Abstract
Vibrio cholerae iron transport mutants were tested for their ability to cause disease in an infant mouse model. The mice were challenged with either the wild-type strain, a vibriobactin synthesis mutant, a heme utilization mutant, or double mutants containing both the vibriobactin synthesis defect and the heme utilization defect. When mice were challenged with 10(7) bacteria, the ability of the double mutant to survive in the intestines was greatly reduced and that of the heme utilization mutant was slightly reduced compared with that of the wild type or the vibriobactin synthesis mutant. When the inoculum size was reduced 10-fold, all of the iron transport mutants failed to colonize the intestines and failed to cause diarrhea in the mice, whereas the wild-type strain was not cleared and elicited a diarrheal response. These data indicate that disruption of either the heme utilization or the vibriobactin uptake system reduces the ability of V. cholerae to cause disease. One of the heme utilization mutants, DHH1, was found to be defective also in utilization of vibriobactin and ferrichrome, mimicking the Escherichia coli TonB- phenotype. This mutant was the least virulent of the iron transport mutants tested. Transformation of DHH1 with the recombinant plasmid pHUT4 restored the abilities to use hemin, vibriobactin, and ferrichrome as iron sources, suggesting that pHUT4 encodes a gene(s) involved globally in the iron transport systems. Hybridization of Vibrio DNA with the V. cholerae heme utilization genes demonstrated the presence of DNA homologous to the genes encoding the outer membrane protein HutA and the inner membrane protein HutB in all the V. cholerae strains tested. The probe containing hutA, but not that containing hutB, also hybridized to DNA from Vibrio parahaemolyticus.
Full text
PDF


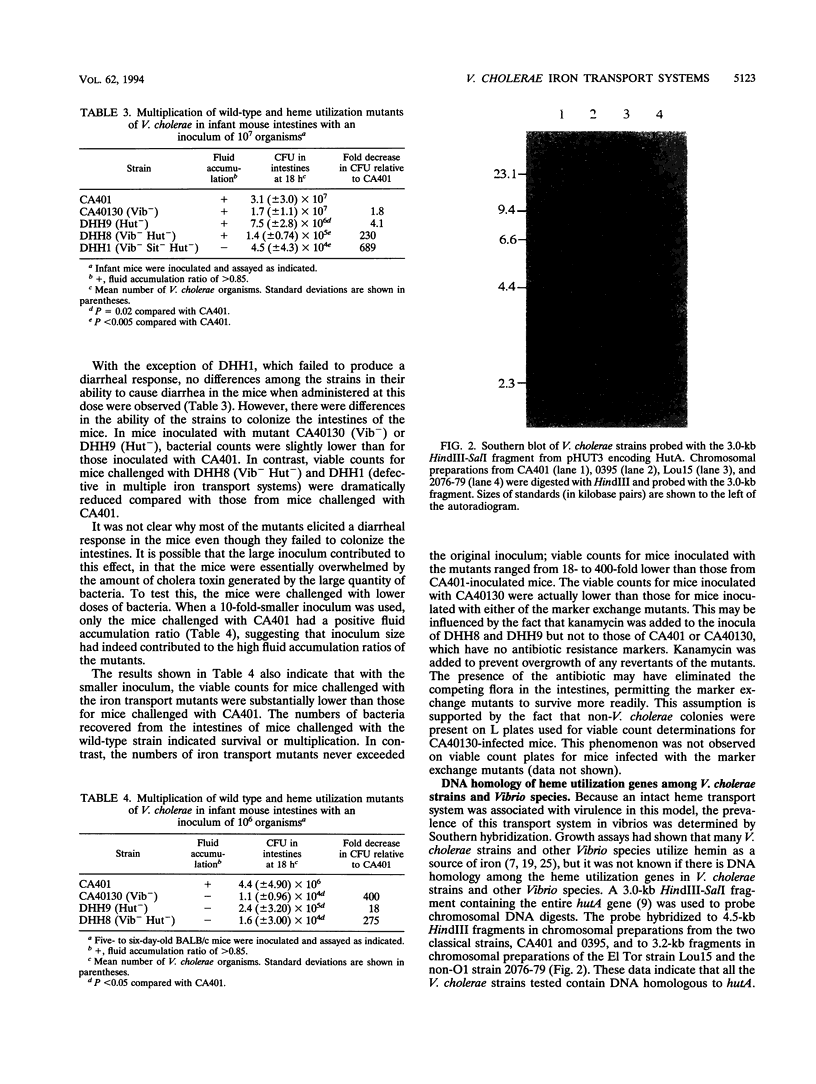


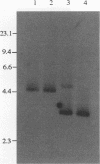
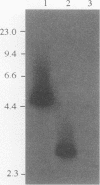
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baselski V., Briggs R., Parker C. Intestinal fluid accumulation induced by oral challenge with Vibrio cholerae or cholera toxin in infant mice. Infect Immun. 1977 Mar;15(3):704–712. doi: 10.1128/iai.15.3.704-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H. A plasmid associated with virulence in the marine fish pathogen Vibrio anguillarum specifies an iron-sequestering system. Nature. 1980 Apr 10;284(5756):566–568. doi: 10.1038/284566a0. [DOI] [PubMed] [Google Scholar]
- Davison J., Heusterspreute M., Chevalier N., Ha-Thi V., Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987;51(2-3):275–280. doi: 10.1016/0378-1119(87)90316-7. [DOI] [PubMed] [Google Scholar]
- GARDNER E. W., LYLES S. T., LANKFORD C. E. A COMPARISON OF VIRULENCE OF VIBRIO CHOLERAE STRAINS FOR THE EMBRYONATED EGG. J Infect Dis. 1964 Dec;114:412–416. doi: 10.1093/infdis/114.5.412. [DOI] [PubMed] [Google Scholar]
- Griffiths G. L., Sigel S. P., Payne S. M., Neilands J. B. Vibriobactin, a siderophore from Vibrio cholerae. J Biol Chem. 1984 Jan 10;259(1):383–385. [PubMed] [Google Scholar]
- Helms S. D., Oliver J. D., Travis J. C. Role of heme compounds and haptoglobin in Vibrio vulnificus pathogenicity. Infect Immun. 1984 Aug;45(2):345–349. doi: 10.1128/iai.45.2.345-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D. P., Payne S. M. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J Bacteriol. 1994 Jun;176(11):3269–3277. doi: 10.1128/jb.176.11.3269-3277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson D. P., Payne S. M. Cloning and characterization of the Vibrio cholerae genes encoding the utilization of iron from haemin and haemoglobin. Mol Microbiol. 1993 Feb;7(3):461–469. doi: 10.1111/j.1365-2958.1993.tb01137.x. [DOI] [PubMed] [Google Scholar]
- Koch J., Kølvraa S., Bolund L. An improved method for labelling of DNA probes by nicktranslation. Nucleic Acids Res. 1986 Sep 11;14(17):7132–7132. doi: 10.1093/nar/14.17.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka K., Bindereif A., Neilands J. B. Aerobactin-mediated utilization of transferrin iron. Biochemistry. 1982 Dec 7;21(25):6503–6508. doi: 10.1021/bi00268a028. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J., Swartz D. J., Pearson G. D., Harford N., Groyne F., de Wilde M. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature. 1983 Dec 8;306(5943):551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- Mickelsen P. A., Blackman E., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from lactoferrin. Infect Immun. 1982 Mar;35(3):915–920. doi: 10.1128/iai.35.3.915-920.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickelsen P. A., Sparling P. F. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect Immun. 1981 Aug;33(2):555–564. doi: 10.1128/iai.33.2.555-564.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilands J. B. Iron absorption and transport in microorganisms. Annu Rev Nutr. 1981;1:27–46. doi: 10.1146/annurev.nu.01.070181.000331. [DOI] [PubMed] [Google Scholar]
- Pidcock K. A., Wooten J. A., Daley B. A., Stull T. L. Iron acquisition by Haemophilus influenzae. Infect Immun. 1988 Apr;56(4):721–725. doi: 10.1128/iai.56.4.721-725.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postle K. TonB and the gram-negative dilemma. Mol Microbiol. 1990 Dec;4(12):2019–2025. doi: 10.1111/j.1365-2958.1990.tb00561.x. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schryvers A. B., Gonzalez G. C. Comparison of the abilities of different protein sources of iron to enhance Neisseria meningitidis infection in mice. Infect Immun. 1989 Aug;57(8):2425–2429. doi: 10.1128/iai.57.8.2425-2429.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Stoebner J. A., Payne S. M. Iron-vibriobactin transport system is not required for virulence of Vibrio cholerae. Infect Immun. 1985 Feb;47(2):360–362. doi: 10.1128/iai.47.2.360-362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. H. Novel iron uptake system specified by ColV plasmids: an important component in the virulence of invasive strains of Escherichia coli. Infect Immun. 1979 Dec;26(3):925–932. doi: 10.1128/iai.26.3.925-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey R. J., Finkelstein R. A. Assmilation of iron by pathogenic Neisseria spp. Infect Immun. 1981 May;32(2):592–599. doi: 10.1128/iai.32.2.592-599.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]