Abstract
Glucocorticoids (GCs) are common components of many chemotherapeutic regimens for lymphoid malignancies including acute lymphoblastic leukemia (ALL). The BCL-2 family has an essential role in regulating GC-induced cell death. Here we show that downregulation of antiapoptotic BCL-2 family proteins, especially MCL-1, enhances GC-induced cell death. Thus we target MCL-1 by using GX15-070 (obatoclax) in ALL cells. Treatment with GX15-070 in both dexamethasone (Dex)-sensitive and -resistant ALL cells shows effective growth inhibition and cell death. GX15-070 induces caspase-3 cleavage and increases the Annexin V-positive population, which is indicative of apoptosis. Before the onset of apoptosis, GX15-070 induces LC3 conversion as well as p62 degradation, both of which are autophagic cell death markers. A pro-apoptotic molecule BAK is released from the BAK/MCL-1 complex following GX15-070 treatment. Consistently, downregulation of BAK reduces caspase-3 cleavage and cell death, but does not alter LC3 conversion. In contrast, downregulation of ATG5, an autophagy regulator, decreases LC3 conversion and cell death, but does not alter caspase-3 cleavage, suggesting that apoptosis and autophagy induced by GX15-070 are independently regulated. Downregulation of Beclin-1, which is capable of crosstalk between apoptosis and autophagy, affects GX15-070-induced cell death through apoptosis but not autophagy. Taken together, GX15-070 treatment in ALL could be an alternative regimen to overcome glucocorticoid resistance by inducing BAK-dependent apoptosis and ATG5-dependent autophagy.
Keywords: glucocorticoid, obatoclax, apoptosis, autophagy, acute lymphoblastic leukemia, MCL-1
Glucocorticoids (GCs) are common components in many chemotherapeutic protocols for lymphoid/myeloid malignancies, including acute lymphoblastic leukemia (ALL). However, patients often develop resistance to GCs on relapse. Resistance to GCs in ALL can be associated with defects in apoptosis machinery and not in the GC receptor.1, 2, 3, 4 Thus, targeting downstream molecules may lead to the development of new therapeutic strategies. GC-induced apoptosis is through the intrinsic mitochondria-dependent pathway.5, 6, 7 The BCL-2 family proteins are central regulatory proteins in this pathway.8, 9 The BCL-2 family is subdivided into three main groups based on regions of BCL-2 homology (BH) and function: multi-domain antiapoptotic (e.g., BCL-2, MCL-1, BCL-XL), multi-domain pro-apoptotic (e.g., BAX, BAK), and BH3-only pro-apoptotic (e.g., BAD, BID, BIM, PUMA). BH3-only proteins cause cytochrome c release by activating BAX and/or BAK, while the antiapoptotic BCL-2 family of proteins prevents this process.10, 11 Targeting the BCL-2 family proteins might be a strategy to overcome GC resistance. We and others have shown that BIM, a pro-apoptotic BH3-only protein, is upregulated by dexamethasone (Dex) treatment in ALL cells and has an essential role in Dex-induced apoptosis.12 We then have demonstrated that co-treatment with Dex (for BIM upregulation) and MEK/ERK inhibitors (for BIM dephosphorylation/activation) promotes apoptosis in a variety of ALL cells.9 GC resistance is also derived from aberrant changes in the regulation of antiapoptotic proteins. Recent studies have shown that increased expression of MCL-1 is associated with GC resistance.13, 14, 15 MCL-1 is distinct among other antiapoptotic proteins, with its short protein turnover being regulated by the 26S proteasome.16 Thus, downregulation or inactivation of MCL-1 could be attractive to resensitize the chemotherapeutic response in ALL.
Recently, small molecules that directly interact with antiapoptotic BCL-2 proteins have been developed.17, 18 These agents interact with antiapoptotic BCL-2 family proteins at their BH3-binding grooves and mimic the action of BH3-only proteins. Among the small-molecule antagonists of antiapoptotic BCL-2 family proteins, GX15-070 (obatoclax), which is an indole bipyrrole compound, exhibits potency against MCL-1.19, 20 Although GX15-070 is currently used in developing single-agent therapy or in combination in phase I/II clinical trials directed at leukemia,21, 22 the molecular mechanisms of cell death induced by GX15-070 are not entirely clear. Some recent reports suggest the induction of autophagy and other cell death pathways besides caspase-dependent apoptosis by GX15-070.23, 24, 25, 26, 27, 28
A major form of autophagy is macroautophagy, in which parts of the cytoplasm and intracellular organelles are sequestered within a double autophagic membrane. Autophagosome formation is dependent on the interaction and activity of ATG proteins. Lipid–protein and protein–protein conjugations occur during autophagosome formation. One of the important conjugations is between cleaved ATG8/LC3 and phosphatidylethanolamine. This conjugation is an event to form an autophagosome structure and can be used as an autophagy marker. In the second conjugation event, ATG12 covalently binds to ATG5. ATG5 then associates with ATG16, which is required for autophagosome elongation. Beclin-1/ATG6 has a role in the initiation of autophagy, by its interaction with class III phosphatidylinositol-3 kinase.29 Furthermore, Beclin-1 has been reported as a BH3-only protein interacting with BCL-2 and BCL-XL, indicating that it is capable of crosstalk between autophagy and apoptosis.30
In this study, we show that GX15-070 induces cell death through BAK-dependent apoptosis and ATG5-dependent autophagy not only in Dex-sensitive, but also in Dex-resistant ALL cells. Thus, GX15-070 treatment in ALL could be an alternative regimen to overcome GC resistance.
Results
Downregulation of MCL-1 enhances Dex-induced lethality in ALL cells
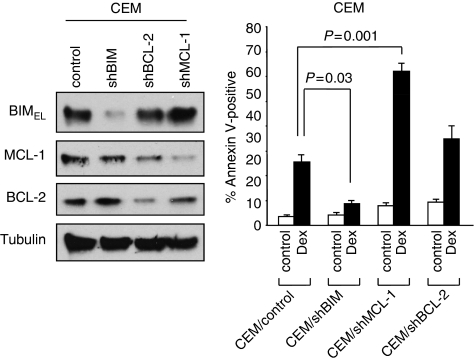
We and others have previously shown that BIM, a pro-apoptotic BH3-only protein, has an essential role in executing Dex-induced cell death in ALL cells. BIM is capable of interacting with all antiapoptotic BCL-2 family proteins (i.e., BCL-2, BCL-XL, MCL-1, BCL-w, and A1). To examine whether these antiapoptotic molecules have a specific role in Dex-induced apoptosis, we introduced shRNA for BCL-2 or MCL-1 into CCRF-CEM (CEM) T-ALL cells, and determined the effect on Dex-induced cell death. Downregulation of MCL-1 strongly enhanced apoptosis induced by Dex compared with the downregulation of BCL-2 (Figure 1). Downregulation of BIM showed significant reduction of Dex-induced apoptosis, as previously demonstrated.9 The results presented here and those of a previous publication31 suggest that inactivation of MCL-1 can sensitize Dex-induced cell death in ALL cells.
Figure 1.
Downregulation of MCL-1 enhances dexamethasone-induced lethality. Left panel: CEM cells were infected with lentiviruses expressing shRNAs for non-targeting control, BIM, MCL-1, or BCL-2. Puromycin-resistant cells were pooled after each infection. Equal amounts of total cell extracts were subjected to western blotting with the indicated antibodies. Right panel: Cells were treated with 100 nM Dex for 48 h. The percentage of apoptotic cells was determined by Annexin V-propidium iodide (PI) staining followed by FACS analysis. Values represent the mean±S.D. of three independent experiments
GX15-070 kills both Dex-sensitive and -resistant ALL cells
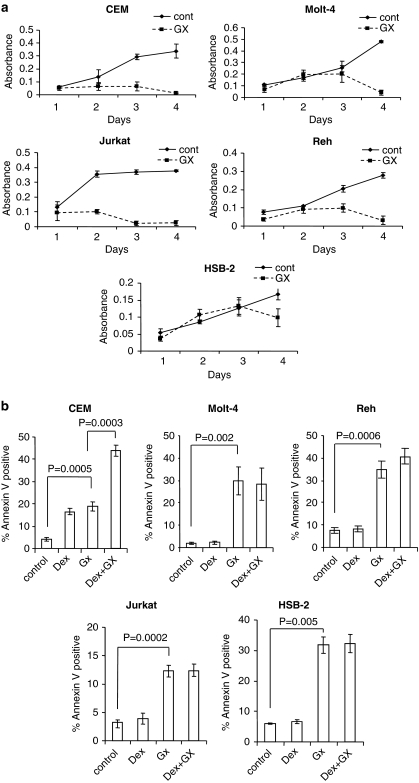
GX15-070 is a small compound that targets all antiapoptotic BCL-2 family proteins. The results in Figure 1 prompted us to examine whether GX15-070 induces cell death in ALL cells. We first determined the dose response of GX15-070 as a single agent for the induction of cell death and found that the concentration of 100 nM showed effective cell death activity in CEM cells (data not shown). Co-treatment of Dex-sensitive CEM cells with 100 nM Dex and 100 nM GX15-070 showed an additive killing effect as compared with each treatment alone (Figure 2a and b). We then treated Dex-resistant Molt-4, Reh, HSB-2, and Jurkat ALL cells with 100 nM of GX15-070. GX15-070 inhibited cell proliferation and induced cell death (Figure 2a and b). In contrast to CEM cells, no additive effect was observed by co-treatment with Dex and GX15-070 in these cells. Thus, the results strongly indicate that GX15-070 as a single agent is capable of overcoming Dex resistance in ALL cells.
Figure 2.
GX15-070 has lethality effect in both Dex-sensitive and -resistant ALL cells. (a) ALL cell lines including CEM, Molt-4, Jurkat, Reh, or HSB-2 were treated with 100 nM GX15-070 (GX) for indicated times and growth rate was determined by WST-1 colorimetric assay. Error bars represent the standard error of three independent experiments. (b) Cells were treated with 100 nM Dex and/or 100 nM GX15-070 for 48 h. The percentage of cell death was determined by Annexin V-PI staining followed by FACS analysis. Values represent the mean±S.D. of three independent experiments
GX15-070 induces apoptosis and autophagy
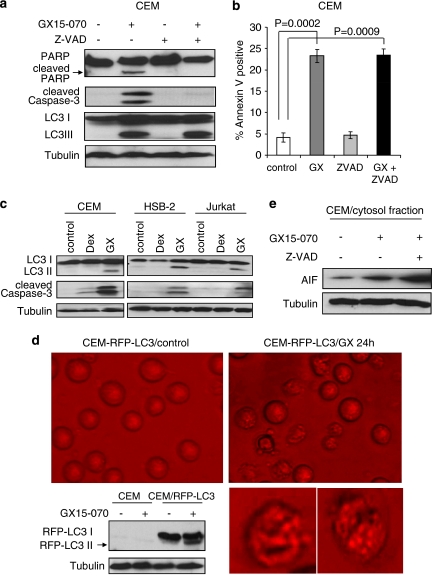
Increased population of Annexin V-positive cells (Figure 2b) indicates that cell death is induced by GX15-070 treatment. We further confirmed GX15-070-induced apoptosis by detection of caspase-3 and PARP cleavages in CEM cells (Figure 3a). To examine whether GX15-070-induced cell death is caspase-dependent, we added a pan-caspase inhibitor Z-VAD-FMK (Z-VAD) in GX15-070-treated CEM cells. Although caspase-3 and PARP cleavages were inhibited by Z-VAD (Figure 3a), total cell death induced by GX15-070 was not affected (Figure 3b). We thus examined whether other cell death pathways such as autophagy or caspase-independent cell death were induced. The conversion from LC3-I to -II, which is indicative of autophagy, was observed with GX15-070 treatment in CEM, HSB-2, and Jurkat cells (Figure 3a and c). However, we could not detect LC3 conversion with Dex treatment in these cells, suggesting that autophagy induction is specific to GX15-070 treatment. Furthermore, following GX15-070 treatment, RFP-LC3 transfected into CEM cells displayed punctated particles that show autophagosome formation and conversion of LC3-I to -II (Figure 3d). AIF release in cytosol, indicative of caspase-independent cell death, was also detected with GX15-070 treatment (Figure 3e). Interestingly, by the addition of Z-VAD, LC3 conversion was not altered (Figure 3a) and AIF release was increased (Figure 3e), suggesting that blocking GX15-070-induced caspase-dependent apoptosis by Z-VAD forced the cells to commit to caspase-independent apoptosis and autophagy.
Figure 3.
Apoptosis and autophagy are induced by GX15-070 treatment. (a) CEM cells were treated with 100 nM GX15-070±50 μM Z-VAD for 32 h. Z-VAD was added 30 min before GX15-070 treatment. Cleavage of PARP or caspase-3 and LC3 conversion were detected by western blot analyses. (b) CEM cells were treated with 100 nM GX15-070 in the presence or absence of 50 μM Z-VAD for 48 h. The percentage of cell death was determined by Annexin V-PI staining followed by FACS analysis. Values represent the mean±S.D. of three independent experiments. (c) Dex-sensitive CEM or -resistant HSB-2 and Jurkat cells were treated with 100 nM Dex or 100 nM GX15-070 for 24 h, and LC3 conversion and caspase-3 cleavage were detected by western blot analyses. (d) CEM cells were stably transfected with RFP-LC3, and G418-resistant clone cells were then treated with 100 nM GX15-070 for 24 h. Punctated RFP-LC3 was visualized under a fluorescent microscope. Western blot analysis confirmed the conversion of RFP-LC3. These pictures are representative of three independent experiments. Magnification is × 40. Bottom pictures are enlarged single-cell images. (e) CEM cells were treated with 100 nM GX15-070±50 μM Z-VAD for 24 h and release of cytochrome c or AIF in cytosol fraction was determined by western blot analyses
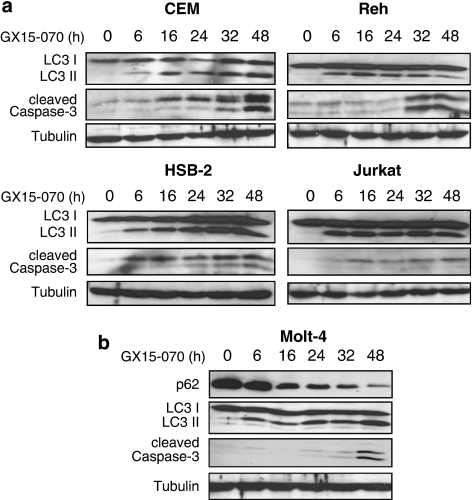
We next performed a time-course study to examine when autophagy and apoptosis started and proceeded by GX15-070 treatment. Autophagy judged by LC3 conversion was detected within 6 h after the treatment, while apoptosis judged by caspase-3 cleavage was detected at the later time points (Figure 4a and b). The most dramatic difference between the onset of autophagy and apoptosis was observed in Molt-4 cells. We also detected a massive decrease in p62 expression, which is another marker for autophagy (Figure 4b).29 Taken together, GX15-070 induces multiple cell death pathways such as caspase-dependent, -independent apoptosis, and autophagy.
Figure 4.
Autophagy is initiated before apoptosis by GX15-070 treatment. (a) CEM, Reh, HSB-2, or Jurkat cells were treated with 100 nM GX15-070 for the indicated times. LC3 conversion and caspase-3 cleavage were determined by western blot analyses. (b) Molt-4 cells were treated with 100 nM GX15-070 for the indicated times. LC3 conversion and degradation of p62 as well as caspase-3 cleavage were demonstrated by western blot analyses
GX15-070 induces BAK-dependent apoptosis in ALL cells
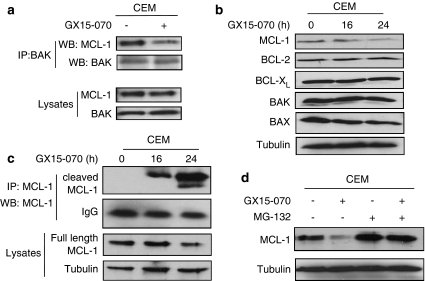
We further investigated the potential molecular mechanisms of how GX15-070 induces multiple cell death pathways. As BAK interacts with MCL-1 under normal condition and is released to be activated upon cell death stimuli,32 we examined the interaction of BAK with MCL-1 after GX15-070 treatment. We detected dissociation of BAK/MCL-1 at 5 h after treatment with GX15-070 (Figure 5a). Following the dissociation, we noticed that expression of MCL-1 was decreased at 16–24 h, while the expression levels of BAX, BAK, BIM, BCL-2, and BCL-XL remained constant (Figure 5b). Cleaved MCL-1 was increased in accordance with decrease of full-length MCL-1 (Figure 5c). Addition of MG-132, a proteasome inhibitor, restored the level of MCL-1 from GX15-070-mediated degradation (Figure 5d), suggesting that the degradation of MCL-1 is proteasome-dependent.
Figure 5.
GX15-070 induces dissociation of MCL-1/BAK complex. (a) CEM cells were treated with 100 nM GX15-070 for 5 h. Total cell extracts were subjected to immunoprecipitation with anti-BAK. Western blotting was performed on precipitated samples and on lysates collected before immunoprecipitation with MCL-1 or BAK antibodies. (b) CEM cells were treated with 100 nM GX15-070 for the indicated times and the levels of MCL-1, BCL-2, BCL-XL, BAX, and BAK were examined by western blot analysis. (c) CEM cells were treated with 100 nM GX15-070 for the indicated times. Total cell extracts were subjected to immunoprecipitation with anti-MCL-1. Western blotting was performed on precipitated samples and on lysates collected before immunoprecipitation with anti-MCL-1. (d) CEM cells were treated with 100 nM GX15-070 for 24 h in the presence or absence of 500 nM MG-132. The level of Mcl-1 was determined by western blot analysis
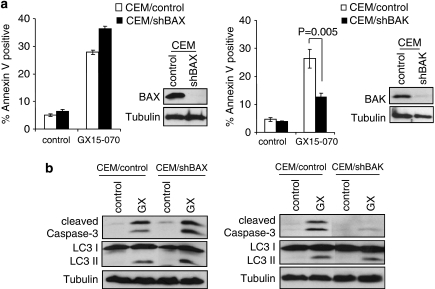
Downregulation of BAK, but not BAX, significantly inhibited apoptosis judged by Annexin V-positive population (Figure 6a) and caspase-3 cleavage (Figure 6b), suggesting that GX15-070-induced apoptosis is BAK-dependent. Taken together, these results indicate that GX15-070-induced apoptosis is initiated by the inhibition of MCL-1 binding to BAK, and then the amount of free BAK increases by MCL-1 degradation, which may amplify the apoptotic effect of BAK.
Figure 6.
GX15-070-induced apoptosis is BAK-dependent. (a) CEM cells were infected with lentiviruses expressing shRNAs for non-targeting control, BAX, or BAK. Puromycin-resistant cells were pooled after each infection. Downregulation of BAX or BAK expression was monitored by western blotting (in sets). Cells were treated with 100 nM GX15-070 for 48 h and the percentage of cell death was determined by Annexin V-PI staining followed by FACS analysis. Values represent the mean±S.D. of three independent experiments. (b) Cells harboring control, BAX, or BAK shRNA were treated with 100 nM GX15-070 for 32 h. Caspase-3 cleavage and LC3 conversion was determined by western blot analysis
GX15-070-induced autophagy is ATG5-dependent in ALL cells
We next investigated the molecular mechanisms of GX15-070-induced autophagy. ATG5 is known to function in autophagosome formation and completion. We could not establish the CEM cells in which ATG5 was downregulated, as knocking down of the ATG5 gene itself killed the cells (data not shown). Downregulation of ATG5 in Molt-4 cells reduced LC3 conversion as well as total cell death induced by GX15-070 treatment (Figure 7a and b). Thus, GX15-070-induced autophagy is ATG5-dependent and contributes to cell death.
Figure 7.
Downregulation of ATG5 decreases autophagy-mediated cell death induced by GX15-070 treatment. (a) Molt-4 cells were infected with lentiviruses expressing shRNAs for either non-targeting control or ATG5. Puromycin-resistant cells were pooled after each infection. Downregulation of ATG5 mRNA was monitored by q-PCR (bottom panel). Cells were treated with 100 nM GX15-070 for 72 h and the percentage of cell death was determined by Annexin V-PI staining followed by FACS analysis. Values represent the mean±S.D. of three independent experiments. (b) Cells were treated with 100 nM GX15-070 for the indicated times, and LC3 conversion and caspase-3 cleavage was assessed by western blot analyses
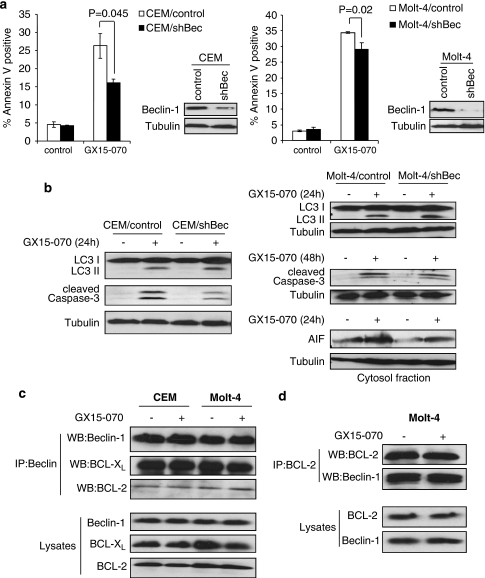
Beclin-1 is a key regulator of classical autophagy. Downregulation of Beclin-1 reduced GX15-070-induced cell death in both CEM and Molt-4 cells (Figure 8a). However, LC3 conversion was not altered (Figure 8b). The activation of class III phosphoinositide 3-kinase (PI3K III) is essential to induce autophagy through Beclin-1.33, 34 We thus examined whether inhibition of PI3K III by LY294002 or 3-MA could inhibit autophagy. Neither LY294002 nor 3-MA affected GX15-070-mediated LC3 conversion (Supplementary Figure S1a and b). These data suggest that GX15-070-induced autophagy is Beclin-1-independent.
Figure 8.
Beclin-1 contributes to GX15-070-induced apoptosis but not to autophagy. (a) CEM or Molt-4 cells were infected with lentiviruses expressing shRNAs for either non-targeting control or Beclin-1 (Bec). Puromycin-resistant cells were pooled after each infection. Downregulation of Beclin-1 expression was monitored by western blotting (in sets). Cells were treated with 100 nM GX15-070 for 48 h and the percentage of cell death was determined by Annexin V-PI staining followed by FACS analysis. Values represent the mean±S.D. of three independent experiments. (b) Cells were treated with 100 nM GX15-070 for the indicated times, and LC3 conversion, caspase-3 cleavage, and AIF release were assessed by western blot analyses. (c) CEM or Molt-4 cells were treated with 100 nM GX15-070 for 24 h. Total cell extracts were subjected to immunoprecipitation with anti-Beclin-1. Western blotting was performed on precipitated samples and on lysates collected before immunoprecipitation with Beclin-1, BCL-2, or BCL-XL antibodies. (d) Reciprocal immunoprecipitation was performed with anti-BCL-2. Western blotting was conducted on precipitated samples and on lysates collected before immunoprecipitation with BCL-2 or Beclin-1 antibodies
Apoptosis and autophagy induced by GX15-070 treatment are independently regulated
We next addressed whether there is any crosstalk between apoptosis and autophagy induced by GX15-070 treatment. Our results show that when apoptosis was reduced by downregulation of BAK, LC3 conversion was not affected (Figure 6a and b). In contrast, caspase-3 cleavage was not altered by downregulation of ATG5 when autophagy was reduced (Figure 7b), suggesting that apoptosis and autophagy are independently regulated by GX15-070 treatment.
Beclin-1, an autophagy regulator, is also known to physically interact with BCL-2 and BCL-XL as a BH3-only protein, suggesting that Beclin-1 could mediate crosstalk between autophagy and apoptosis pathways. Although GX15-070-induced autophagy was not affected by downregulation of Beclin-1 in CEM and Molt-4 cells, the amount of cell death was decreased (Figure 8a and b). We thus examined the role of Beclin-1 in the apoptosis pathway. In the case of CEM cells, downregulation of Beclin-1 reduced the amount of caspase-3 cleavage induced by GX15-070, suggesting that caspase-dependent apoptosis is partly regulated by Beclin-1 (Figure 8b). We did not observe any changes in the amount of AIF released after Beclin-1 knockdown in CEM cells (data not shown). On the contrary, Beclin-1 downregulation did not alter GX15-070-induced caspase-3 cleavage in Molt-4 cells while AIF release was decreased, suggesting that caspase-independent apoptosis is also regulated by Beclin-1 expression (Figure 8b). To further examine the role of Beclin-1 in crosstalk between autophagy and apoptosis, we determined the interactions of Beclin-1 with BCL-2, Beclin-1 with BCL-XL, and Beclin-1 with MCL-1 following GX15-070 treatment. Co-immunoprecipitation with anti-Beclin-1 followed by western blot with anti-BCL-2 or anti-BCL-XL revealed that the interactions were not altered by GX15-070 in both CEM and Molt-4 cells (Figure 8c). However, we could not detect any interaction of Beclin-1 with MCL-1 in both cell lines (data not shown). Reciprocal co-immunoprecipitation with anti-BCL-2 followed by western blot with anti-Beclin-1 confirmed the interaction of Beclin-1 with BCL-2 without any alteration by GX15-070 treatment (Figure 8d). Taken together, these results suggest that Beclin-1 does not have a role in inducing autophagy by GX15-070, but has a substantial role in GX15-070-induced apoptosis.
Discussion
GCs have been used in chemotherapy for leukemia, lymphoma, and myeloma for decades. Although they are effective in the initial stages, resistance on relapse often emerges. The molecular mechanisms of sensitivity/resistance to this agent are still not fully understood. In this study, we propose a possible strategy to overcome GC resistance in ALL by targeting downstream molecules of the GC receptor, such as the BCL-2 family proteins. We hypothesized that targeting antiapoptotic MCL-1 might be effective among the BCL-2 family proteins, as (1) we recognized that treatment with Dex in CEM or Molt-4 cells slightly induces MCL-19 and that the expression level of MCL-1 is higher in Dex-resistant ALL cells than in Dex-sensitive cells (Supplementary Figure S2); (2) recent studies have demonstrated that increased expression of MCL-1 is associated with GC resistance.13, 14, 15 In support of our hypothesis, downregulation of MCL-1 by shRNA enhanced Dex-induced cell death (Figure 1). We then pharmacologically inactivated MCL-1 function by GX15-070, a BH3 mimetic small molecule that targets antiapoptotic BCL-2 family proteins including BCL-2, BCL-XL, and MCL-1. Treatment with GX15-070 induced caspase-3 cleavage and increased the Annexin V-positive population, which is indicative of apoptosis. It has been shown that GX15-070 binds to MCL-1 and inhibits the binding with BAK,19 which leads to apoptosis. Consistent with the previous findings, dissociation of BAK/MCL-1 complex was detected 5 h after GX15-070 treatment. We also observed dramatic degradation of MCL-1 at later time points in a proteasome-dependent manner. These results suggest that BAK activation is initiated by the release from MCL-1 and probably is amplified by MCL-1 degradation. Downregulation of BAK reduced caspase-3 cleavage and cell death, but did not alter LC3 conversion, indicating that apoptosis but not autophagy is regulated by BAK.
Before the onset of apoptosis and as early as 6 h after the treatment, GX15-070 induced LC3 conversion as well as p62 degradation, which is indicative of autophagy. Downregulation of ATG5 decreased LC3 conversion and cell death, but did not alter caspase-3 cleavage. These data suggest that GX15-070-induced autophagy contributes to cell death but is not a pre-requisite for commitment to apoptosis. Thus, ATG5-dependent autophagy and BAK-dependent apoptosis are independently regulated by GX15-070 treatment. Furthermore, our data suggest that GX15-070-induced autophagy is executed by the Beclin-1-independent pathway. Downregulation of Beclin-1 did not alter LC3 conversion induced by GX15-070 treatment (Figure 8b). We can come to the same conclusion by using LY294002 and 3-MA, inhibitors of PI3K that are required for autophagy induction through Beclin-1. Neither LY29002 nor 3-MA affected GX15-070-mediated LC3 conversion (Supplementary Figure S1). Although GX15-070-induced autophagy was not affected by downregulation of Beclin-1, the amount of cell death decreased. This reduction of cell death is mediated through apoptosis, as caspase-3 cleavage or AIF release was decreased by downregulation of Beclin-1. It has been recently shown that after initiating apoptosis, Beclin-1 is cleaved by caspases and the N-terminal fragment of Beclin-1 can inhibit autophagy while the C-terminal fragment can amplify mitochondrial-mediated apoptosis,35 suggesting that Beclin-1 has dual function as a regulator of autophagy and apoptosis. In contrast to this finding, the level of full-length Beclin-1 was constant following GX15-070 treatment, even though caspase-3 was already active (data not shown). Of note, a recent report described that autophagy is induced after Dex treatment in Dex-sensitive ALL cells.36 However, we could not detect LC3 conversion in CEM cells after Dex treatment when caspase-3 cleavage was clearly observed (Figure 3c). Furthermore, we showed before that the amount of Dex-induced cell death was not altered by co-treatment with LY294002,12 and that co-treatment with Dex and GX15-070 showed an additive effect compared with each treatment alone (Figure 2b). Therefore, GX15-070-induced cell death pathways are distinct and independent of the Dex-induced cell death pathway.
Recently, Bonapace et al.28 demonstrated that GX15-070 re-sensitizes GC-resistant ALL cells. Our results demonstrate that single treatment with a low dose of GX15-070 (i.e., 100 nM) sufficiently induces growth inhibition and cell death by inducing BAK-dependent apoptosis and ATG5-dependent autophagy. The authors observed dissociation of the Beclin-1/MCL-1 complex with GX15-070 treatment. In contrast, we could not detect the Beclin-1/MCL-1 complex in CEM, Molt-4 and Jurkat cells (data not shown), with the same conditions under which we clearly observed Beclin-1/BCL-2 and Beclin-1/BCL-XL interaction (Figure 8c and d), probably because of the amount of existing complex in the cells. A previous report also showed that the interaction of MCL-1 and Beclin-1 is not as strong as that between Beclin-1 and BCL-2 and between Beclin-1 and BCL-XL.37 Thus, further investigation is required to clarify the mechanism required to initiate GX15-070-induced autophagy.
Enhanced expression levels of antiapoptotic BCL-2 family protein have been observed in several types of tumors. Targeting these proteins is therefore an attractive strategy for restoring the apoptosis process in tumor cells. Among the small-molecule BCL-2 inhibitors, ABT-737 and its analog ABT-263 are the leading compounds that are currently in clinical development. However, these molecules have an affinity only for BCL-2 and BCL-XL, but not for MCL-1. Thus, ABT-737 cannot be effective as a single-agent therapeutic for ALL when MCL-1 is overexpressed.17, 38 In contrast, GX15-070 can overcome the resistance conferred by high levels of MCL-1. Our results suggest that GX15-070 could be useful as a single-agent therapeutic against ALL and that the activity/expression of antiapoptotic proteins could be a biomarker to determine the treatment strategy for ALL patients.
Materials and methods
Cell lines and culture
CCRF-CEM, Molt-4, HSB-2, Reh, and Jurkat cells were purchased from the American Tissue Culture Collection (Manassas, VA, USA). Cells were cultured in RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 1 mM sodium pyruvate, streptomycin, and penicillin G at 37°C in a humidified, 5% CO2 incubator.
Chemicals and antibodies
Dex was purchased from Sigma (St Louis, MO, USA). GX15-070 was provided from Gemin X (Montreal, Quebec, Canada). MG-132 was purchased from Calbiochem (San Diego, CA, USA). The antibodies used for western blot and immunoprecipitation were as follows: BAX (N-20), α-tubulin, β-tubulin and AIF from Santa Cruz Biotechnology (Santa Cruz, CA, USA); BAK from Upstate/Millipore (Billerica, MA, USA); BCL-2 from Sigma; MCL-1 from Assay Designs (Ann Arbor, MI, USA); BIM, BCL-XL, Beclin-1, ATG5 and caspase-3 from Cell Signaling Technology (Beverly, MA, USA); cytochrome c and p62 from BD-Pharmingen (San Diego, CA, USA); Beclin-1 and LC3 from Novus Biologicals (Littleton, CO, USA); PARP from Oncogene (Darmstadt, Germany); WST-1 reagent from Roche (Mannheim, Germany); and Z-VAD-FMK from ALEXIS Biochemicals (Lausen, Switzerland).
Lentivirus infection and plasmid transfection
The lentiviral short-hairpin RNA (shRNA)-expressing constructs were purchased from Open Biosystems (Huntsville, AL, USA). The constructs were transfected into 293T packaging cells along with the packaging plasmids, and the lentivirus-containing supernatants were used to transduce ALL cells. The RFP-LC3 construct was kindly provided by Dr. David Gewirtz (VCU). Transfection was performed by electroporation using a Bio-Rad electroporator (Hercules, CA, USA). The cells were suspended in RPMI 1640 (4 × 106/400 μl) with 10 μg of DNA and electroporated in 0.4-cm cuvettes at 300 V and 500 μF. Puromycin (2 μg/ml for shRNAs) or G418 (800 μg/ml for RFP-LC3) selection to establish stable cells began 24 h after virus infection or electroporation.
Quantitative RT-PCR
Total RNA was extracted by Trizol (Invitrogen, Carlsbad, CA, USA) from Molt-4 cells infected with shATG5-containing lentivirus. In all, 1 μg of RNA was reverse-transcribed by using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems) according to the manufacturer's instructions. Using Taqman Gene Expression Assay probe/primer Hs00169468 (Applied Biosystems, Foster City, CA, USA), cDNAs were amplified in a fluorescence thermocycler (Applied Biosystems 7500HT Fast Real-time PCR system) and were analyzed based on the expression level of GADPH with SDS2.2 software.
Immunoprecipitation and western blot analyses
Whole-cell lysates were prepared with CHAPS lysis buffer (20 mM Tris (pH 7.4), 137 mM NaCl, 1 mM dithiothreitol (DTT), 1% CHAPS (3-((3-cholamidopropyl)dimethylammonio)-1-propanesulfonate), 20 mM NaF, 10 mM β-glycerophosphate, and a protease inhibitor cocktail (Sigma)). For immunoprecipitation, equal amounts of protein were incubated with the appropriate antibody for 3 h on ice. Then antibody complexes were captured with protein A/G beads (Pierce, Rockford, IL, USA), at 4°C for 1 h with rotation. After washing three times with the same lysis buffer, the beads were re-suspended in the sample buffer and the samples were separated by SDS-PAGE. For western blot analyses, equal amounts of proteins were loaded on SDS-PAGE, transferred to a nitrocellulose membrane and analyzed by immunoblotting.
Cell viability assay
Cell proliferation was measured by WST-1 assay. 104 CEM, Reh or HSB-2 and 5 × 103 Jurkat or Molt-4 cells were cultured in 100 μl RPMI and treated with 100 nM GX15-070 for 1–4 days. WST-1 (5 μl) was added to samples and they were incubated for 3 h at 37°C and in 5% CO2. Absorbance of triplicate samples at 450 nm was measured by a multilabel reader (PerkinElmer, Shelton, CT, USA).
Cell death was quantified by Annexin V-FITC or -APC (BD Pharmingen) and propidium iodide (Sigma) staining according to the manufacturer's protocol, followed by flow-cytometric analysis using FACScan (BD Biosciences).
Subcellular fractionation
Two million cells were washed in PBS and lysed by incubating for 30 s at room temperature in digitonin lysis buffer (75 mM NaCl, 8 mM Na2HPO4, 1 mM NaH2PO4, 1 mM EDTA, and 350 μg/ml digitonin). Lysates were centrifuged ( × 12 000 g) for 1 min, and the supernatant (cytosolic fraction) was collected. Pellets (membrane fractions) were washed once in cold PBS and lysed in CHAPS lysis buffer. The cytosolic and membrane samples were quantified, separated by SDS-PAGE, and subjected to western blot analysis.
Fluorescent microscopy
CEM cells stably expressing RFP-LC3 were treated with 100 nM GX15-070 for 24 h. Cells were then analyzed using Olympus 1X70 microscope.
Statistical analysis
Values represent the means±S.D. for three separate experiments. The significance of differences between experimental variables was determined using the Student's t-test. Values were considered statistically significant at P<0.05.
Acknowledgments
We thank Gemin X and David Gewirtz (VCU) for providing us GX15-070 (obatoclax) and the RFP-LC3 plasmid, respectively. We also thank Barbara Szomju, and Kristoffer Valerie for lentivirus generation at the VCU Macromolecule Core Facility, which was supported, in part, by NIH-NCI Cancer Center Core Grant 5P30CA016059. This work is supported by NIH R01CA134473 and the William Lawrence and Blanche Hughes Foundation.
Glossary
- GC
glucocorticoid
- GX
GX15-070
- ALL
Acute lymphoblastic leukemia
- Dex
dexamethasone
- BH
BCL-2 homology
- PI3K III
class III phosphoinositide 3-kinase
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Disease website (http://www.nature.com/cddis)
Supplementary Material
References
- Tissing WJ, Meijerink JP, den Boer ML, Pieters R. Molecular determinants of glucocorticoid sensitivity and resistance in acute lymphoblastic leukemia. Leukemia. 2003;17:17–25. doi: 10.1038/sj.leu.2402733. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Rainer J, Ploner C, Presul E, Riml S, Kofler R. Glucocorticoid-induced apoptosis and glucocorticoid resistance: molecular mechanisms and clinical relevance. Cell Death Differ. 2004;11 (Suppl 1:S45–S55. doi: 10.1038/sj.cdd.4401456. [DOI] [PubMed] [Google Scholar]
- Bachmann PS, Gorman R, Papa RA, Bardell JE, Ford J, Kees UR, et al. Divergent mechanisms of glucocorticoid resistance in experimental models of pediatric acute lymphoblastic leukemia. Cancer Res. 2007;67:4482–4490. doi: 10.1158/0008-5472.CAN-06-4244. [DOI] [PubMed] [Google Scholar]
- Tissing WJ, Meijerink JP, Brinkhof B, Broekhuis MJ, Menezes RX, den Boer ML, et al. Glucocorticoid-induced glucocorticoid-receptor expression and promoter usage is not linked to glucocorticoid resistance in childhood ALL. Blood. 2006;108:1045–1049. doi: 10.1182/blood-2006-01-0261. [DOI] [PubMed] [Google Scholar]
- Distelhorst CW. Recent insights into the mechanism of glucocorticosteroid-induced apoptosis. Cell Death Differ. 2002;9:6–19. doi: 10.1038/sj.cdd.4400969. [DOI] [PubMed] [Google Scholar]
- Frankfurt O, Rosen ST. Mechanisms of glucocorticoid-induced apoptosis in hematologic malignancies: updates. Curr Opin Oncol. 2004;16:553–563. doi: 10.1097/01.cco.0000142072.22226.09. [DOI] [PubMed] [Google Scholar]
- Planey SL, Litwack G. Glucocorticoid-induced apoptosis in lymphocytes. Biochem Biophys Res Commun. 2000;279:307–312. doi: 10.1006/bbrc.2000.3922. [DOI] [PubMed] [Google Scholar]
- Ploner C, Rainer J, Lobenwein S, Geley S, Kofler R. Repression of the BH3-only molecule PMAIP1/Noxa impairs glucocorticoid sensitivity of acute lymphoblastic leukemia cells. Apoptosis. 2009;14:821–828. doi: 10.1007/s10495-009-0355-5. [DOI] [PubMed] [Google Scholar]
- Rambal AA, Panaguiton ZL, Kramer L, Grant S, Harada H. MEK inhibitors potentiate dexamethasone lethality in acute lymphoblastic leukemia cells through the pro-apoptotic molecule BIM. Leukemia. 2009;23:1744–1754. doi: 10.1038/leu.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J. Quearry B and Harada H, p38-MAP kinase activation followed by BIM induction is essential for glucocorticoid-induced apoptosis in lymphoblastic leukemia cells. FEBS Lett. 2006;580:3539–3544. doi: 10.1016/j.febslet.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Stam RW, Den Boer ML, Schneider P, de Boer J, Hagelstein J, Valsecchi MG, et al. Association of high-level MCL-1 expression with in vitro and in vivo prednisone resistance in MLL-rearranged infant acute lymphoblastic leukemia. Blood. 2010;115:1018–1025. doi: 10.1182/blood-2009-02-205963. [DOI] [PubMed] [Google Scholar]
- Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–342. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, et al. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351:533–542. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- Opferman JT, Green DR. DUB-le trouble for cell survival. Cancer Cell. 2010;17:117–119. doi: 10.1016/j.ccr.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Chonghaile TN, Letai A. Mimicking the BH3 domain to kill cancer cells. Oncogene. 2008;27 (Suppl 1:S149–S157. doi: 10.1038/onc.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci USA. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–5438. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- Schimmer AD, O'Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- O'Brien SM, Claxton DF, Crump M, Faderl S, Kipps T, Keating MJ, et al. Phase I study of obatoclax mesylate (GX15-070), a small molecule pan-Bcl-2 family antagonist, in patients with advanced chronic lymphocytic leukemia. Blood. 2009;113:299–305. doi: 10.1182/blood-2008-02-137943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campas C, Cosialls AM, Barragan M, Iglesias-Serret D, Santidrian AF, Coll-Mulet L, et al. Bcl-2 inhibitors induce apoptosis in chronic lymphocytic leukemia cells. Exp Hematol. 2006;34:1663–1669. doi: 10.1016/j.exphem.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJ, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- Martin AP, Mitchell C, Rahmani M, Nephew KP, Grant S, Dent P. Inhibition of MCL-1 enhances lapatinib toxicity and overcomes lapatinib resistance via BAK-dependent autophagy. Cancer Biol Ther. 2009;8:2084–2096. doi: 10.4161/cbt.8.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang CC, Wroblewski D, Yang F, Hersey P, Zhang XD. Human melanoma cells under endoplasmic reticulum stress are more susceptible to apoptosis induced by the BH3 mimetic obatoclax. Neoplasia. 2009;11:945–955. doi: 10.1593/neo.09692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK, et al. Induction of autophagy-dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J Clin Invest. 2010;120:1310–1323. doi: 10.1172/JCI39987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Sinha S, Kroemer G. Bcl-2 family members: dual regulators of apoptosis and autophagy. Autophagy. 2008;4:600–606. doi: 10.4161/auto.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner C, Rainer J, Niederegger H, Eduardoff M, Villunger A, Geley S, et al. The BCL2 rheostat in glucocorticoid-induced apoptosis of acute lymphoblastic leukemia. Leukemia. 2008;22:370–377. doi: 10.1038/sj.leu.2405039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S, Colbert CL, Becker N, Wei Y, Levine B. Molecular basis of the regulation of Beclin 1-dependent autophagy by the gamma-herpesvirus 68 Bcl-2 homolog M11. Autophagy. 2008;4:989–997. doi: 10.4161/auto.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara A, Kabeya Y, Ohsumi Y, Yoshimori T. Beclin-phosphatidylinositol 3-kinase complex functions at the trans-Golgi network. EMBO Rep. 2001;2:330–335. doi: 10.1093/embo-reports/kve061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirawan E, Vande Walle L, Kersse K, Cornelis S, Claerhout S, Vanoverberghe I, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laane E, Tamm KP, Buentke E, Ito K, Kharaziha P, Oscarsson J, et al. Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 2009;16:1018–1029. doi: 10.1038/cdd.2009.46. [DOI] [PubMed] [Google Scholar]
- Erlich S, Mizrachy L, Segev O, Lindenboim L, Zmira O, Adi-Harel S, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3:561–568. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- High LM, Szymanska B, Wilczynska-Kalak U, Barber N, O'Brien R, Khaw SL, et al. The Bcl-2 homology domain 3 mimetic ABT-737 targets the apoptotic machinery in acute lymphoblastic leukemia resulting in synergistic in vitro and in vivo interactions with established drugs. Mol Pharmacol. 2010;77:483–494. doi: 10.1124/mol.109.060780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.