Abstract
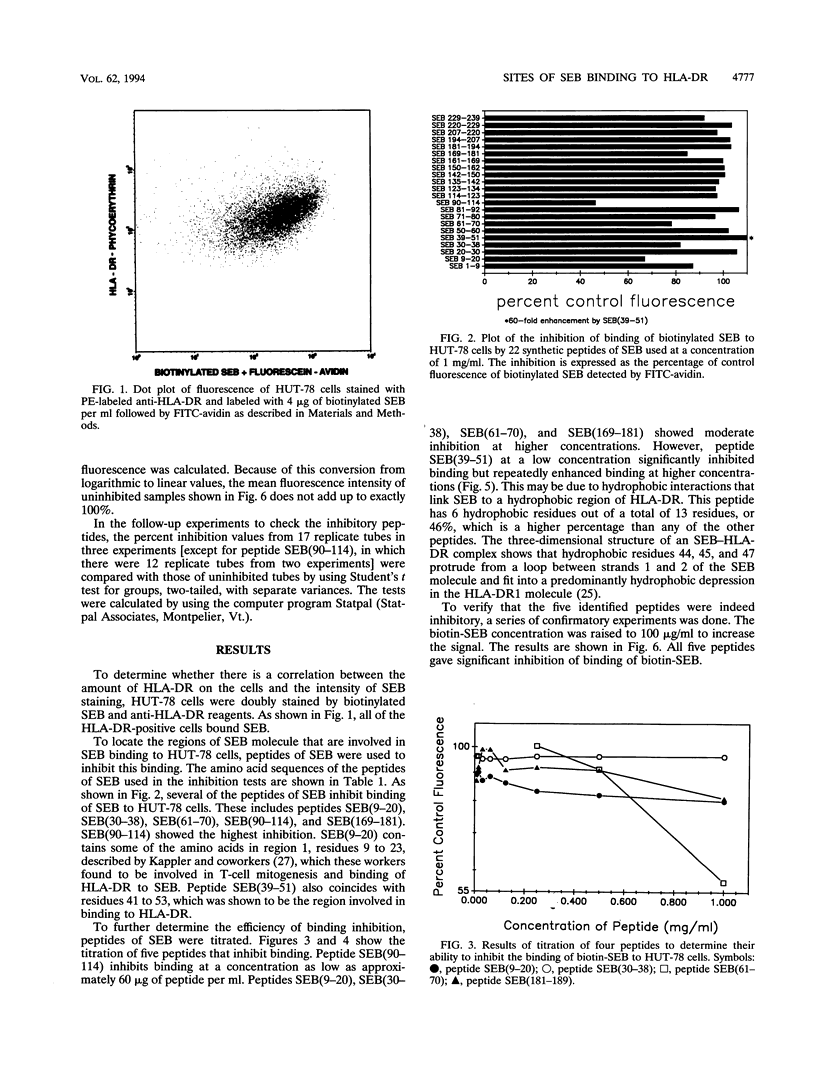
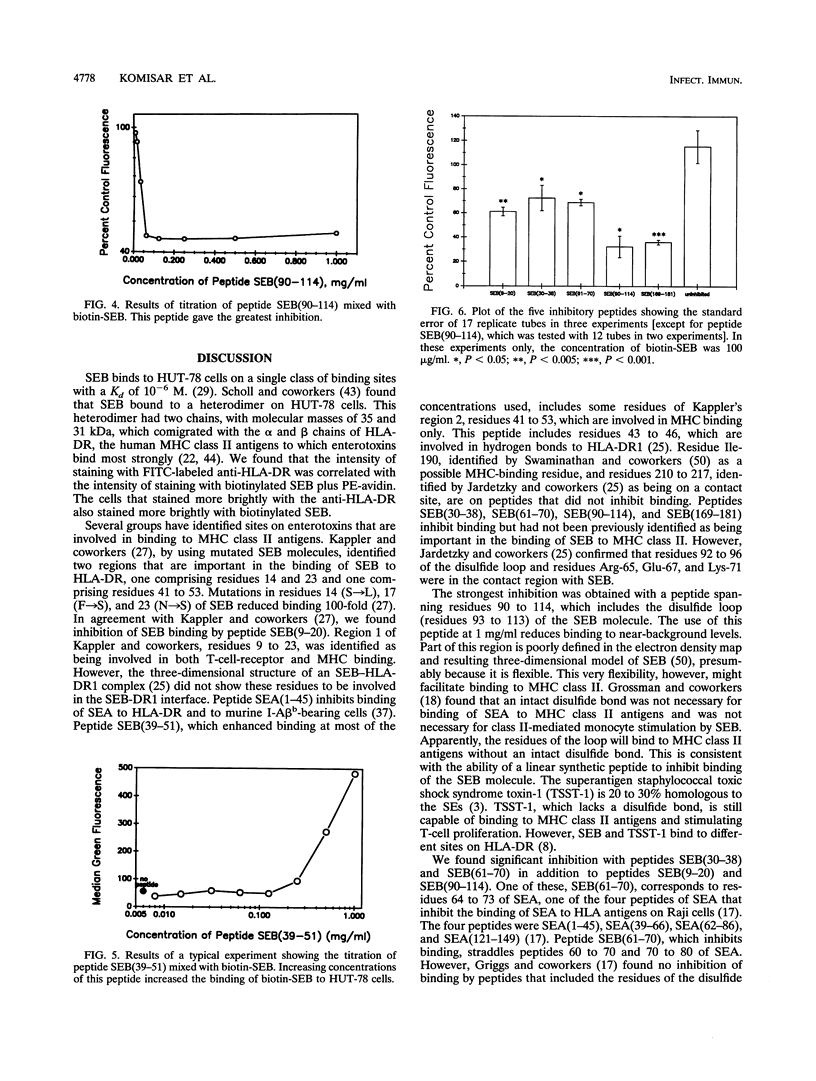
Staphylococcal enterotoxins are major causes of food poisoning and toxic shock syndrome. Their ability to bind to major histocompatibility complex (MHC) class II molecules has been suggested to be the first step in the mechanism whereby they cause illness. By flow cytometric analysis, the sites of interaction of staphylococcal enterotoxin B (SEB) with HLA-DR molecules were probed in the present study by inhibiting the binding of biotinylated SEB to a human T-cell line (HUT-78) with synthetic peptides of SEB. Five peptides of SEB gave significant inhibition of binding: a peptide containing amino acids 9 to 20 [SEB(9-20)], SEB(30-38), SEB(61-70), SEB(90-114), and SEB(169-181). One peptide, SEB(39-51), enhanced binding. Among the inhibitory peptides, SEB(90-114), a peptide spanning the entire disulfide loop, showed the most efficient inhibition of binding. Peptides SEB(9-20) and SEB(39-51) include amino acid residues that have been identified by previous mutation studies (J.W. Kappler, A. Herman, J. Clements, and P. Marrack, J. Exp. Med. 175:387-396, 1992) as being important in binding to MHC class II. Amino acids lining the alpha 5 groove of SEB have also been postulated to be involved in binding to MHC class II molecules. However, only two of the residues that line the alpha 5 groove of SEB, His-12 and Tyr-17, are on peptide SEB(9-20) that inhibits binding. These results confirm previous studies that implicated the amino-terminal portion of the molecule in binding to MHC class II molecules and further indicate an important role for residues in other regions, particularly the disulfide loop.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya K. R., Passalacqua E. F., Jones E. Y., Harlos K., Stuart D. I., Brehm R. D., Tranter H. S. Structural basis of superantigen action inferred from crystal structure of toxic-shock syndrome toxin-1. Nature. 1994 Jan 6;367(6458):94–97. doi: 10.1038/367094a0. [DOI] [PubMed] [Google Scholar]
- Betley M. J., Borst D. W., Regassa L. B. Staphylococcal enterotoxins, toxic shock syndrome toxin and streptococcal pyrogenic exotoxins: a comparative study of their molecular biology. Chem Immunol. 1992;55:1–35. [PubMed] [Google Scholar]
- Binek M., Newcomb J. R., Rogers C. M., Rogers T. J. Localisation of the mitogenic epitope of staphylococcal enterotoxin B. J Med Microbiol. 1992 Mar;36(3):156–163. doi: 10.1099/00222615-36-3-155. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T. S., Gorga J. C., Stern L. J., Urban R. G., Strominger J. L., Wiley D. C. Three-dimensional structure of the human class II histocompatibility antigen HLA-DR1. Nature. 1993 Jul 1;364(6432):33–39. doi: 10.1038/364033a0. [DOI] [PubMed] [Google Scholar]
- Buelow R., O'Hehir R. E., Schreifels R., Kummerehl T. J., Riley G., Lamb J. R. Localization of the immunologic activity in the superantigen Staphylococcal enterotoxin B using truncated recombinant fusion proteins. J Immunol. 1992 Jan 1;148(1):1–6. [PubMed] [Google Scholar]
- Carlsson R., Fischer H., Sjögren H. O. Binding of staphylococcal enterotoxin A to accessory cells is a requirement for its ability to activate human T cells. J Immunol. 1988 Apr 15;140(8):2484–2488. [PubMed] [Google Scholar]
- Chintagumpala M. M., Mollick J. A., Rich R. R. Staphylococcal toxins bind to different sites on HLA-DR. J Immunol. 1991 Dec 1;147(11):3876–3881. [PubMed] [Google Scholar]
- Choi Y. W., Kotzin B., Herron L., Callahan J., Marrack P., Kappler J. Interaction of Staphylococcus aureus toxin "superantigens" with human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8941–8945. doi: 10.1073/pnas.86.22.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaj B., Mourad W., Naccache P. H. Superantigen-mediated human monocyte-T lymphocyte interactions are associated with an MHC class II-, TCR/CD3-, and CD4-dependent mobilization of calcium in monocytes. J Immunol. 1992 Sep 1;149(5):1497–1503. [PubMed] [Google Scholar]
- Dellabona P., Peccoud J., Kappler J., Marrack P., Benoist C., Mathis D. Superantigens interact with MHC class II molecules outside of the antigen groove. Cell. 1990 Sep 21;62(6):1115–1121. doi: 10.1016/0092-8674(90)90388-u. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Schrezenmeier H., Conradt P. T lymphocyte activation by staphylococcal enterotoxins: role of class II molecules and T cell surface structures. Cell Immunol. 1989 Apr 15;120(1):92–101. doi: 10.1016/0008-8749(89)90177-9. [DOI] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- Fuleihan R., Mourad W., Geha R. S., Chatila T. Engagement of MHC-class II molecules by staphylococcal exotoxins delivers a comitogenic signal to human B cells. J Immunol. 1991 Mar 1;146(5):1661–1666. [PubMed] [Google Scholar]
- Gascoigne N. R., Ames K. T. Direct binding of secreted T-cell receptor beta chain to superantigen associated with class II major histocompatibility complex protein. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):613–616. doi: 10.1073/pnas.88.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs N. D., Pontzer C. H., Jarpe M. A., Johnson H. M. Mapping of multiple binding domains of the superantigen staphylococcal enterotoxin A for HLA. J Immunol. 1992 Apr 15;148(8):2516–2521. [PubMed] [Google Scholar]
- Grossman D., Cook R. G., Sparrow J. T., Mollick J. A., Rich R. R. Dissociation of the stimulatory activities of staphylococcal enterotoxins for T cells and monocytes. J Exp Med. 1990 Dec 1;172(6):1831–1841. doi: 10.1084/jem.172.6.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D., Lamphear J. G., Mollick J. A., Betley M. J., Rich R. R. Dual roles for class II major histocompatibility complex molecules in staphylococcal enterotoxin-induced cytokine production and in vivo toxicity. Infect Immun. 1992 Dec;60(12):5190–5196. doi: 10.1128/iai.60.12.5190-5196.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman D., Van M., Mollick J. A., Highlander S. K., Rich R. R. Mutation of the disulfide loop in staphylococcal enterotoxin A. Consequences for T cell recognition. J Immunol. 1991 Nov 15;147(10):3274–3281. [PubMed] [Google Scholar]
- Hedlund G., Dohlsten M., Herrmann T., Buell G., Lando P. A., Segrén S., Schrimsher J., MacDonald H. R., Sjögren H. O., Kalland T. A recombinant C-terminal fragment of staphylococcal enterotoxin A binds to human MHC class II products but does not activate T cells. J Immunol. 1991 Dec 15;147(12):4082–4085. [PubMed] [Google Scholar]
- Herman A., Croteau G., Sekaly R. P., Kappler J., Marrack P. HLA-DR alleles differ in their ability to present staphylococcal enterotoxins to T cells. J Exp Med. 1990 Sep 1;172(3):709–717. doi: 10.1084/jem.172.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Hufnagle W. O., Tremaine M. T., Betley M. J. The carboxyl-terminal region of staphylococcal enterotoxin type A is required for a fully active molecule. Infect Immun. 1991 Jun;59(6):2126–2134. doi: 10.1128/iai.59.6.2126-2134.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardetzky T. S., Brown J. H., Gorga J. C., Stern L. J., Urban R. G., Chi Y. I., Stauffacher C., Strominger J. L., Wiley D. C. Three-dimensional structure of a human class II histocompatibility molecule complexed with superantigen. Nature. 1994 Apr 21;368(6473):711–718. doi: 10.1038/368711a0. [DOI] [PubMed] [Google Scholar]
- Kanner S. B., Odum N., Grosmaire L., Masewicz S., Svejgaard A., Ledbetter J. A. Superantigen and HLA-DR ligation induce phospholipase-C gamma 1 activation in class II+ T cells. J Immunol. 1992 Dec 1;149(11):3482–3488. [PubMed] [Google Scholar]
- Kappler J. W., Herman A., Clements J., Marrack P. Mutations defining functional regions of the superantigen staphylococcal enterotoxin B. J Exp Med. 1992 Feb 1;175(2):387–396. doi: 10.1084/jem.175.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komisar J., Rivera J., Vega A., Tseng J. Effects of staphylococcal enterotoxin B on rodent mast cells. Infect Immun. 1992 Jul;60(7):2969–2975. doi: 10.1128/iai.60.7.2969-2975.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque N., Thibodeau J., Sékaly R. P. Interactions between staphylococcal superantigens and MHC class II molecules. Semin Immunol. 1993 Feb;5(1):23–32. doi: 10.1006/smim.1993.1004. [DOI] [PubMed] [Google Scholar]
- Lee J. M., Watts T. H. Binding of staphylococcal enterotoxin A to purified murine MHC class II molecules in supported lipid bilayers. J Immunol. 1990 Nov 15;145(10):3360–3366. [PubMed] [Google Scholar]
- Marrack P., Blackman M., Kushnir E., Kappler J. The toxicity of staphylococcal enterotoxin B in mice is mediated by T cells. J Exp Med. 1990 Feb 1;171(2):455–464. doi: 10.1084/jem.171.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Metzroth B., Marx T., Linnig M., Fleischer B. Concomitant loss of conformation and superantigenic activity of staphylococcal enterotoxin B deletion mutant proteins. Infect Immun. 1993 Jun;61(6):2445–2452. doi: 10.1128/iai.61.6.2445-2452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollick J. A., Cook R. G., Rich R. R. Class II MHC molecules are specific receptors for staphylococcus enterotoxin A. Science. 1989 May 19;244(4906):817–820. doi: 10.1126/science.2658055. [DOI] [PubMed] [Google Scholar]
- Mourad W., Mehindate K., Schall T. J., McColl S. R. Engagement of major histocompatibility complex class II molecules by superantigen induces inflammatory cytokine gene expression in human rheumatoid fibroblast-like synoviocytes. J Exp Med. 1992 Feb 1;175(2):613–616. doi: 10.1084/jem.175.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontzer C. H., Griggs N. D., Johnson H. M. Agonist properties of a microbial superantigen peptide. Biochem Biophys Res Commun. 1993 Jun 30;193(3):1191–1197. doi: 10.1006/bbrc.1993.1751. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Jarpe M. A., Johnson H. M. Site of nonrestrictive binding of SEA to class II MHC antigens. Int Arch Allergy Appl Immunol. 1990;93(2-3):107–112. doi: 10.1159/000235288. [DOI] [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Localization of an immune functional site on staphylococcal enterotoxin A using the synthetic peptide approach. J Immunol. 1989 Jul 1;143(1):280–284. [PubMed] [Google Scholar]
- Pontzer C. H., Russell J. K., Johnson H. M. Structural basis for differential binding of staphylococcal enterotoxin A and toxic shock syndrome toxin 1 to class II major histocompatibility molecules. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):125–128. doi: 10.1073/pnas.88.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad G. S., Earhart C. A., Murray D. L., Novick R. P., Schlievert P. M., Ohlendorf D. H. Structure of toxic shock syndrome toxin 1. Biochemistry. 1993 Dec 21;32(50):13761–13766. doi: 10.1021/bi00213a001. [DOI] [PubMed] [Google Scholar]
- Qasim W., Kehoe M. A., Robinson J. H. Does staphylococcal enterotoxin B bind directly to murine T cells? Immunology. 1991 Aug;73(4):433–437. [PMC free article] [PubMed] [Google Scholar]
- Schantz E. J., Roessler W. G., Wagman J., Spero L., Dunnery D. A., Bergdoll M. S. Purification of staphylococcal enterotoxin B. Biochemistry. 1965 Jun;4(6):1011–1016. doi: 10.1021/bi00882a005. [DOI] [PubMed] [Google Scholar]
- Scholl P. R., Diez A., Geha R. S. Staphylococcal enterotoxin B and toxic shock syndrome toxin-1 bind to distinct sites on HLA-DR and HLA-DQ molecules. J Immunol. 1989 Oct 15;143(8):2583–2588. [PubMed] [Google Scholar]
- Scholl P. R., Diez A., Karr R., Sekaly R. P., Trowsdale J., Geha R. S. Effect of isotypes and allelic polymorphism on the binding of staphylococcal exotoxins to MHC class II molecules. J Immunol. 1990 Jan 1;144(1):226–230. [PubMed] [Google Scholar]
- Spero L., Leatherman D. L., Adler W. H. Mitogenicity of formalinized toxoids of staphylococcal enterotoxin B. Infect Immun. 1975 Nov;12(5):1018–1020. doi: 10.1128/iai.12.5.1018-1020.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spero L., Morlock B. A. Biological activities of the peptides of staphylococcal enterotoxin C formed by limited tryptic hydrolysis. J Biol Chem. 1978 Dec 25;253(24):8787–8791. [PubMed] [Google Scholar]
- Spertini F., Spits H., Geha R. S. Staphylococcal exotoxins deliver activation signals to human T-cell clones via major histocompatibility complex class II molecules. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7533–7537. doi: 10.1073/pnas.88.17.7533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles B. G., Bavari S., Krakauer T., Ulrich R. G. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect Immun. 1993 Dec;61(12):5333–5338. doi: 10.1128/iai.61.12.5333-5338.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaminathan S., Furey W., Pletcher J., Sax M. Crystal structure of staphylococcal enterotoxin B, a superantigen. Nature. 1992 Oct 29;359(6398):801–806. doi: 10.1038/359801a0. [DOI] [PubMed] [Google Scholar]
- Tseng J., Komisar J. L., Chen J. Y., Hunt R. E., Johnson A. J., Pitt L., Rivera J., Ruble D. L., Trout R., Vega A. Immunity and responses of circulating leukocytes and lymphocytes in monkeys to aerosolized staphylococcal enterotoxin B. Infect Immun. 1993 Feb;61(2):391–398. doi: 10.1128/iai.61.2.391-398.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Yan X. J., Imanishi K., Kawachi A., Araake M., Tachihara R., Shinagawa K., Kanagawa O. Activation of murine T cells by staphylococcal enterotoxin E: requirement of MHC class II molecules expressed on accessory cells and identification of V beta sequence of T cell receptors in T cells reactive to the toxin. Cell Immunol. 1991 Apr 1;133(2):446–455. doi: 10.1016/0008-8749(91)90117-t. [DOI] [PubMed] [Google Scholar]


