Abstract
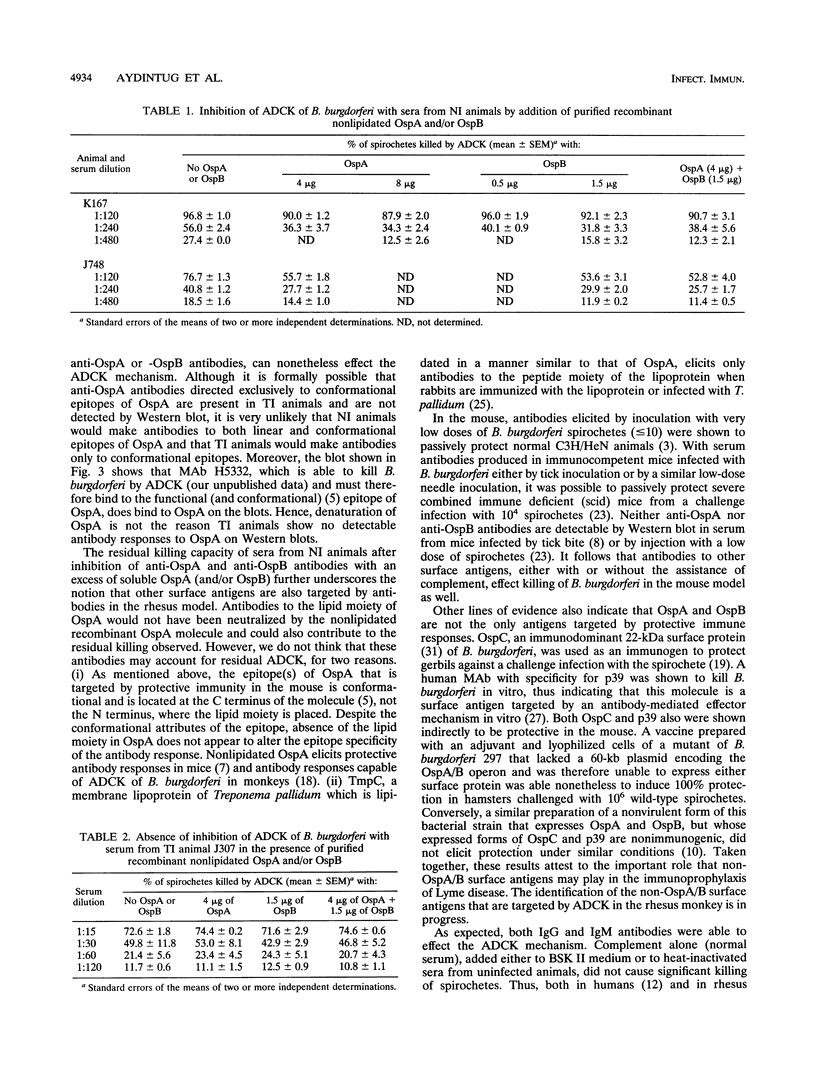
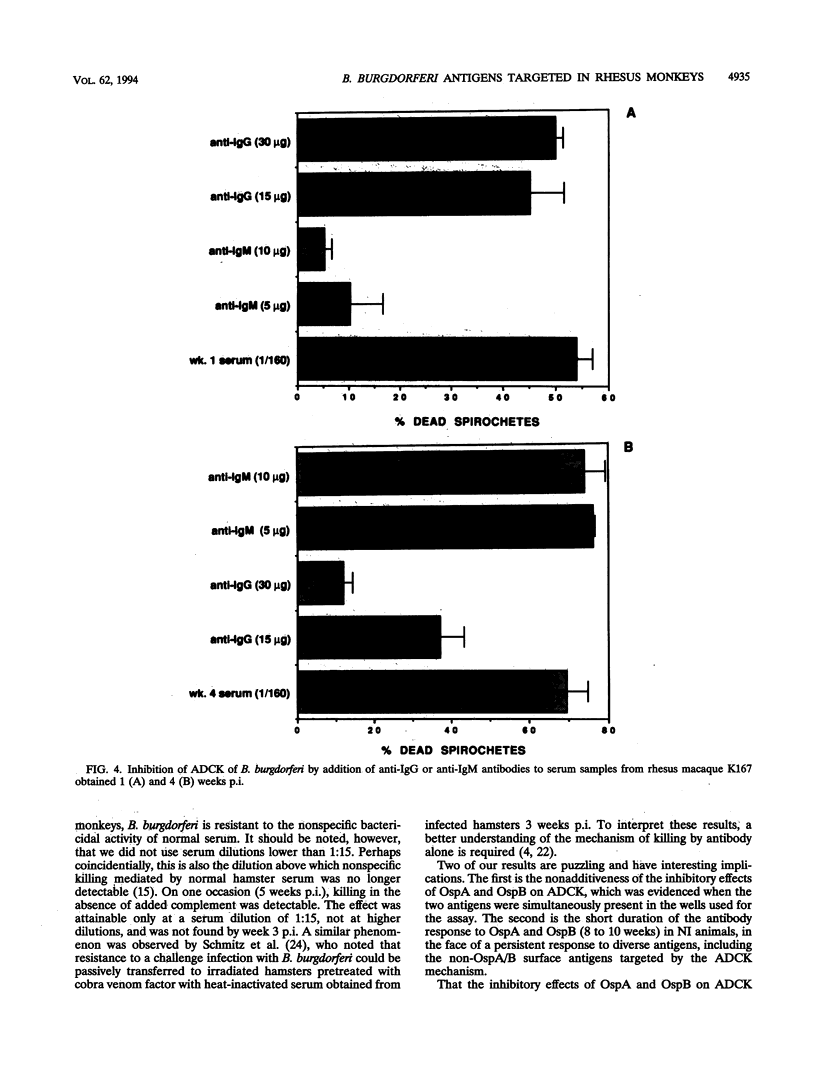
We identified surface antigens of Borrelia burgdorferi that are targeted by antibody-dependent, complement-mediated killing (ADCK) in the rhesus monkey. For this purpose, we had available serum samples from three animals infected with B. burgdorferi JD1 by needle inoculation and from two monkeys that were infected with the same B. burgdorferi strain by Ixodes scapularis tick bite. Sera from monkeys from the first group contained antibodies to OspA and OspB detectable by Western blot (immunoblot) using whole B. burgdorferi antigens, whereas serum samples from animals in the second group did not. The targeting of OspA and OspB by functional antibodies was demonstrated directly by showing that ADCK was partially inhibited when antibodies were preincubated with an excess of soluble recombinant OspA or OspB. Simultaneous addition of OspA and OspB did not result in an additive inhibitory effect on ADCK, a result that suggests that the epitopes on OspA and that on OspB targeted by antibody in this mechanism are the same, or at least cross-reacting. The targeting of non-OspA, non-OspB surface antigens was inferred from the fact that sera from tick-inoculated animals, which did not contain detectable anti-OspA or anti-OspB antibodies, were able to effect ADCK. This killing effect was not inhibitable by the addition of recombinant OspA or OspB or both proteins together. We also showed that both immunoglobulin G and M antibodies participate in the ADCK mechanism in the rhesus monkey. Rhesus complement does not kill B. burgdorferi in vitro in the absence of antibody, and antibody alone is effective in killing only at serum dilutions lower than 1:15. However, such "complement-independent" antibodies were not present in all bleeds. Two main conclusions may be drawn from the analysis of our results. First, both OspA and OspB are targeted by the ADCK mechanism in the rhesus monkey. Second, one or more B. burgdorferi surface antigens that are neither OspA nor OspB also participate in ADCK.
Full text
PDF

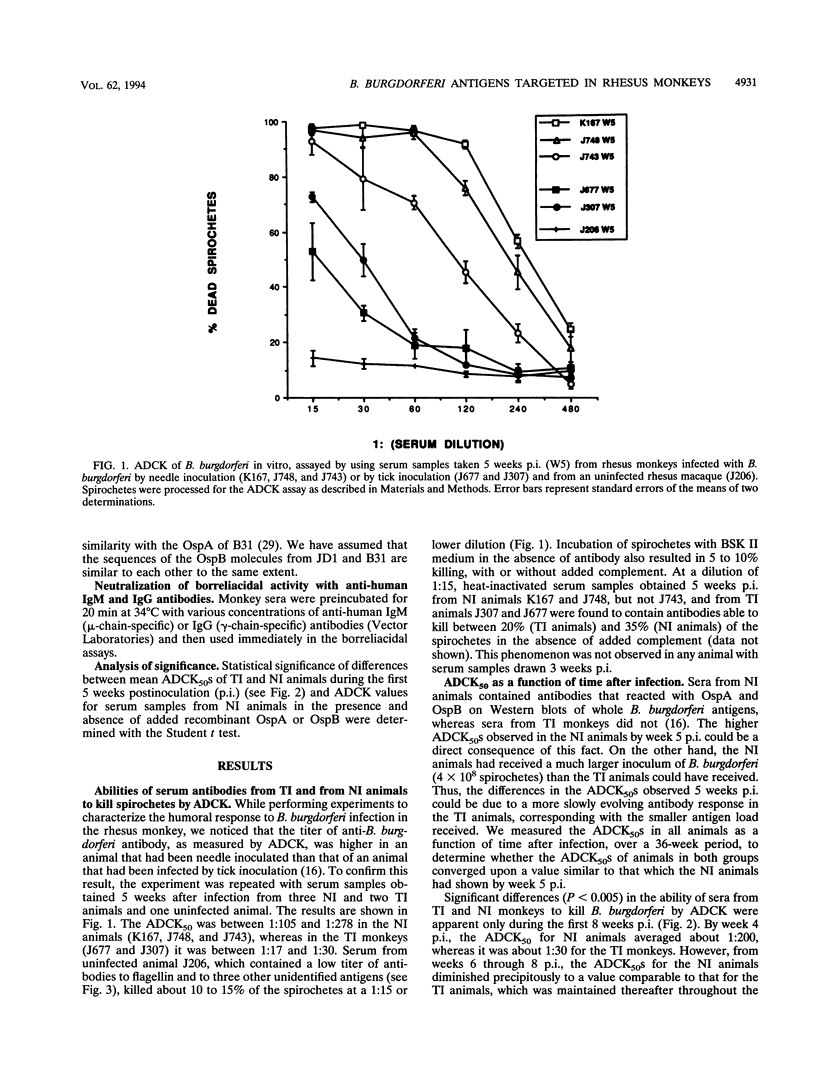


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbour A. G., Schrumpf M. E. Polymorphisms of major surface proteins of Borrelia burgdorferi. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 Dec;263(1-2):83–91. doi: 10.1016/s0176-6724(86)80107-9. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Bockenstedt L. K. Passive immunizing activity of sera from mice infected with Borrelia burgdorferi. Infect Immun. 1993 Nov;61(11):4696–4702. doi: 10.1128/iai.61.11.4696-4702.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt L. K., Barthold S., Deponte K., Marcantonio N., Kantor F. S. Borrelia burgdorferi infection and immunity in mice deficient in the fifth component of complement. Infect Immun. 1993 May;61(5):2104–2107. doi: 10.1128/iai.61.5.2104-2107.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockenstedt L. K., Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Inability of truncated recombinant Osp A proteins to elicit protective immunity to Borrelia burgdorferi in mice. J Immunol. 1993 Jul 15;151(2):900–906. [PubMed] [Google Scholar]
- Dunn J. J., Lade B. N., Barbour A. G. Outer surface protein A (OspA) from the Lyme disease spirochete, Borrelia burgdorferi: high level expression and purification of a soluble recombinant form of OspA. Protein Expr Purif. 1990 Nov;1(2):159–168. doi: 10.1016/1046-5928(90)90011-m. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Gern L., Schaible U. E., Simon M. M. Mode of inoculation of the Lyme disease agent Borrelia burgdorferi influences infection and immune responses in inbred strains of mice. J Infect Dis. 1993 Apr;167(4):971–975. doi: 10.1093/infdis/167.4.971. [DOI] [PubMed] [Google Scholar]
- Greene R. T., Walker R. L., Nicholson W. L., Heidner H. W., Levine J. F., Burgess E. C., Wyand M., Breitschwerdt E. B., Berkhoff H. A. Immunoblot analysis of immunoglobulin G response to the Lyme disease agent (Borrelia burgdorferi) in experimentally and naturally exposed dogs. J Clin Microbiol. 1988 Apr;26(4):648–653. doi: 10.1128/jcm.26.4.648-653.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C. A., Engstrom S. M., Coleman L. A., Kodner C. B., Johnson R. C. Protective immunity is induced by a Borrelia burgdorferi mutant that lacks OspA and OspB. Infect Immun. 1993 Dec;61(12):5115–5122. doi: 10.1128/iai.61.12.5115-5122.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M., Noppa L., Barbour A. G., Bergström S. Heterogeneity of outer membrane proteins in Borrelia burgdorferi: comparison of osp operons of three isolates of different geographic origins. Infect Immun. 1992 May;60(5):1845–1853. doi: 10.1128/iai.60.5.1845-1853.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi S. K., Johnson R. C., Dalmasso A. P. Facilitation of complement-dependent killing of the Lyme disease spirochete, Borrelia burgdorferi, by specific immunoglobulin G Fab antibody fragments. Infect Immun. 1993 Jun;61(6):2532–2536. doi: 10.1128/iai.61.6.2532-2536.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochi S. K., Johnson R. C. Role of immunoglobulin G in killing of Borrelia burgdorferi by the classical complement pathway. Infect Immun. 1988 Feb;56(2):314–321. doi: 10.1128/iai.56.2.314-321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lovrich S. D., Callister S. M., Schmitz J. L., Alder J. D., Schell R. F. Borreliacidal activity of sera from hamsters infected with the Lyme disease spirochete. Infect Immun. 1991 Aug;59(8):2522–2528. doi: 10.1128/iai.59.8.2522-2528.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M. T., Aydintug M. K., Bohm R. P., Jr, Cogswell F. B., Dennis V. A., Lanners H. N., Lowrie R. C., Jr, Roberts E. D., Conway M. D., Karaçorlu M. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993 Jul;61(7):3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Patsouris E., Jauris S., Will G., Soutschek E., Rainhardt S., Lehnert G., Klockmann U., Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992 Nov-Dec;20(6):342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- Roehrig J. T., Piesman J., Hunt A. R., Keen M. G., Happ C. M., Johnson B. J. The hamster immune response to tick-transmitted Borrelia burgdorferi differs from the response to needle-inoculated, cultured organisms. J Immunol. 1992 Dec 1;149(11):3648–3653. [PubMed] [Google Scholar]
- Schaible U. E., Gern L., Wallich R., Kramer M. D., Prester M., Simon M. M. Distinct patterns of protective antibodies are generated against Borrelia burgdorferi in mice experimentally inoculated with high and low doses of antigen. Immunol Lett. 1993 May;36(2):219–226. doi: 10.1016/0165-2478(93)90056-8. [DOI] [PubMed] [Google Scholar]
- Schmitz J. L., Lovrich S. D., Callister S. M., Schell R. F. Depletion of complement and effects on passive transfer of resistance to infection with Borrelia burgdorferi. Infect Immun. 1991 Oct;59(10):3815–3818. doi: 10.1128/iai.59.10.3815-3818.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schouls L. M., van der Heide H. G., van Embden J. D. Characterization of the 35-kilodalton Treponema pallidum subsp. pallidum recombinant lipoprotein TmpC and antibody response to lipidated and nonlipidated T. pallidum antigens. Infect Immun. 1991 Oct;59(10):3536–3546. doi: 10.1128/iai.59.10.3536-3546.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriba M., Ebrahim J. S., Schlott T., Eiffert H. The 39-kilodalton protein of Borrelia burgdorferi: a target for bactericidal human monoclonal antibodies. Infect Immun. 1993 Oct;61(10):4523–4526. doi: 10.1128/iai.61.10.4523-4526.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sădziene A., Rosa P. A., Thompson P. A., Hogan D. M., Barbour A. G. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992 Sep 1;176(3):799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R., Helmes C., Schaible U. E., Lobet Y., Moter S. E., Kramer M. D., Simon M. M. Evaluation of genetic divergence among Borrelia burgdorferi isolates by use of OspA, fla, HSP60, and HSP70 gene probes. Infect Immun. 1992 Nov;60(11):4856–4866. doi: 10.1128/iai.60.11.4856-4866.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmire W. M., Garon C. F. Specific and nonspecific responses of murine B cells to membrane blebs of Borrelia burgdorferi. Infect Immun. 1993 Apr;61(4):1460–1467. doi: 10.1128/iai.61.4.1460-1467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilske B., Preac-Mursic V., Jauris S., Hofmann A., Pradel I., Soutschek E., Schwab E., Will G., Wanner G. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun. 1993 May;61(5):2182–2191. doi: 10.1128/iai.61.5.2182-2191.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]