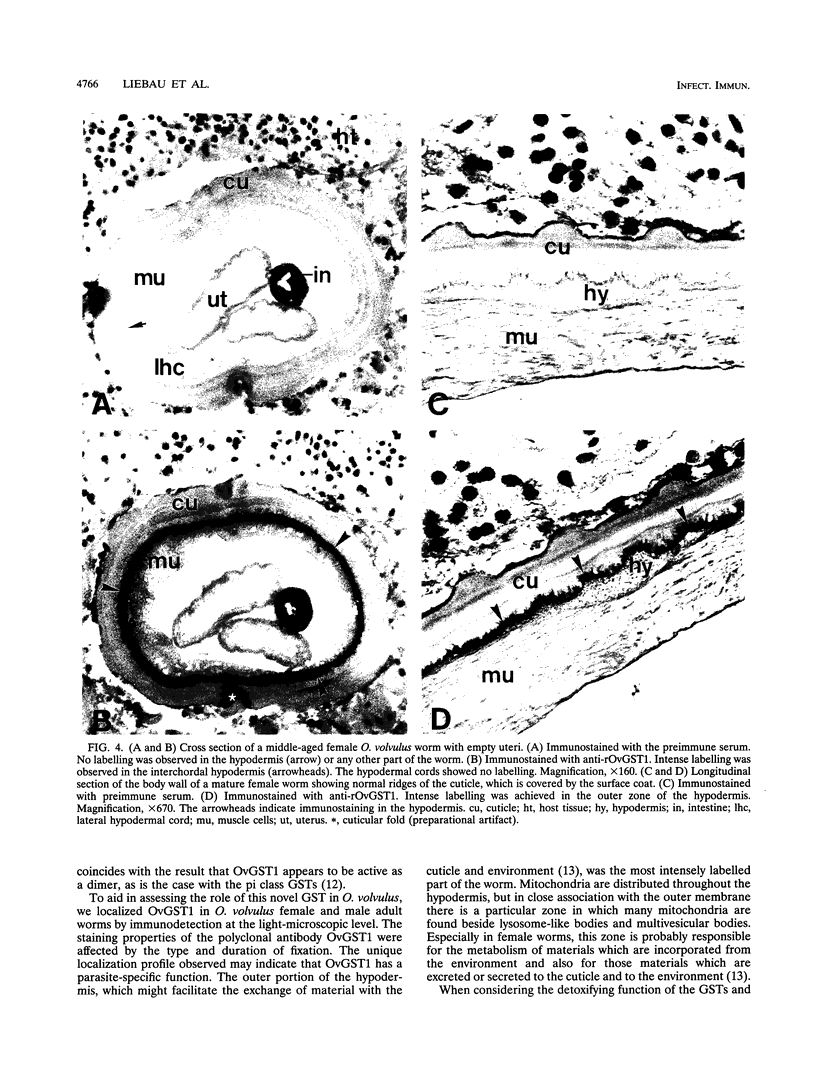
Onchocerca volvulus is a pathogenic human filarial parasite which, like other helminth parasites, is capable of evading the host's immune responses by a variety of defense mechanisms which are likely to include the detoxification and repair mechanisms of the enzyme glutathione S-transferase (GST). In this study, we show that one of the previously described GSTs from O. volvulus appears to possess the characteristics of a secreted enzyme. When the complete O. volvulus GST1 (OvGST1) sequence presented here is compared with those of other GSTs, 50 additional residues at the N terminus are observed, the first 25 showing characteristics of a signal peptide. This is consistent with the N-terminal sequence data on the native mature enzyme which begins at amino acid 26, based on the deduced protein sequence from the cDNA. The native protein, without the signal peptide sequence, possesses a 24-amino-acid extension not present in other GSTs. The deduced amino acid sequence of the OvGST1 cDNA clone was shown to possess four potential N-glycosylation sites. Digestion of O. volvulus homogenate with endoglycosidase, followed by detection of OvGST1 with specific antibody, indicated that the enzyme possesses at least two N-linked oligosaccharide chains. Gel filtration of the Escherichia coli-produced recombinant OvGST1 showed that it is enzymatically active as a nonglycosylated dimer. OvGST1 is found in the media surrounding adult worms maintained in culture, indicating that, in vitro, this enzyme is released from the worm. The strongest immunostaining for OvGST1 was observed in the outer cellular covering of the adult worm body, the syncytial hypodermis, especially in the interchordal hypodermis, where the peripheral membrane forms a series of lamellae which run into the outer zone of the hypodermal cytoplasm.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albiez E. J., Büttner D. W., Duke B. O. Diagnosis and extirpation of nodules in human onchocerciasis. Trop Med Parasitol. 1988 Dec;39 (Suppl 4):331–346. [PubMed] [Google Scholar]
- Armstrong R. N. Glutathione S-transferases: reaction mechanism, structure, and function. Chem Res Toxicol. 1991 Mar-Apr;4(2):131–140. doi: 10.1021/tx00020a001. [DOI] [PubMed] [Google Scholar]
- Balloul J. M., Grzych J. M., Pierce R. J., Capron A. A purified 28,000 dalton protein from Schistosoma mansoni adult worms protects rats and mice against experimental schistosomiasis. J Immunol. 1987 May 15;138(10):3448–3453. [PubMed] [Google Scholar]
- Balloul J. M., Sondermeyer P., Dreyer D., Capron M., Grzych J. M., Pierce R. J., Carvallo D., Lecocq J. P., Capron A. Molecular cloning of a protective antigen of schistosomes. Nature. 1987 Mar 12;326(6109):149–153. doi: 10.1038/326149a0. [DOI] [PubMed] [Google Scholar]
- Boulanger D., Reid G. D., Sturrock R. F., Wolowczuk I., Balloul J. M., Grezel D., Pierce R. J., Otieno M. F., Guerret S., Grimaud J. A. Immunization of mice and baboons with the recombinant Sm28GST affects both worm viability and fecundity after experimental infection with Schistosoma mansoni. Parasite Immunol. 1991 Sep;13(5):473–490. doi: 10.1111/j.1365-3024.1991.tb00545.x. [DOI] [PubMed] [Google Scholar]
- Brophy P. M., Barrett J. Glutathione transferase in helminths. Parasitology. 1990 Apr;100(Pt 2):345–349. doi: 10.1017/s0031182000061369. [DOI] [PubMed] [Google Scholar]
- Brophy P. M., Pritchard D. I. Immunity to helminths: Ready to tip the biochemical balance? Parasitol Today. 1992 Dec;8(12):419–422. doi: 10.1016/0169-4758(92)90195-8. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cordell J. L., Falini B., Erber W. N., Ghosh A. K., Abdulaziz Z., MacDonald S., Pulford K. A., Stein H., Mason D. Y. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem. 1984 Feb;32(2):219–229. doi: 10.1177/32.2.6198355. [DOI] [PubMed] [Google Scholar]
- Daniel V. Glutathione S-transferases: gene structure and regulation of expression. Crit Rev Biochem Mol Biol. 1993;28(3):173–207. doi: 10.3109/10409239309086794. [DOI] [PubMed] [Google Scholar]
- DeJong J. L., Morgenstern R., Jörnvall H., DePierre J. W., Tu C. P. Gene expression of rat and human microsomal glutathione S-transferases. J Biol Chem. 1988 Jun 15;263(17):8430–8436. [PubMed] [Google Scholar]
- Dirr H., Reinemer P., Huber R. X-ray crystal structures of cytosolic glutathione S-transferases. Implications for protein architecture, substrate recognition and catalytic function. Eur J Biochem. 1994 Mar 15;220(3):645–661. doi: 10.1111/j.1432-1033.1994.tb18666.x. [DOI] [PubMed] [Google Scholar]
- Franz M. The morphology of adult Onchocerca volvulus based on electron microscopy. Trop Med Parasitol. 1988 Dec;39 (Suppl 4):359–366. [PubMed] [Google Scholar]
- Harris J. M., Meyer D. J., Coles B., Ketterer B. A novel glutathione transferase (13-13) isolated from the matrix of rat liver mitochondria having structural similarity to class theta enzymes. Biochem J. 1991 Aug 15;278(Pt 1):137–141. doi: 10.1042/bj2780137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkle K. J., Davern K. M., Wright M. D., Ramos A. J., Mitchell G. F. Comparison of the cloned genes of the 26- and 28-kilodalton glutathione S-transferases of Schistosoma japonicum and Schistosoma mansoni. Mol Biochem Parasitol. 1990 Apr;40(1):23–34. doi: 10.1016/0166-6851(90)90076-x. [DOI] [PubMed] [Google Scholar]
- Henkle K. J., Liebau E., Müller S., Bergmann B., Walter R. D. Characterization and molecular cloning of a Cu/Zn superoxide dismutase from the human parasite Onchocerca volvulus. Infect Immun. 1991 Jun;59(6):2063–2069. doi: 10.1128/iai.59.6.2063-2069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Structural features in eukaryotic mRNAs that modulate the initiation of translation. J Biol Chem. 1991 Oct 25;266(30):19867–19870. [PubMed] [Google Scholar]
- Kuzmich S., Vanderveer L. A., Tew K. D. Evidence for a glycoconjugate form of glutathione S-transferase pI. Int J Pept Protein Res. 1991 Jun;37(6):565–571. doi: 10.1111/j.1399-3011.1991.tb00776.x. [DOI] [PubMed] [Google Scholar]
- Liebau E., Schönberger O. L., Walter R. D., Henkle-Dührsen K. J. Molecular cloning and expression of a cDNA encoding glutathione S-transferase from Ascaris suum. Mol Biochem Parasitol. 1994 Jan;63(1):167–170. doi: 10.1016/0166-6851(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Liebau E., Walter R. D., Henkle-Dührsen K. Isolation, sequence and expression of an Onchocerca volvulus glutathione S-transferase cDNA. Mol Biochem Parasitol. 1994 Feb;63(2):305–309. doi: 10.1016/0166-6851(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Liebau E., Walter R. D., Henkle-Dührsen K. Onchocerca volvulus: isolation and sequence of a second glutathione S-transferase cDNA. Exp Parasitol. 1994 Aug;79(1):68–71. doi: 10.1006/expr.1994.1062. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F. Glutathione S-transferases - potential components of anti-schistosome vaccines? Parasitol Today. 1989 Feb;5(2):34–37. doi: 10.1016/0169-4758(89)90185-3. [DOI] [PubMed] [Google Scholar]
- Panaccio M., Wilson L. R., Crameri S. L., Wijffels G. L., Spithill T. W. Molecular characterization of cDNA sequences encoding glutathione S-transferases of Fasciola hepatica. Exp Parasitol. 1992 Mar;74(2):232–237. doi: 10.1016/0014-4894(92)90051-b. [DOI] [PubMed] [Google Scholar]
- Rademacher T. W., Parekh R. B., Dwek R. A. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- Richter D., Incani R. N., Harn D. A. Isotype responses to candidate vaccine antigens in protective sera obtained from mice vaccinated with irradiated cercariae of Schistosoma mansoni. Infect Immun. 1993 Jul;61(7):3003–3011. doi: 10.1128/iai.61.7.3003-3011.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton J. L., Milner A. R., Panaccio M., Waddington J., Wijffels G., Chandler D., Thompson C., Wilson L., Spithill T. W., Mitchell G. F. Glutathione S-transferase. Novel vaccine against Fasciola hepatica infection in sheep. J Immunol. 1990 Dec 1;145(11):3905–3910. [PubMed] [Google Scholar]
- Trottein F., Godin C., Pierce R. J., Sellin B., Taylor M. G., Gorillot I., Silva M. S., Lecocq J. P., Capron A. Inter-species variation of schistosome 28-kDa glutathione S-transferases. Mol Biochem Parasitol. 1992 Aug;54(1):63–72. doi: 10.1016/0166-6851(92)90095-2. [DOI] [PubMed] [Google Scholar]
- Tsuchida S., Sato K. Glutathione transferases and cancer. Crit Rev Biochem Mol Biol. 1992;27(4-5):337–384. doi: 10.3109/10409239209082566. [DOI] [PubMed] [Google Scholar]
- Weston K., Yochem J., Greenwald I. A Caenorhabditis elegans cDNA that encodes a product resembling the rat glutathione S-transferase P subunit. Nucleic Acids Res. 1989 Mar 11;17(5):2138–2138. doi: 10.1093/nar/17.5.2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]