Abstract
The role of liver transplantation in 29 patients with fulminant and subacute hepatic failure due to a variety of different causes was examined by comparing the outcome and a variety of “hospitalization” variables. Transplanted patients (n = 13) were more likely to survive (p < 0.05), were younger (p < 0.05) and spent more time in the hospital (p < 0.025) than did those who were not transplanted (n = 16). Despite spending a much longer time in the hospital, transplanted patients spent less time in the intensive care unit (p < 0.05) in coma (p < 0.01) and on a respirator (p < 0.01) than did those not transplanted. Most importantly, the survival rate for transplanted patients was significantly improved (p < 0.05) as compared to those not transplanted. We conclude that liver transplantation can be applied successfully to the difficult clinical problem of fulminant and subacute hepatic failure.
Fulminant and subacute hepatic failure are major clinical problems in hepatology because of the uniformly poor prognosis experienced by its victims. Most series report mortality figures ranging between 80 to 100% with the majority reporting survival rates of only 5 to 10% (1-6).
A wide variety of experimental modalities have been used in an effort to improve the dismal prognosis of such patients. These include charcoal and other resin hemoperfusion systems, total body blood exchange techniques, temporary liver support using animal organs connected in series with the patient and heterotopic liver transplantation (7-15). As yet, none of these methods has provided consistent results. Moreover, in most hands, the with these modalities have heen little or no better than standard medical care provided in an intensive care unit.
Since February, 1981 until July 1, 1985, we have been referred for consideration for orthotopic hepatic transplantation (OLTx) 29 adult patients with acute or subacute hepatic failure. Herein, we report our experience with these patients.
MATERIALS AND METHODS
Definitions
For the purpose of this study, fulminant hepatic failure was defined as the occurrence of severe impairment of hepatocellular function progressing to advanced encephalopathy (either advanced Stage 3 or Stage 4) within 8 weeks of onset in an individual without a history or not of evidence of previous hepatic disease. Subacute hepatic failure was defined as the occurrence of severe irreversible liver failure which developed within 8 to 28 weeks from the onset of symptoms in an individual without an antecedent history or evidence of chronic liver disease.
Patients
All patients admitted to either the medical or surgical services of the authors of this paper with a diagnosis of acute fuiminant or subacute hepatic failure in advanced Stage III or Stage IV coma have been considered as possible candidates for OLTx since February, 1981. Since that time and until July 1, 1985, a total of 29 patients have been evaluated with these two diagnoses. Of these 29, 11 had fulminant viral hepatitis documented by the appropriate viral serologic studies and/or a clinical history of a needle stick or other blood exposure in the cases of non-A, non-B fulminant hepatitis. Nine patients had subacute Wilson’s disease documented by the presence of Kayser Fleischer rings, an increased urine and hepatic content of copper and a reduced serum ceruloplasmin level. Nine patients were thought to have fulminant drug or toxin-induced hepatotoxicity based upon a clinical history of recent drug or toxin exposure, a consistent history of fever and rapid onset of hepatotoxicity associated in most cases with an eosinophilia and the absence of any serologic or other laboratory data to suggest an alternative diagnosis of a recognizable viral or metabolic liver disease.
Diagnostic Evaluation of the Patients
Each patient underwent a complete liver transplant evaluation consisting of the studies required to identify the specific etiology and the severity of their disease, and to recognize and manage any complications that may have developed. This evaluation has been described before and has not changed over the 41/2 years which encompass this report (16-18).
Chart Review and Validation
The charts of the 29 patients included in this study were reviewed following their discharge or death to obtain the various data herein reported. All records including intensive care unit data sheets, anesthesia records, operative reports and the medical and surgical progress notes were reviewed. In addition, the health status of the 10 surviving patients has been determined by telephone contact with the patients and their local physicians.
Statistical Analysis
All data are reported as mean values ± S.E. Statistical differences have been determined using the Student’s t test. Associations have been assessed using the adds ratio to approximate the relative risk. In this setting, OLTx has been considered to be antecedent to the outcome (survival), and thus the odds ratio has been used to measure the odds in favor of experiencing the outcome (survival). When the confidence interval around the odds ratio excluded unity, indicating excess odds in favor, the significance of the association between transplantation and survival was tested using χ2 (19, 20).
RESULTS
The mean age of the 29 patients included in this study was 27.5 ± 2.2 years. Sixteen were female and 13 were male. Table 1 shows the individual ages, final diagnoses, type of hepatic failure, time from admission to onset of coma and outcome of the 29 patients studied. Table 2 shows the liver injury and hematologic parameters of the patients studied just before OLTx or at the time of death or peak level of abnormality (which ever was greater) if OLTx was not performed.
Table 1.
Age, etiology and outcome of the 29 cases of acute and subacute hepatic failure
| Patient | Age (yr) | Diagnosisa | Type of hepatic failureb |
Days in comac |
Outcomed |
|---|---|---|---|---|---|
| 1 | 37 | Non-A, non-B | S | 0 | A, T |
| 2 | 20 | HBV | A | 3 | D, N |
| 3 | 50 | HBV | A | 0 | D, N |
| 4 | 26 | HBV | A | 0 | A, T |
| 5 | 30 | HBV | A | 0 | D, N |
| 6 | 51 | Non-A, non-B | S | 0 | D, N |
| 7 | 26 | HAV | S | 4 | D, N |
| 8 | 29 | HAV | S | 0 | D, N |
| 9 | 21 | Non-A, non-B | S | 0 | A, T |
| 10 | 46 | HBV | S | 0 | D, N |
| 11 | 16 | Wilson’s | A | 2 | D, T |
| 12 | 16 | Wilson’s | A | 4 | A, T |
| 13 | 31 | Wilson’s | A | 2 | D, N |
| 14 | 25 | Wilson’s | s | 1 | A, T |
| 15 | 21 | Wilson’s | S | 0 | D, N |
| 16 | 27 | Wilson’s | S | 0 | D, N |
| 17 | 20 | Wilson’s | A | 0 | D, T |
| 18 | 32 | Wilson’s | A | 0 | A, N |
| 19 | 21 | Wilson’s | A | 0 | A, T |
| 20 | 33 | Gold | A | 7 | D, T |
| 21 | 51 | Halothane | A | 4 | A, N |
| 22 | 27 | Nitropropane | S | 4 | D, T |
| 23 | 49 | Cimetidine | A | 0 | D, N |
| 24 | 24 | Nitropropane | S | 0 | D, T |
| 25 | 32 | Disulfiram | A | 5 | D, N |
| 26 | 38 | α-Methyldopa | A | 2 | D, N |
| 27 | 17 | Acetaminophen | A | 0 | A, N |
| 28 | 22 | Non-A, non-B | S | 0 | A, T |
| 29 | 17 | Phenytoin | S | 0 | D, T |
| Mean ± S.E. | 27.5 ± 2.2 |
Non-A, non-B = non-A, non-B hepatitis; HBV = hepatitis B virus; HAV = hepatitis A virus.
S = subacute; A = acute.
Values: 0 = <12 hr = 0 days; 1 = >12 but <36 hr = 1 day; 2 = >36 but <60 hr = 2 days; 3 = >60 but <84 hr = 3 days; and 4 = >84 but <108 hr = 4 days.
A = alive; T = transplanted; D = dead; and N = not transplanted.
Table 2.
Liver injury and hematological parameters of the 29 patients
| AST (IU/liter) |
ALT (IU/liter) |
Alkaline phosphatase (IU/liter) |
Bilirubin T/Da (mg/dl) |
Protime (secs) |
White blood cells (×1,000 cells/mm3) |
Platelet count (×100,000 cells/mm3) |
|
|---|---|---|---|---|---|---|---|
| 1 | 288 | 456 | 109 | 29.4/13.1 | 28 | 6.0 | 45 |
| 2 | 319 | 340 | 570 | 44.4/18.4 | 47 | 5.3 | 48 |
| 3 | 310 | 350 | 50 | 32.1/21.9 | 21 | 18.9 | 20 |
| 4 | 500 | 620 | 144 | 36.2/18.9 | 36 | 14.5 | 62 |
| 5 | 764 | 622 | 234 | 11.3/7.5 | 14 | 8.6 | 179 |
| 6 | 124 | 53 | 35 | 51.6/41.6 | 19 | 3.3 | 78 |
| 7 | 91 | 25 | 115 | 5.6/2.9 | 17 | 17.6 | 165 |
| 8 | 122 | 62 | 121 | 5.1/2.4 | 32 | 8.3 | 90 |
| 9 | 45 | 50 | 142 | 55.0/39.0 | 27 | 18.5 | 58 |
| 10 | 945 | 801 | 118 | 16.1/10.2 | 12 | 7.4 | 60 |
| 11 | 1,526 | 1,830 | 170 | 52.1/39.6 | 50 | 14.3 | 50 |
| 12 | 215 | 82 | 875 | 34.6/17.8 | 18 | 11.2 | 72 |
| 13 | 1,900 | 2,300 | 186 | 56.1/40.2 | 48 | 16.1 | 51 |
| 14 | 140 | 95 | 420 | 38.1/11.6 | 19 | 10.7 | 27 |
| 15 | 149 | 233 | 450 | 29.1/17.7 | 25 | 12.2 | 23 |
| 16 | 140 | 180 | 185 | 9.5/4.4 | 17 | 11.8 | 140 |
| 17 | 235 | 122 | 81 | 23.0/8.9 | 46 | 6.8 | 54 |
| 18 | 965 | 1,236 | 320 | 44.0/31 | 35 | 67 | 35 |
| 19 | 1,625 | 1,925 | 250 | 46.0/40 | 25 | 12.3 | 75 |
| 20 | 444 | 644 | 155 | 56.0/41 | 27 | 9.4 | 81 |
| 21 | 321 | 618 | 316 | 67.0/57 | 62 | 17.1 | 73 |
| 22 | 3,370 | 3,500 | 165 | 37.0/11 | 65 | 22.1 | 256 |
| 23 | 921 | 227 | 172 | 2.3/1.6 | 15 | 2.2 | 50 |
| 24 | 125 | 135 | 116 | 10.5/8.7 | 18 | 5.2 | 84 |
| 25 | 2,295 | 1,158 | 173 | 20.0/16 | 25 | 12.4 | 150 |
| 26 | 3,251 | 3,726 | 245 | 15.4/11.4 | 18 | 7.1 | 84 |
| 27 | 152 | 36 | 39 | 75.0/45 | 35 | 18.3 | 88 |
| 28 | 187 | 202 | 115 | 21.0/17.5 | 22 | 7.3 | 75 |
| 29 | 151 | 173 | 128 | 36.4/30.1 | 35 | 5.9 | 80 |
| Normal values: | <36 | <32 | <115 | <1.1/<0.2 | 10-12 | 5-10 | 150-300 |
T/D = tota/direct.
Of these 29, only three patients, two females with acute hepatic failure due to recurrent halothane exposure and acetaminophen overdose respectively, and a young adult male with Wilson’s disease, recovered and left the hospital alive not having required a liver transplant. Of the 26 remaining patients, 13 died waiting to be transplanted while 13 others received an OLTx. Of those transplanted, 7 are alive and 6 died, for an overall survival rate of 10 of 29 (34%). However, it should be noted that 55% of those transplanted actually survived (Figure 1).
Fig. 1.
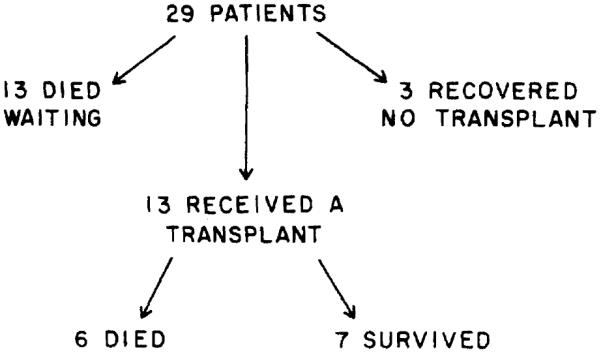
Schematic outline of the hospital course of the 29 patients evaluated.
In order to determine whether any differences existed between those who died waiting to be transplanted and those who actually lived long enough to receive a transplant, the age, the time spent in hospital, time spent in an intensive care unit, and the time in coma and/or on a respirator either prior to transplantation or death as well as the amount of blood products consumed by these patients were compared (Tables 3 to 5). The patients who lived long enough to be transplanted following admission to the hospital were younger (p < 0.05) and spent less time in the intensive care unit (p < 0.05), in coma (p < 0.01) and on a respirator (p < 0.01) than did those who died waiting to be transplanted (Tables 3 and 4). However, because the patients who were transplanted survived the early hospital period, as a result of being tranplanted, they spent more total time in the hospital than did those who were not transplanted, most of whom (13 of 16 or 81%) died (Table 3). Despite such a greater hospitalization time for those transplanted compared those not transplanted, no significant difference for blood product consumption was evident between the four groups.
Table 3.
Hospitalization characteristics of the 29 patients studied
| OLTx survived (n = 7) |
OLTx died (n = 6) |
No OLTx survived (n = 3) |
No OLTx died (n = 13) |
|
|---|---|---|---|---|
| Age (yr) | 21.0 ± 4.0 | 19.7 ± 4.4 | 35.3 ± 9.9 | 34.2 ± 3.0 |
| Time in hospital (days) |
12.1 ± 6.9 | 5.6 ± 2.8 | 21.7 ± 6.0 | 3.3 ± 0.6 |
| Time in ICU (days) | 1.6 ± 0.5 | 3.1 ± 0.7 | 5.7 ± 3.2 | 2.4 ± 0.5 |
| Time in coma (days) |
1.3 ± 0.3 | 2.4 ± 0.3 | 3.3 ± 1.9 | 1.1 ± 0.5 |
| Time on respirator (days) |
1.1 ± 0.4 | 1.7 ± 0.4 | 1.3 ± 1.3 | 1.8 ± 0.5 |
| RBC (units) | 3.9 ± 0.9 | 8.6 ± 3.5 | 4.0 ± 2.2 | 7.8 ± 1.6 |
| FFP (units) | 15.5 ± 3.5 | 32.4 ± 15.5 | 8.0 ± 3.1 | 17.4 ± 3.2 |
The abbreviations used are: ICU = intensive care unit; RBC = red blood cells; FFP = fresh frozen plasma.
Table 5.
Cause of death observed in the 19 patients who died
| I. Those not transplanted (n = 13) | |
| Hepatic failure | |
| Cerebral edema | 1 |
| Hepatorenal syndrome | 4 |
| Hemorrhagic pancreatitis | 1 |
| Bacterial sepsis | 3 |
| Gastrointestinal bleeding | 2 |
| Cerebral hemorrhage | 2 |
| II. Those transplanted (n = 6) | |
| Fungemia | 1 |
| Renal failure | 1 |
| Subarachnoid hemorrhage | 1 |
| Bacterial sepsis | 3 |
Table 4.
Characteristics of 13 transplanted patients
| Characteristic | Survivors (n = 7) |
Nonsurvivors (n = 6) |
|---|---|---|
| Age (yr) | 21.0 ± 4.0 | 19.7 ± 4.4 |
| Time in hospital prior to OLTx (days) |
12.1 ± 6.9 | 4.6 ± 2.6 |
| Time in ICU prior to OL Tx (days) |
0.6 ± 0.5 | 1.1 ± 0.5 |
| Time in coma prior to OLTx (days) |
0.3 ±O.3 | 0.4 ± 0.3 |
| Time on respirator prior to OLTx (days) |
0.1 ± 0.2 | 0.7 ± 0.5 |
| Units of RBC used prior to OLTx |
1.9 ± 0.8 | 4.6 ± 2.5 |
| Units of FFP used prior to OLTx |
10.5 ± 3.3 | 22.4 ± 12.5 |
| Total time in hospital (days) | 67.8 ± 19.4 | 26.0 ± 13.7 |
| Total time in ICU (days) | 2.8 ± 0.8 | 14.1 ± 5.9 |
| Total time in coma (days) | 0.5 ± 0.4 | 7.3 ± 4.7 |
| Total time on respirator (days) |
1.8 ± 0.4 | 9.9 ± 4.8 |
| Time for hepatectomy (min) | 221.3 ± 42.5 | 168.6 ± 41.8 |
| A hepatic time (min) | 64.6 ± 11.8 | 96.7 ± 21.8 |
| Implantation time (min) | 262.8 ± 50.5 | 320.6 ± 84.8 |
| Total units RBC (per hospi- talization) |
13.8 ± 3.5 | 32.2 ± 10.5 |
| Total units FFP (per hospi- talization) |
22.4 ± 5.6 | 41.1 ± 14.1 |
Values are mean ± S.E.
The abbreviations used are: ICU = intensive care unit; RBC = red blood cells; FFP = fresh frozen plasma.
In order to determine what factors contributed to poor posttransplant outcome, the same variables as as several others known to affect survival following OLTx were compared between those who survived and those who did not survive OLTx (Table 4). No statistical differences between the two groups were evident for of the 16 separate variables studied. However, a trend for a greater amount of time spent in the intensive unit, time on a respirator and units of blood products consumed prior to transplantation was seen for the non-surviving group. Had the two groups been larger, these differences may have achieved statistical significance. It should be noted, however, that the survivors had a longer hospitalization prior to transplant than did those who died, suggesting that they may have been less severely ill initially. Again, Table 4 shows a trend for greater intensive care unit use, time in coma, total time on a respirator and total blood product consumption by those who died as compared to those who survived following OLTx. Moreover, the duration of the anhepatic phase of transplant procedure itself tended to be longer in those did not survive. However, as a direct consequence of their survival, the survivors actually spent more time in the hospital than did nonsurvivors when all groups are compared (Table 5).
The immediate cause of death of the 13 patients died waiting to be transplanted and those who following OLTx are reported in Table 5.
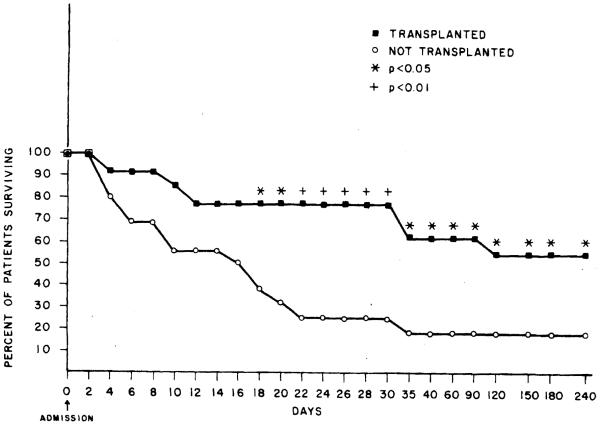
Figure 2 shows a life table analysis of the 29 patients herein reported. The survival rates at time points of 1 month or longer from time of hospital admission were significantly increased in the transplanted group as compared to those who did not receive a transplant. Specifically, the odds in favor of survival (odds ratio; upper 95% confidence interval; lower 95% confidence interval; and χ2 significance level, respectively) as a result of OLTx were increased 7-fold at 1 month (7.33; 38.86,1.38; p < 0.05) and 5-fold at 6 months (5.06; 15.38, 1.66; p < 0.05).
Fig. 2.
Actuarial life table analysis of the 29 patients evaluated with acute fulminant and subacuk hepatic failure divided into two groups: those transplanted (closed squares) and those not transplanted (open circles).
DISCUSSION
This report clearly documents the poor prognosis of patients with acute fulminant and subacute hepatic failure whether treated medically or surgically. It also supports the commonly held idea that youthful persons better than do older patients with such an illness. It should be noted, however, that only 4 of our 29 subjects were less than 20 years of age, the usual cut-off age cited for individuals having a better prognosis with fulminant or subacute hepatic failure. Moreover, two of these four died while one recovered without OLTx (Table 1). Thus, we do not believe that the younger mean age of the transplanted patients accounts for their greater survival. Most importantly, this report demonstrates that the shorter the time the patient is in coma and/or on a respirator, the better the overall prognosis of the patient. This observation is consistent with earlier reports of poorer posttransplant survival in patients hospitalized within intensive care units and those on respirators prior to OLTx (21). Finally, the present data document that liver transplantation alters the natural history of patients in advanced Stage III or IV hepatic coma due to acute fulminant and subacute hepatic failure by improving survival and changing the causes of death in those who ultimately die. Specifically, those who die prior to transplantation do so because of hepatic failure or one of it’s several associated complications (Table 5), while those who die after transplantation do so primarily because of either bacterial or fungal sepsis or renal failure, both of which are probably related to the use of cyclosporin-prednisone immunosuppression. The causes of death following transplantation observed in the patients herein reported do not differ from those of patients transplanted for the more usual indications for OLTx reported previously (22).
Nonetheless, the present data clearly document a 2.8-fold increased survival rate at 6 months for those patients who were transplanted as compared to those who were not. Data concerning the long-term survival of these patients are currently not available. However, as one would not expect the original toxic or metabolic liver disease to reoccur in the patients with such diseases originally, it is not unreasonable to believe that their long-term survival should be no different from that of individuals surviving OLTx performed for other indications. These data suggest that the survival curve is nearly flat 3 months after OLTx and remains so for approximately 6 years, the limits provided by the currently available experience for the large number of patients surviving OLTx. It is of some interest to note moreover that the single survivor of acute fulminant type B hepatitis became hepatitis B surface antibody-positive post-operatively and is currently working full time free of any clinical or biochemical evidence of liver disease.
Finally, it should be noted that liver failure, particularly its fulminant and subacute hepatic forms, is not a homogenous condition and that survival is known to be influenced by the underlying etiology (23, 24). In fact, the mix of patients herein reported is somewhat unusual, with 9 of the 29 having had fulminant Wilson’s disease. It should be noted in addition, however, that with the exception of this report, no case of fulminant Wilson’s disease has been reported to survive to date without OLTx. It shouId also be noted that only those who use less restrictive definitions of fulminant and subacute hepatic failure (e.g., not requiring the obligate presence of deep Stage 3 or Stage 4 coma) have reported survival rates of between 33 and 70%, which are comparable to what we have found in advanced stages of hepatic encephalopathy (1,3,5,10,23-28). Clearly, the role of liver transplantation in patients having less severe fulminant or subacute hepatic dysfunction but not in advanced Grade 3 or 4 hepatic encephalopathy but rather in Stage 2 and early Stage 3 remains to be determined. The current data suggest, however, that once an advanced stage of hepatic encephalopathy is reached, that liver transplantation improves survival in this particularly ill subset of patients with fulminant and subacute hepatic failure. This is particularly evident when one considers that spontaneous recovery, as evidenced by a return of consciousness, removed patients herein reported from the active transplant candidate list and thereby actually prejudices the data for survival in favor of the group not transplanted.
Acknowledgments
This work was supported in part by grants from the NIAMDD (ROl-AM32556), NIAAA (AA04425) and the Gastroenterology Medical Foundation of Southwestern Pennsylvania.
REFERENCES
- 1.Auslander MO, Gitnick GL. Vigorous medical management of acute fulminant hepatitis. Arch Intern Med. 1977;137:599–601. [PubMed] [Google Scholar]
- 2.Milandri M, Gaub J, Ranek L. Evidence for liver cell proliferation during fatal acute liver failure. Gut. 1980;21:423–427. doi: 10.1136/gut.21.5.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ring-Larsen H, Palazzo U. Renal failure in fulminant hepatic failure and terminal cirrhosis: a comparison between incidences, types and prognosis. Gut. 1981;22:585–591. doi: 10.1136/gut.22.7.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyke RJ, Canalese JC, Gimson AES, et al. Bacteraemia in patients with fulminant hepatic failure. Liver. 1982;2:45–52. doi: 10.1111/j.1600-0676.1982.tb00177.x. [DOI] [PubMed] [Google Scholar]
- 5.Christensen E, Bremmelgaard A, Bahnsen M, et al. Prediction of fatality in fulminant hepatic failure. Scand J Gastroenterol. 1984;19:90–96. [PubMed] [Google Scholar]
- 6.Kraut JR, Yogev R. Fatal fulminant hepatitis with hemolysis in Wilson’s disease. Clin Pediatr. 1984;23:637–640. doi: 10.1177/000992288402301106. [DOI] [PubMed] [Google Scholar]
- 7.Burnell JM, Dawborn JK, Epstein RB, et al. Acute hepatic coma treated by cross-circulation or exchange transfusion. N Engl J Med. 1967;276:935–943. doi: 10.1056/NEJM196704272761701. [DOI] [PubMed] [Google Scholar]
- 8.Abouna G, Ameniya H, Fisher L, et al. Hepatic support by intermittent liver perfusion and exchange blood transfusions. Transplant Proc. 1971;3:1589–1596. [Google Scholar]
- 9.Abouna G, Cook JS, Fisher L, et al. Treatment of acute hepatic coma by ex vivo baboon and human liver perfusions. Surgery. 1972;71:537–546. [PubMed] [Google Scholar]
- 10.Gazzard BG, Weston MJ, Murray-Lyon IM, et al. Charcoal haemoperfusion in the treatment of fulminant hepatic failure. Lancet. 1974;1:1301–1307. doi: 10.1016/s0140-6736(74)90678-3. [DOI] [PubMed] [Google Scholar]
- 11.Wakabayashi A, Kubo T, Gilman P, et al. Total body washout and ex vivo liver perfusion in acute hepatic failure. Arch Surg. 1974;109:52–56. doi: 10.1001/archsurg.1974.01360010038008. [DOI] [PubMed] [Google Scholar]
- 12.Opolon P. High-permeability membrane hemodialysis and hemofiltration in acute hepatic coma: experimental and clinical results. Artif Organs. 1979;3:354–360. doi: 10.1111/j.1525-1594.1979.tb01077.x. [DOI] [PubMed] [Google Scholar]
- 13.Willson RA. Acute fulminant hepatic failure. Gastroenterology. 1975;69:244–248. [PubMed] [Google Scholar]
- 14.Hamlyn AN, Gollan JL, Douglas AP, et al. Fulminant Wilson’s disease with haemolysis and renal failure: copper studies and assessment of dialysis regimens. Br Med J. 1977;2:660–663. doi: 10.1136/bmj.2.6088.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodle ES, Moody RR, Cox KL, et al. Orthotopic liver transplantation in a patient with amanita poisoning. JAMA. 1985;253:69–70. [PubMed] [Google Scholar]
- 16.Van Thiel DH, Schade RR, Starzl TE, et al. Liver transplantation in adults. Hepatology. 1982;2:637–640. doi: 10.1002/hep.1840020517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Thiel DH, Schade RR, Gavaler JS, et al. Medical aspects of liver transplantation. Hepatology. 1984;4:79S–83S. doi: 10.1002/hep.1840040721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Thiel DH. Liver transplantation. Pediatr Ann. 1985;14:474–480. doi: 10.3928/0090-4481-19850701-05. [DOI] [PubMed] [Google Scholar]
- 19.Schlesselman JJ. Case control studies: design, conduct and analysis. Oxford University Press; New York: 1982. p. 176. [Google Scholar]
- 20.Fleiss J. Statistical methods for rates and proportions. 2nd ed John Wiley and Sons; New York: 1973. p. 58. [Google Scholar]
- 21.Starzl TE, Iwatsuki S, Shaw BW, et al. Consensus conference report on liver transplantation. Dialysis, Transplantation & Burn. 1983;2:27–32. [PMC free article] [PubMed] [Google Scholar]
- 22.Cuervas-Mons V, Martinez AJ, Dekker A, et al. Adult liver transplantation: an analysis of the early causes of death in 40 consecutive cases. Hepatology. 1986;6:495–501. doi: 10.1002/hep.1840060329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bernuau J, Rueff B, Benhamon JP. Fulminant and subfulminant liver failure: definitions and causes. I. Sem Liver Dis. 1986;6:107–118. doi: 10.1055/s-2008-1040593. [DOI] [PubMed] [Google Scholar]
- 24.Tygstrup N, Ravek L. Assessment of prognosis in fulminant hepatic failure. Sem Liver Dis. 1986;6:129–137. doi: 10.1055/s-2008-1040596. [DOI] [PubMed] [Google Scholar]
- 25.Tygstrup N, Ravek L. Fulminant hepatic failure. Clin Gastroenterol. 1981;10:151–208. [PubMed] [Google Scholar]
- 26.Hughes RO, Williams R. Clinical experience with charcoal and resin hemoperfusion. Sem Liver Dis. 1986;6:164–173. doi: 10.1055/s-2008-1040600. [DOI] [PubMed] [Google Scholar]
- 27.Trey C, Lyscorth L, Dravidzan CS. Parameters influencing survival in the first 318 patients reported to the fulminant hepatic failure surveillance study. Gastroenterology. 1970;58:306–311. [Google Scholar]
- 28.Rueff B, Benhamou JP. Acute hepatic necrosis and fulminant hepatic failure. Gut. 1973;14:805–815. doi: 10.1136/gut.14.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]