Abstract
Cellular responses rely on signaling. In plant cells, cytosolic free calcium is a major second messenger, and ion channels play a key role in mediating physiological responses. Self-incompatibility (SI) is an important genetically controlled mechanism to prevent self-fertilization. It uses interaction of matching S-determinants from the pistil and pollen to allow “self” recognition, which triggers rejection of incompatible pollen. In Papaver rhoeas, the S-determinants are PrsS and PrpS. PrsS is a small novel cysteine-rich protein; PrpS is a small novel transmembrane protein. Interaction of PrsS with incompatible pollen stimulates S-specific increases in cytosolic free calcium and alterations in the actin cytoskeleton, resulting in programmed cell death in incompatible but not compatible pollen. Here, we have used whole-cell patch clamping of pollen protoplasts to show that PrsS stimulates SI-specific activation of pollen grain plasma membrane conductance in incompatible but not compatible pollen grain protoplasts. The SI-activated conductance does not require voltage activation, but it is voltage sensitive. It is permeable to divalent cations (Ba2+ ≥ Ca2+ > Mg2+) and the monovalent ions K+ and NH4+ and is enhanced at voltages negative to −100 mV. The Ca2+ conductance is blocked by La3+ but not by verapamil; the K+ currents are tetraethylammonium chloride insensitive and do not require Ca2+. We propose that the SI-stimulated conductance may represent a nonspecific cation channel or possibly two conductances, permeable to monovalent and divalent cations. Our data provide insights into signal-response coupling involving a biologically important response. PrsS provides a rare example of a protein triggering alterations in ion channel activity.
Signal-response coupling is essential for relaying signals to cellular targets, and ion transport is of fundamental importance to all cells. A major focus for studies in plant cells has been the role of cytosolic free calcium ([Ca2+]cyt) as a second messenger (for review, see Sanders et al., 2002; Hetherington and Brownlee, 2004). Considerable progress in understanding the nature of ion physiology in plant cells has been made, using Ca2+ imaging to detect and measure alterations in intracellular [Ca2+]cyt (Rudd and Franklin-Tong, 1999), electrophysiology to measure ion currents (Ward et al., 2009), and, more recently, identification of homologs of animal ion channels (Ward et al., 2009; Verret et al., 2010). Several major physiologically relevant systems have helped establish the nature of ion channels in higher plant cells; these include guard cells, root cells, and two tip-growing systems: root hairs and pollen (Feijo et al., 1995; White and Broadley, 2003; Demidchik and Maathuis, 2007; Ward et al., 2009). At the plant plasma membrane, several channels mediating Ca2+ influx have been characterized. A key characteristic is that although they are Ca2+ permeable, none are Ca2+ selective. There are many nonspecific cation channels (NSCCs); they include hyperpolarization- and depolarization-activated Ca2+ channels, stretch-activated channels, Glu receptors, and cyclic nucleotide gated channels (CNGCs). Where physiological functions have been identified, many mediate stress responses (for review, see Bothwell and Ng, 2005; Demidchik and Maathuis, 2007).
Polar tip growth is a specialized form of growth used by pollen tubes, root hairs, algal rhizoids, and fungal hyphae (Palanivelu and Preuss, 2000; Campanoni and Blatt, 2007) and has been used as a model system for ion regulation studies. An inward-rectifying Ca2+ conductance has been identified in Arabidopsis (Arabidopsis thaliana) root hair tips (Véry and Davies, 2000), and a Glu receptor is required for increases in [Ca2+]cyt in root hairs (Qi et al., 2006). In pollen tubes, the focus has been on the involvement of Ca2+ in signaling regulation of pollen tube growth as well as possible roles for H+, K+, and Cl− channels (Campanoni and Blatt, 2007; Cheung and Wu, 2008; Michard et al., 2009). Electrophysiological studies of pollen protoplast plasma membranes have provided evidence for hyperpolarization-activated Ca2+-permeable channels (Wang et al., 2004; Shang et al., 2005; Qu et al., 2007) and a stretch-activated Ca2+-permeable cation channel (Dutta and Robinson, 2004). Genetic evidence has provided the first functional evidence that a CNGC and a Ca2+ transporter, ACA9, are required for pollen tube tip growth (Schiøtt et al., 2004; Frietsch et al., 2007). Thus, there are a number of channels through which Ca2+ influx could occur in tip-growing plant cells (for review, see Michard et al., 2009).
Pollination involves the regulation of pollen tube growth by signaling networks at many levels (Michard et al., 2009). A further level of control is employed by many higher plants, as self-incompatibility (SI) provides a specialized mechanism to prevent self-fertilization. A multiallelic S-locus, comprising at least a pollen S-determinant and a pistil S-determinant, is responsible for specifying SI (Takayama and Isogai, 2005). Interaction between identical alleles of the pollen and pistil S-determinants results in rejection of incompatible (“self”) pollen. In Papaver rhoeas, the pistil S-determinants are small novel secreted cysteine-rich proteins (CRPs), recently renamed PrsS (Foote et al., 1994; Wheeler et al., 2009). The pollen S-determinant, PrpS, was recently identified as a small novel transmembrane protein (Wheeler et al., 2009). Addition of PrsS to incompatible pollen tubes triggers almost instantaneous, large, transient increases in [Ca2+]cyt (Franklin-Tong et al., 1993, 1995, 1997) and Ca2+ influx (Franklin-Tong et al., 2002). This provided the basis for a longstanding model for SI in this species: namely, that PrsS acts as a signaling ligand that interacts with the pollen S-determinant to trigger a Ca2+-dependent signaling network specifically in incompatible pollen. Several Ca2+-regulated cellular targets have been identified, resulting in arrest of growth and subsequent programmed cell death of incompatible pollen (Thomas and Franklin-Tong, 2004; Bosch and Franklin-Tong, 2007; for a recent review, see Bosch et al., 2008).
Despite good progress in understanding the downstream events triggered by SI, crucial early signaling events have not been well characterized. Although a Ca2+-selective vibrating probe approach provided evidence that SI stimulated Ca2+ influx adjacent to the pollen tube membrane (Franklin-Tong et al., 2002), the nature of the putative ion channels involved was not examined. In this study, we employed whole-cell patch clamping of Papaver pollen protoplasts to investigate the nature of the SI response with respect to ion channel activity. Our data demonstrate that SI stimulates S-specific activation of Ca2+- and K+-permeable conductance, implicating a ligand-gated channel in the early SI response.
RESULTS
PrsS Activates Ca2+ Currents in Incompatible Papaver Pollen Protoplasts
In order to establish if SI stimulated Ca2+ currents, we used a whole-cell patch-clamping approach to measure plasma membrane ion channel activation during the SI response. Pollen grain protoplasts (diameter of 30.3 ± 3.0 μm; n = 106) were superfused with medium containing 10 mm CaCl2, 100 mm Glc, 5 mm MES, and d-sorbitol, pH 5.5, in which Ca2+ was the only extracellular cation. The holding voltage was set at −100 mV, a value consistent with plant cell membrane potential under physiological conditions. Addition of incompatible recombinant PrsS stimulated a large, inward current that activated rapidly upon the arrival of PrsS in the recording chamber and saturated within 20 s (13.3 ± 2.0 pA pF−1; n = 40; Fig. 1A). Upon washout, the inward current rapidly decayed and returned to prestimulus levels with a similar time course to activation. Subsequent application of PrsS reactivated the current to the same level; this response to washout and reactivation could be repeated up to eight times (Fig. 1A; n = 15). Since the protoplasts were held under voltage clamp, activation must be a direct result of application of PrsS and not an indirect effect mediated through a change in membrane potential.
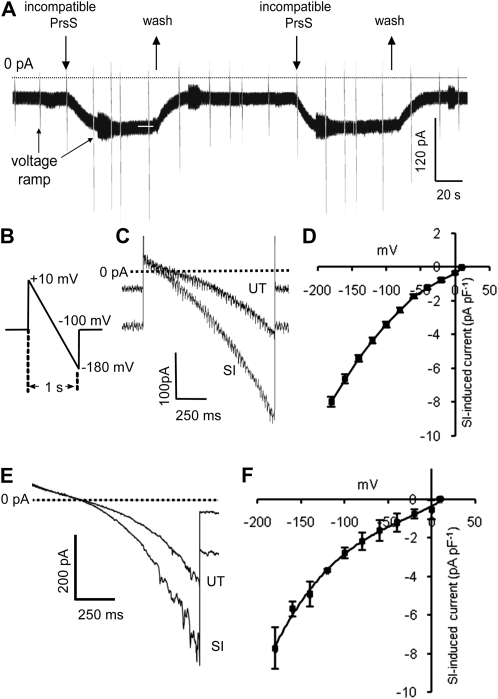
Figure 1.
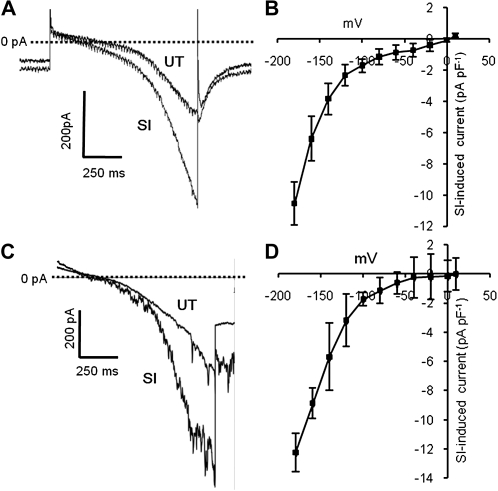
PrsS stimulates a ligand-gated ion Ca2+-permeable conductance. SI was induced by the addition of PrsS3 + PrsS8 to pollen protoplasts (haplotype S3 or S8), bathed in Ca2+-containing saline. A, Whole-cell current record shows induction of inward current by PrsS (10 μg mL−1) in a whole-cell-clamped pollen protoplast (Vh = −100 mV). Incompatible PrsS was applied by bath perfusion (indicated by arrows above traces). Inward current was activated immediately upon arrival of PrsS in the recording chamber and stabilized within 20 s. Upon washout of PrsS (upward arrows), the current returned to control levels. Reapplication of PrsS induced a second, identical inward current. Responses to voltage ramps applied before and during PrsS exposure appear as brief current excursions (arrows). B, Cartoon of the ramp protocol used for all experiments except those in Figure 2. Cells were stepped from −100 mV (holding voltage) to 10 mV followed by a linear ramp (duration of 1 s) to −180 mV; an interval of 10 to 20 s was allowed between ramps. C, Representative ramp-induced, whole-cell currents obtained in an untreated pollen protoplast bathed in Ca2+-containing saline before (UT) and after SI induction (SI) by PrsS addition. The dashed line indicates zero current. SI increased the holding current before application of the voltage ramp and greatly increased inward current at more negative potentials. D, I-V curve (mean ± se; n = 41) for SI-induced current in protoplasts bathed in Ca2+-containing saline. E, Representative ramp-induced, whole-cell PrsS-induced Ca2+ currents obtained before (UT) and after PrsS addition (SI) to a protoplast bathed in Ca2+-containing saline, using K+ as the backfill internal solution. The dashed line indicates zero current. SI increased the holding current before application of the voltage ramp and greatly increased inward current at more negative potentials. F, I-V curve (mean ± se; n = 3) for SI-induced current in protoplasts, using K+ in the backfill, internal solution.
To examine the current-voltage (I-V) relationship of PrsS-induced current, we used a protocol that applied a voltage ramp (+10 to −180 mV) over a period of 1 s before returning to −100 mV (Fig. 1B). PrsS treatment of incompatible pollen protoplasts induced a large increase in the current and a small positive shift in reversal potential (Fig. 1C). The PrsS-induced current (isolated by subtraction of leak current recorded before PrsS application) was markedly nonlinear, with mean amplitudes of −8.0 ± 0.31 at −180 mV and −3.4 ± 0.17 at −100 mV (mean ± se; n = 41) and approaching zero current at approximately 10 mV (Fig. 1D; n = 41).
To confirm that this PrsS-induced current could be observed in a more physiological intracellular environment, we repeated this protocol using a pipette saline in which Cs+ was replaced by 100 mm K+. Currents recorded under these conditions resembled those observed using the standard Cs+-based pipette saline and in some instances showed single channel activity (Fig. 1E). Mean reversal potential and current amplitude were indistinguishable from those recorded using Cs+ pipette saline (P > 0.3 for amplitude of PrsS-induced current at all voltages; Fig. 1F).
The PrsS-Activated Current Is Voltage Dependent
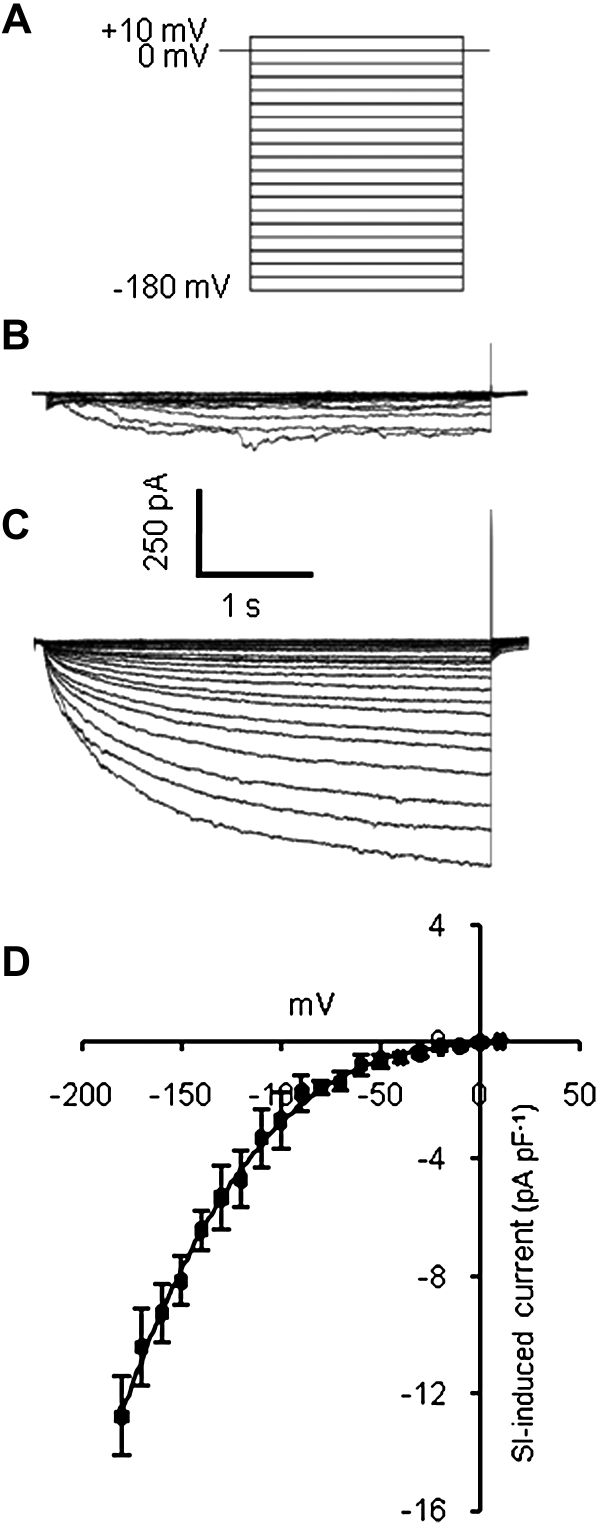
The I-V relationship of PrsS-induced current obtained using a voltage ramp protocol (Fig. 1B) showed clear nonlinearity, current being enhanced at more negative voltages (Fig. 1, C–F). This could reflect either inward rectification or voltage sensitivity of the PrsS-activated conductance. To investigate this, we used a protocol in which voltage was stepped from a holding level of 0 mV to a series of values between −180 mV and +10 mV (Fig. 2A). Leak current was linear down to approximately −120 mV, with an inward current that activated over a period of approximately 1 s present at more negative voltages (Fig. 2B). In the presence of incompatible PrsS, inward currents were markedly increased in amplitude and showed a clear difference in their kinetics (Fig. 2C). The I-V relationship of PrsS-induced current resembled that obtained using voltage ramps, but nonlinearity was more marked, with currents at voltages negative to −100 mV being significantly larger than those recorded using a ramp protocol (Fig. 2D; P = 5 × 10−5). Currents showed clear voltage dependency, activating over a period of 2 to 4 s, and inactivated very rapidly (Fig. 2, C and D). Thus, although the I-V plot of the PrsS-activated current appears to show inward rectification, this largely reflects voltage sensitivity of the SI-activated conductance.
Figure 2.
A stepped voltage protocol also reveals a PrsS-stimulated Ca2+-permeable conductance. A, Cartoon of the voltage step protocol. B, A family of currents obtained from an untreated pollen protoplast using the stepped voltage mode protocol (see A). C, A family of currents recorded from the same cell as in B, subjected to the voltage step protocol after exposure to incompatible PrsS. D, I-V curve for SI-induced current (obtained by subtraction of current amplitude at the end of the 4-s voltage step). Values shown are means ± se of four protoplasts.
Current Activation by PrsS Is SI Specific
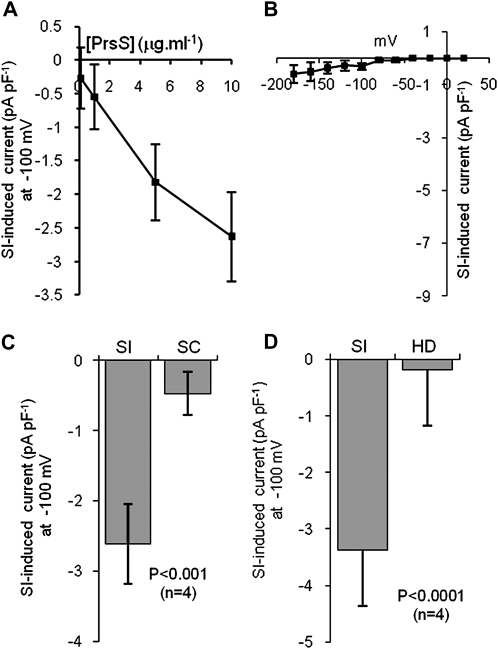
The current induced by incompatible PrsS was strongly dose dependent over the range 1 to 10 μg mL−1 (Fig. 3A; n = 6). In order to establish that the activation of this conductance by PrsS was an authentic SI response, we tested if a current could be induced by a compatible combination of PrsS and pollen S-haplotype. When recombinant PrsS3 and PrsS8 were added to pollen protoplasts from plants of haplotype S3S8 (an incompatible combination), a robust current was observed (Fig. 1, D and F). When the same PrsS3 and PrsS8 were added to pollen protoplasts with different S-specificities (S2, S4, or S6; a compatible combination), currents were not significantly different from currents in untreated cells (P = 0.146, not significant; n = 5). When currents obtained with untreated protoplasts were subtracted from those obtained from protoplasts undergoing interaction with compatible recombinant PrsS proteins, very little conductance was observed (Fig. 3B). Likewise, when PrsS1 was added to protoplasts from plants with haplotype S3S8, currents were not significantly different from those in untreated protoplasts (P = 0.092, not significant; n = 4). The currents recorded in the presence of compatible and incompatible PrsS were highly significantly different (P < 0.001; n = 4; Fig. 3C). This demonstrates that the PrsS-induced currents are SI specific (i.e. are induced by incompatible combinations of PrsS and PrpS, where S-alleles match).
Figure 3.
Conductance activation by PrsS is dose dependent and SI specific. SI was induced by the addition of PrsS3 + PrsS8 to pollen protoplasts (haplotype S3 or S8), bathed in Ca2+-containing saline. Currents were elicited using the voltage ramp protocol. A, Dose dependence of SI-induced current (difference between currents recorded in the presence and absence of PrsS) in protoplasts (n = 6). B, Compatible PrsS does not stimulate Ca2+ currents. The I-V curve (n = 5) for currents in protoplasts, bathed in Ca2+-containing saline, from plants of haplotypes S2S4 or S4S6 upon addition of biologically active recombinant PrsS3 and PrsS8 proteins (a compatible combination) was obtained by subtraction of untreated values. C, SI-induced currents in pollen protoplasts (haplotype S3 or S8) on addition of SI-PrsS (PrsS3 + PrsS8; SI) or the same amount of PrsS1 (self-compatible; SC). Bars show mean PrsS-induced current at −100 mV (n = 4). D, SI-induced currents in pollen protoplasts on addition of active PrsS (SI) and the same PrsS sample heat denatured (HD). Bars show mean PrsS-induced current at −100 mV (n = 4). All points are means ± se.
To further establish the specific nature of the SI-induced currents, we compared the currents induced by PrsS3 and PrsS8 with pollen of haplotypes PrpS3 and PrpS8 (an incompatible combination) with those obtained with heat-denatured proteins. The currents induced by heat-denatured PrsS were negligible and significantly different from those obtained using biologically active PrsS (Fig. 3D; P < 0.0001; n = 4), which were not significantly different from untreated samples (P = 0.108, not significant; n = 4). Thus, only incompatible, biologically active PrsS activated the current.
Characteristics of the SI-Stimulated Ion Conductance
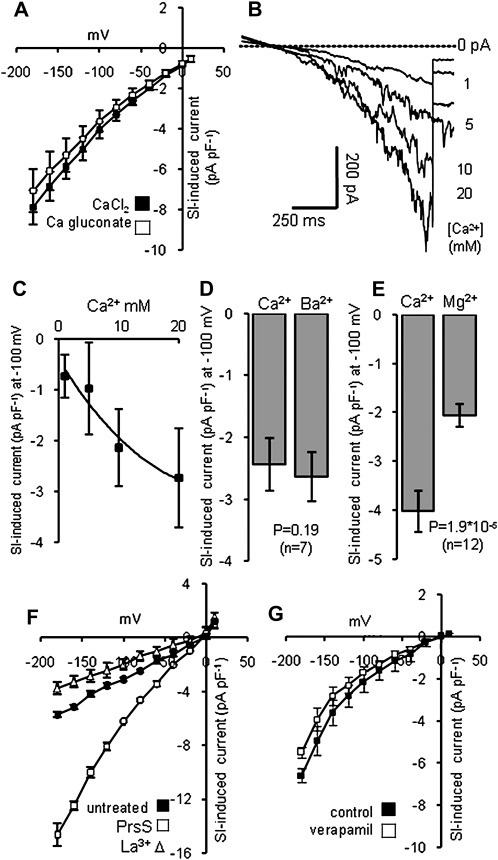
The large SI-induced inward currents could be due either to influx of Ca2+ (the only cation in the medium) or to chloride efflux (present in the pipette saline at 102.2 mm, with a calculated equilibrium potential of approximately 41 mV). First, we examined the effect of replacing extracellular Cl− with gluconate. SI-induced currents were assessed, first in standard Ca2+-containing medium and then after replacing CaCl2 with the nonpermeant anion calcium gluconate (which shifts the calculated equilibrium potential by more than 140 mV positive). The characteristics of the SI-induced current were unaffected by this substitution. The apparent reversal potentials of currents measured under these two conditions were indistinguishable and there was no change in current amplitude, despite the very large increase in the driving force for Cl− efflux (P = 0.186, not significant; Fig. 4A). This provides evidence that the recorded current was carried primarily by influx of Ca2+. To confirm this, we investigated the effect of extracellular [Ca2+] on current amplitude. Cells were superfused sequentially with saline containing 10 mm (standard Ca2+ saline), 1 mm, 2 mm, and 20 mm Ca2+, and currents were recorded in each saline. Currents showed very similar I-V characteristics under each condition, but the current amplitude was greatly dependent upon extracellular [Ca2+] (Fig. 4, B and C). Therefore, we conclude that currents were carried primarily by Ca2+, with anion (Cl−) efflux contributing little, if anything, to the SI-activated current.
Figure 4.
Characteristics of the SI-stimulated Ca2+-permeable conductance. SI was induced by the addition of PrsS3 + PrsS8 to pollen protoplasts (haplotype S3 or S8), bathed in Ca2+-containing saline. Currents were elicited using the voltage ramp protocol. A, Mean SI-induced current (n = 4) measured in saline containing 10 mm CaCl2 (black squares), then in saline where CaCl2 was replaced with calcium gluconate (white squares). B, A representative set of raw traces for whole cell current recorded from the same protoplast at different Ca2+ concentrations. C, Mean data for the effect of different Ca2+ concentrations on SI-induced currents (n = 3). D, The mean SI-induced current at −100 mV in Ca2+-containing medium (Ca2+) and in medium where CaCl2 was replaced by BaCl2 (Ba2+; n = 7). E, The mean SI-induced current (n = 12) at −100 mV in Ca2+-containing medium (Ca2+) and in medium where CaCl2 was replaced by MgCl2 (Mg2+). F, SI-induced currents recorded at −100 mV, in standard Ca2+-containing medium (untreated control; black squares), after addition of incompatible PrsS (white squares) and after subsequent addition of 500 μm La3+ (white triangles). The current induced by incompatible PrsS was completely abolished (n = 3). G, SI-stimulated currents recorded at −100 mV were tested for sensitivity to verapamil. The I-V curves (n = 3) are shown after addition of incompatible PrsS, first in standard Ca2+-containing medium (black squares) and then after addition of 50 μm verapamil (white squares). All data are means ± se.
To establish if the SI-activated channel was selective for Ca2+ over other divalent cations, we substituted Ca2+ in the extracellular saline with equimolar concentrations of other divalent cations. When Ca2+ was replaced by equimolar Ba2+, the SI-induced current in the same protoplasts (assessed using the voltage ramp protocol) was increased by approximately 10% (P = 0.19, not significant; n = 7; Fig. 4D), showing that the SI-activated channel is permeable to Ba2+. When Ca2+ was replaced by Mg2+, a PrsS-induced current was observed, but the amplitude was reduced by almost 50% (Fig. 4E; P = 1.9 × 10−5; n = 12). These data show that PrsS activates a conductance that shows limited selectivity between divalent cations, with a permeability of Ba2+ ≥ Ca2+ > Mg2+.
La3+ is an effective, broad-spectrum blocker of Ca2+-permeable channels. We investigated whether La3+ inhibited the SI-stimulated conductance (Fig. 4F). The SI-induced current carried by 10 mm CaCl2 was completely abolished by 500 μm La3+. At −180 mV, currents recorded in the presence of La3+ were reduced significantly below the “leak” current observed prior to stimulation (P < 0.02; n = 3; Fig. 4F). To further characterize the SI-activated conductance, we tested for sensitivity to verapamil, a Ca2+ channel blocker. Currents induced by addition of incompatible PrsS to pollen protoplasts bathed in Ca2+-containing medium were assessed before and after the addition of 50 μm verapamil. I-V curves of SI-activated current recorded in the presence and absence of verapamil were similar (Fig. 4G); the currents recorded at −100 mV and −180 mV were not significantly different (P ≥ 0.083, not significant; n = 3). Together, these data are consistent with this conductance being due to a Ca2+-permeable channel.
The SI-Stimulated Ion Conductance Also Carries Monovalent Cations
To test whether the SI-activated conductance was specific for divalent cations, we also tested permeability to monovalent cations using K+. The I-V relationship of the SI-induced current (measured using the ramp protocol) was markedly nonlinear at values negative to −100 mV. Currents were −1.7 ± 0.4 pA pF−1 at −100 mV and −10.6 ± 0.4 pA pF−1 at −180 mV, reversing close to 0 mV (Fig. 5, A and B). Substituting Cs+ for K+ in the pipette saline did not alter the amplitude or shape of PrsS-induced currents (P = 0.947, not significant; Fig. 5, C and D). When [K+] in the pipette saline was reduced from 100 to 10 mm, which changes EK from −58 to 0 mV, the shape of the I-V curve was similar and reversal potential was 4.5 ± 1.4 mV (n = 3). SI-activated conductivity was also permeable to NH4+, but with lower conductance than to K+. Replacement of extracellular K+ with NH4+ reduced the amplitude of the current by more than 50% (P < 0.05). These data demonstrate that incompatible PrsS also stimulates an increase in K+ conductance in pollen protoplasts.
Figure 5.
SI stimulates a conductance that is highly permeable to K+. SI was induced by the addition of PrsS3 + PrsS8 to pollen protoplasts (haplotype S3 or S8), bathed in K+-containing saline. A, Representative ramp-induced, whole-cell currents obtained in an untreated pollen protoplast bathed in K+-containing saline before (UT; black trace) and after SI induction (SI; gray trace) by PrsS addition. The dotted line shows zero current. B, I-V curve for SI-induced current in protoplasts bathed in K+-containing saline (mean ± se; n = 4). C, SI-induced current in K+-containing medium, as in A, but with K+ in the backfill, internal solution. The ramp protocol was applied under control conditions in untreated (UT) protoplasts and after application of incompatible PrsS (SI). D, I-V curve for the SI-induced K+ current for protoplasts with K+ in the backfill (mean ± se; n = 3).
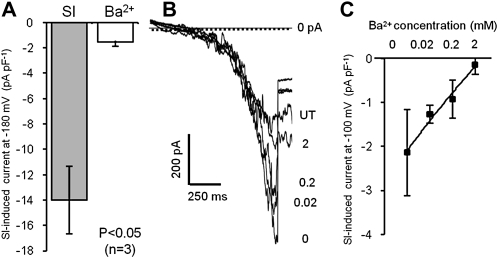
The SI-stimulated K+ current was almost completely blocked (greater than 90%) when K+ medium was supplemented with 2 mm Ba2+ (Fig. 6A; P < 0.05; n = 3). This effect of Ba2+ was strongly dose dependent (Fig. 6, B and C; n = 3), and there was a statistically significant effect of Ba2+ (P < 0.01 compared with untreated) on PrsS-induced current amplitudes at all concentrations except 0.02 mm Ba2+. The blockage of K+ by Ba2+ is commonly observed with divalent cation-permeable channels, where permeability to monovalent cations is very high under divalent-free conditions, but it is dramatically reduced by the presence of divalent ions, apparently due to binding in the channel (Sather and McCleskey, 2003). It has previously been shown that Ba2+ in the external medium reduced K+ currents in plant cells by approximately 75% (Schroeder et al., 1987). We also tested the sensitivity of currents to 10 mm tetraethylammonium chloride (TEA+), an effective inhibitor of voltage-gated and Ca2+-activated K+ channels. TEA+ did not significantly reduce the SI-induced K+ current (Supplemental Fig. S1A; n = 3; P = 0.94, not significant). To confirm that K+ conductance was not a secondary phenomenon, due to a Ca2+-activated K+ channel induced downstream of Ca2+ influx through the PrsS-activated conductance, we added 2 mm 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA) to the K+ medium to reduce [Ca2+] to negligible levels. BAPTA did not significantly reduce the SI-stimulated K+ current, confirming that it was not dependent upon influx of Ca2+ (P = 0.93, not significant, n = 3; Supplemental Fig. S1B).
Figure 6.
Properties of the K+-permeable, SI-induced conductance. SI was induced in pollen protoplasts bathed in K+-containing saline. Currents were elicited using the voltage ramp protocol. A, SI-induced current at −180 mV (±se; n = 3) in K+-containing medium (SI) and after addition of 2 mm BaCl2 (Ba2+). B, Representative raw traces showing the effects of various concentrations of Ba2+ (as indicated; mm) on the PrsS-induced K+ current. The current obtained from an untreated (UT) protoplast was similar to that in the presence of 2 mm Ba2+. C, Mean ± se data for the effect of different Ba2+ concentrations on SI-induced currents (n = 3).
Gaboon viper venom (GVV) is a potent irreversible blocker of inward rectifier K+ currents (Kir) in animal cells (Castle et al., 1989). We investigated the effect of GVV on SI-induced conductances in protoplasts. The toxin inhibited the SI-induced K+ current in a time-dependent manner, significantly reducing K+ current by approximately 75% after 10 min (P = 0.0015; n = 3; Supplemental Fig. S1C). However, when GVV was applied to protoplasts bathed in Ca2+-containing saline, it was similarly effective and also inhibited SI-stimulated Ca2+ currents (P = 0.014; n = 3; Supplemental Fig. S1D). Thus, the toxin did not discriminate between SI-activated K+ and Ca2+ currents, although we cannot rule out nonspecific effects. These data indicate that the SI-induced conductance cannot be attributed to Kir channels and are consistent with the idea that activation by incompatible PrsS involves a NSCC.
The SI-Stimulated Ca2+-Permeable Conductance Is pH and ATP Independent
As some pollen Ca2+-permeable channels are sensitive to extracellular pH (Qu et al., 2007), we examined whether the SI-stimulated Ca2+-permeable conductance was pH sensitive. Pollen protoplasts were tested for their SI-induced response to incompatible PrsS first in standard Ca2+-containing saline (pH 5.5) and then in saline with pH adjusted to 4.5. At pH 4.5, the SI-induced Ca2+ current was 3.4 ± 0.2 pA pF−1 at −100 mV; at pH 5.5, it was not significantly different (3.2 ± 0.3 pA pF−1; P = 0.11, not significant; n = 5; Supplemental Fig. S2A). Similarly, comparison of the SI-induced Ca2+ currents at pH 5.5 and 7.5 revealed no differences (P = 0.160, not significant; n = 3; Supplemental Fig. S2B). Thus, the SI-induced currents are not pH dependent.
The effects of SI-induced stimulation described above were observed in protoplasts held under whole-cell clamp, using pipette saline containing ATP but no GTP. In the whole-cell recording configuration, the pipette solution rapidly dialyzes the cell, and concentrations of cellular nucleotides rapidly fall to negligible levels unless they are included in the pipette saline (Trussell and Jackson, 1987). Thus, GTP does not appear to be required for activation of the PrsS-induced conductance. However, as phosphorylation downstream of agonist-receptor binding might be important, we examined whether intracellular ATP was required for the activation of this conductance. The I-V relationship for SI-induced currents recorded under ATP-free conditions was indistinguishable from that recorded in the presence of intracellular ATP (Supplemental Fig. S2C). Current density at −100 mV was −2.9 ± 0.3 pA pF−1 (n = 3), not significantly different from that recorded in parallel controls with 8 mm ATP in the pipette saline (P > 0.5). These data are consistent with the idea that phosphorylation is unlikely to be required for the SI-induced conductance.
Here, we show that adding PrsS, a Cys-rich peptide ligand, to induce SI in incompatible P. rhoeas pollen protoplasts stimulates SI-specific activation of voltage-sensitive plasma membrane ion conductance permeable to divalent cations, including Ca2+, and monovalent ions, including K+. These currents are triggered in an S-allele-specific manner and are only activated by biologically active PrsS. This is, to our knowledge, the first direct demonstration and investigation of a conductance change during the SI response. Our study begins to define one of the earliest signaling events in the SI response in incompatible pollen of P. rhoeas and provides a significant advance in our knowledge, not only about the mechanisms involved in SI but also with respect to the control of ion fluxes in pollen tubes.
DISCUSSION
PrsS Stimulates a Physiologically Relevant Protein-Stimulated Conductance
Here, we report what is to our knowledge the first direct demonstration of conductance changes stimulated during the SI response. PrsS, the pistil S-determinant, triggers inward currents carried by Ca2+ and K+ at the pollen grain plasma membrane under the experimental conditions employed. This is a physiologically relevant interaction that crucially mediates pollination outcomes. These currents are stimulated by SI, specifically only in pollen with incompatible S-allele-specific combinations with PrsS. This conductance, therefore, is stimulated by a highly defined interaction. Moreover, the finding that ungerminated pollen grain protoplasts are responsive to PrsS provides important information about the SI response in this species. Previous studies regarding [Ca2+]cyt were carried out on pollen tubes, as microinjecting pollen grains to perform Ca2+ imaging was technically challenging. This study is physiologically more realistic, as SI usually occurs prior to, or shortly after, germination. It also shows that cell wall components are not required for a SI response. Although we have postulated for many years that PrsS appears to act as a ligand, this study provides further evidence that it functions as such. Thus, these studies provide an important step forward in understanding the very earliest signaling events in the SI response.
Many studies have been made of channel activities exhibited by pollen grains and tubes. However, with a few exceptions, there has been a focus on describing the innate properties of pollen grain and pollen tube channel activities, with a view to understanding the currents required for regulating pollen germination and pollen tube growth. Models that attempt to explain how conductance changes/channel activities may relate to the regulation of pollen germination and growth have been proposed (Cheung and Wu, 2008; Michard et al., 2009; Liu et al., 2010). However, remarkably few studies have investigated the effects of physiologically relevant stimuli on pollen plasma membrane conductances. One study used drugs to show that actin depolymerization stimulates the activation of a Ca2+-permeable channel (Wang et al., 2004). A recent study, measuring membrane potentials, demonstrated that a defensin-like protein from maize (Zea mays), ZmES4, triggered rapid, transient membrane depolarization that resulted in pollen tube tip bursting, allowing fertilization. An inward-rectifying Shaker K+ channel was implicated as the target (Amien et al., 2010).
The identification of ZmES4 as a signaling ligand triggering rapid plasma membrane depolarization (Amien et al., 2010) suggests that there may be functional parallels between the way this and the PrsS-PrpS system may operate. ZmES4 is a small CRP; PrsS is also a CRP. Both trigger alterations in ion channel activity. CRPs are a diverse group of small, secreted proteins that have little sequence homology but have conserved Cys residues and putative secondary structure; many also share a γ-core signature (Yeaman and Yount, 2007). Several of these proteins (including the Brassica pollen S-locus determinant SCR/SP11) play a role in regulating pollen-pistil interactions (for a recent review, see Higashiyama, 2010). However, generally, they interact with receptor-like kinases. Here, we have shown that PrsS, like ZmES4, triggers alterations in plasma membrane ion conductance. Thus, although the targets of these two ligands are clearly different, as ZmES4 activates a Shaker K+ channel (Amien et al., 2010) it provides a new category of target for these signaling proteins. This suggests that several of these small signaling ligands may function to regulate ion channel activity.
The SI-Stimulated Conductance Appears To Be Ligand Gated
As we had previously shown that SI triggers increases in [Ca2+]cyt (Franklin-Tong et al., 1993, 1995, 1997), apparently by inducing an influx of Ca2+ (Franklin-Tong et al., 2002), we focused our studies primarily on Ca2+ fluxes. At −100 mV, addition of incompatible PrsS recombinant protein to pollen protoplasts, where Ca2+ was the only external cation, caused a tonic inward current that remained stable until washout of the ligand. This is a property suggestive of ligand gating. Interestingly, the effect of maize ZmES4 was also fully reversible (Amien et al., 2010). Repeated PrsS addition and washout could reactivate the current to the same level, with the same onset and offset kinetics. This is consistent with ligand gating and suggests that neither the receptor nor the ion channel activated by PrsS exhibited desensitization. Moreover, as the protoplasts were held under voltage clamp, activation was clearly a direct result of the application of PrsS and not an indirect effect due to a change in membrane potential.
The SI-induced whole-cell currents were recorded using pipettes of a tip size that should permit dialysis of the protoplast. Pipette saline contained no GTP. Thus, the SI-induced conductance does not appear to require cytoplasmic GTP or G-protein activation. Furthermore, removal of ATP from the pipette saline (and so from the cytoplasm of the protoplasts) was without effect. This suggests the lack of a requirement for receptor-ligand signaling involving phosphorylation or cAMP (and the consequent activation of CNGCs) for activation of the SI-induced currents. Therefore, we propose that conductance alteration by PrsS is most likely through a direct, ligand-gated mechanism, which is consistent with previous observations that elevation of [Ca2+]cyt is near “instantaneous” (Franklin-Tong et al., 1993, 1997).
Ligand-gated channels in plant cells that have been described include Arabidopsis glutamate receptor-like genes (AtGLRs), CNGCs, and ATP-gated cation channels (for a recent review, see Verret et al., 2010). The former two are both strong candidates for plasma membrane Ca2+-permeable channels. AtGLRs function as ligand-gated cation channels (Urquhart et al., 2007; Tapken and Hollmann, 2008), triggering rapid increases in [Ca2+]cyt (Dennison and Spalding, 2000). A CNGC has been shown to be required for pollen tube growth (Frietsch et al., 2007). However, the PrsS-activated current is unlikely to be carried by these, as the ligand here is a small, novel protein, PrsS, which acts in the absence of cytosolic nucleotides. Interestingly, the maize CRP ligand, KZM1 (Amien et al., 2010), stimulated instantaneous activation of K+ channel activity, suggesting ligand gating by a protein. Although we cannot be sure that the channel activation by PrsS is due directly to the activation of a ligand-gated channel at this stage, this is clearly an exciting finding that merits further investigation.
The Nature of the SI-Stimulated Conductance
Patch-clamp studies have provided direct characterization of a number of distinct classes of Ca2+ and cation conductances in plant cells. Most plant plasma membrane channels exhibit increased activity upon hyperpolarization, and these hyperpolarization-activated Ca2+ channels play important roles in conducting Ca2+ (Mäser et al., 2001; Véry and Sentenac, 2003; Ward et al., 2009; Verret et al., 2010). In contrast to many of the Ca2+-permeable channels so far identified in plants (Hamilton et al., 2000; Pei et al., 2000; Véry and Davies, 2000; Shang et al., 2005), the SI-stimulated Ca2+ conductance described here is activated by PrsS when cells are clamped at resting potential (−100 mV) and does not require voltage activation. However, when negative voltage steps were applied during PrsS activation, we observed activation of Ca2+ conductance by a mechanism that took 2 to 4 s to saturate and resulted in an I-V curve that resembled inward rectification. These slow activation kinetics clearly show voltage sensitivity and also explain our observation that 4-s voltage steps induced relatively larger currents at negative voltages compared with the voltage ramp protocol. These properties, together with its specific ligand gating and lack of sensitivity to pH, suggest that the SI-stimulated conductance is due to a novel, previously uncharacterized channel.
Our analysis has provided good evidence that in the presence of extracellular Ca2+, the recorded current was carried primarily by influx of Ca2+ but that the SI-activated conductance was poorly selective, with a permeability of Ba2+ ≥ Ca2+ > Mg2+. When Ca2+ was replaced by K+, we recorded an inward current of similar I-V characteristics but slightly greater amplitude. PrsS could also stimulate current carried by NH4+, although this current was smaller and showed different voltage sensitivity to that carried by K+. GVV toxin, an inhibitor of Kir in animal cells (Castle et al., 1989), inhibited both the K+ and Ca2+ inward conductances with similar potency. Furthermore, our observation that the apparent reversal potential for the SI-induced current in protoplasts bathed in Ca2+ medium was close to zero suggests that the divalent cation conductance allows the efflux of intracellular monovalent cations (Cs+ and K+). Both these observations are consistent with SI activation of a single nonspecific conductance. K+ currents were blocked by Ba2+, an effective blocker of K+-specific channels, in a dose-dependent manner. It has previously been shown that Ba2+ in the external medium suppresses both inward and outward K+ currents in plant cells by approximately 75% (Schroeder et al., 1987). However, many channels that show selectivity for divalent cations under normal experimental conditions become highly permeable to monovalent cations in the absence of divalents (Sather and McCleskey, 2003). The dose-dependent block of the K+ currents by Ba2+, observed in the absence of divalent cations, might be due to such an effect. That inward currents observed in the presence of 10 mm K+ and 2 mm Ba2+ were much smaller than those observed with 10 mm Ba2+ may reflect a low affinity of Ba2+ for the channel, such that reduction of [Ba2+]o from 10 to 2 mm significantly reduced Ba2+ current amplitude. Thus, it is possible that both divalent and monovalent currents are carried by a single conductance, a cation channel of limited selectivity for divalent cations or possibly a NSCC that is activated by incompatible PrsS.
NSCCs display little or no cation selectivity and often are permeable to both divalent and monovalent cations. These channels (rather than Ca2+-selective channels) are currently thought to be the best candidates for mediating Ca2+ conductances in many plant cells. Our data fit these criteria well. NSCCs have been shown to be involved in growth and development, stress responses, and calcium signaling (for a recent review, see Demidchik and Maathuis, 2007). NSCCs share a number of characteristics with the SI-stimulated conductance, being resistant to organic Ca2+ channel antagonists like verapamil and conventional K+ channel blockers such as TEA+ but sensitive to La3+ (Demidchik and Maathuis, 2007). Inward currents in pollen grains are primarily carried by K+ (Weisenseel and Jaffe, 1976); inward K+ fluxes have been reported in pollen grains (Obermeyer and Blatt, 1995), and whole-cell K+ currents have been characterized in pollen tube protoplasts (Griessner and Obermeyer, 2003). Thus, although our studies have focused on Ca2+ influx to date, it is not far-fetched to suggest that an NSCC with K+ permeability may be involved in mediating SI stimulus-response-coupled Ca2+ influx.
However, comparison of the SI-induced Ca2+ and K+ currents showed a consistent difference in their properties, in that the K+ current exhibited more marked voltage sensitivity than the Ca2+ current. K+ currents obtained with the voltage ramp protocol, both with Cs+ and K+ as the intracellular cation, had a clear inflection at approximately −125 mV, whereas divalent currents obtained with the same protocol showed a much smoother I-V relationship. This raises the possibility that divalent currents and K+ currents may be carried by separate conductances. If this is the case, activation of a K+ conductance might reflect secondary effects of SI-induced rapid increases in [Ca2+]cyt (Franklin-Tong et al., 1993, 1995, 1997). However, the K+ current was not dependent on Ca2+, and 10 mm TEA+ (an effective blocker of KCa channels) did not inhibit the K+ current. Thus, we conclude that either (1) incompatible PrsS activates an NSCC that has some voltage dependence, the K+ current displaying greater voltage sensitivity, or (2) incompatible PrsS simultaneously activates two conductances with different selectivities and voltage dependencies. In the absence of single-channel recordings, it is not possible to discount the second of these alternatives. Arabidopsis has five K+ channels, all predicted to be Tandem Pore K+ (TPK) channels. Slow inward K+ fluxes in pollen protoplasts are thought to be likely to be mediated by SPIK, a Shaker K+ channel (Mouline et al., 2002; Becker et al., 2004). However, SPIK is sensitive to extracellular pH, and AtTPK4 is sensitive to extracellular Ca2+ (Becker et al., 2004). Thus, if the SI-induced conductance involves a K+ channel, it is novel.
In summary, here we demonstrate and characterize currents carried by Ca2+ and K+, stimulated by SI induction at the pollen grain plasma membrane in a highly specific manner. Our data provide good evidence that PrsS, a well-characterized protein ligand, activates a nonspecific cation conductance that is not voltage activated but does appear to be activated in a voltage-sensitive manner. Our data provide a significant advance in our knowledge about mechanisms regulating Ca2+ influx stimulated by a biologically relevant event. They also have potential implications for our understanding of the control of tip growth in pollen tubes, which is pivotal to fertilization and sexual reproduction in plants.
MATERIALS AND METHODS
Plant Material
Pollen was collected from plants (Papaver rhoeas ‘Shirley’) segregating for known S-haplotypes and stored at −20°C until needed. Pollen from plants carrying either S-allele S3 or S8 was used to obtain appropriate recombinant pistil S protein (PrsS) for SI induction (see below).
Isolation of Pollen Protoplasts
Pollen protoplasts were made from P. rhoeas pollen grains. After 1 h of hydration, pollen was cultured in liquid germination medium [0.01% H3BO3, 0.01% KNO3, 0.01% Mg(NO3)2·6H2O, 0.036% CaCl2·2H2O, and 13.5% Suc] as described (Snowman et al., 2002) for 1 h, then washed and incubated in enzyme solution (1% [w/v] macerozyme R-10 [Onozuka] and 2.0% [w/v] cellulase R-10 [Onozuka]) and 1% (w/v) bovine serum albumin (Sigma) for 20 min at 25°C to release the protoplasts. The enzyme solution was then exchanged for the patch-clamp bath solution (see below).
Patch-Clamp Solutions
For Ca2+ measurements, the standard basal external (bath) solution contained 10 mm CaCl2, 100 mm Glc, and 5 mm MES. Saline was adjusted to 1,400 mosmol with 1.25 m d-sorbitol and to pH 5.5 with Tris, using methodology from Qu et al. (2007). Calcium gluconate saline was identical to this buffer, except that 10 mm calcium gluconate was substituted for CaCl2. In K+ and NH4+ saline, CaCl2 was replaced by 10 mm KCl and 10 mm NH4Cl, respectively. We also performed PrsS-induced calcium current experiments with the standard bath medium but changed the [Ca]o to 1, 5, 10, and 20 mm sequentially between ramps, so recordings were made from the same cell under different conditions. We started with the standard 10 mm Ca2+, then changed to 1 mm CaCl2, then 5 mm CaCl2, then 20 mm Ca2+. Sorbitol was adjusted to maintain osmolality. Other changes to the bath solutions are given in the text. To test the nature of the K+ channel permeability, 10 mm NH4Cl was substituted for KCl.
The standard basal intracellular (pipette) medium used for all experiments, except where stated, contained 0.65 m d-sorbitol, 0.1 mm CaCl2, 4 mm Ca(OH)2, 3 mm MgATP, 5 mm Tris-ATP, 10 mm EGTA, 15 mm HEPES, 100 mm CsCl, 1 mm MgCl2, and 5 mm MES, pH 7.1. Osmolality was 1,010 mosmol. Free Ca2+ concentration in the pipette solution was approximately 250 nm (calculated by the chemical speciation program MaxChelator). For some experiments, we replaced Cs+ in the backfill with 100 or 10 mm K+. Where necessary, we osmotically compensated with sorbitol. Some experiments used symmetrical solutions (standard extracellular Ca2+ or K+ medium) in both the bath and pipette. For the experiments testing a requirement for kinase activity for currents, MgATP and Tris-ATP were omitted from the pipette saline. During whole-cell patch clamp, the cytoplasmic ionic content was rapidly replaced by the pipette saline, which should be effective in small spherical protoplasts (1.44 × 10−8 cm3).
SI Induction in Pollen Protoplasts
Expression and purification of the recombinant PrsS was as described (Foote et al., 1994; Snowman et al., 2002). Nucleotide sequences specifying the mature peptides of the PrsS gene for PrsS1, PrsS3, and PrsS8 cloned into the expression vector pMS119 were used to produce recombinant PrsS in Escherichia coli cells using induction with 1 mm isopropyl β-d-thiogalactoside. Resultant inclusion bodies were isolated, purified, refolded, and stored in Tris (Kakeda et al., 1998). Protein concentration was estimated using the Coomassie dye-binding method (Bradford, 1976).
Papaver pollen protoplasts were subjected to an incompatible interaction by adding recombinant PrsS that matched the S-alleles carried by the pollen parent (Wheeler et al., 2009). Thus, SI was induced by adding PrsS3 and PrsS8 at a final concentration 10 μg mL−1 in MES/Tris buffer, pH 5.5, to pollen protoplasts from plants with a S3S8 haplotype (an incompatible combination). Controls comprised the addition of 10 μg mL−1 biologically inactivated incompatible PrsS (heat-denatured at 100°C, 10 min) in the same combination or 10 μg mL−1 of a compatible PrsS-pollen combination (PrsS3 and PrsS8 with pollen from plants of haplotype S2S6 or S4S6).
PrsS was delivered using an AutoMate perfusion system with a ValveLink8.2 controller for solution delivery at a flow rate of 2 to 4 mL min−1. This meant that delivery of PrsS was not immediate; hence, the apparent delay in response time. We used Evans blue to assess the “travel time” from addition to the perfusing saline to arrival at the recording pipette/protoplast. Markers in Figure 1, showing application and washout of PrsS, are placed to take account of this lag.
Inhibitor Experiments
Inhibitors were added to the bath medium at the following concentrations: 10 mm TEA+, 2 mm Ba2+, 50 μm verapamil, 500 μm La3+, 2 mm BAPTA, and 250 μg mL−1 GVV. For the K+ current, we tested the effect of Ba2+ at 0.02, 0.2, and 2 mm. All chemicals were from Sigma, unless otherwise stated.
Patch-Clamp Recording
Standard patch pipettes were made from borosilicate glass capillaries (1.5 mm o.d., 0.86 mm i.d.) using a Sutter P97 electrode puller. When filled with d-sorbitol saline (see above), pipette resistance was in the range 10 to 30 MΏ in normal bath medium. After obtaining a GΏ seal, the whole-cell configuration was achieved (confirmed by a sudden increase in capacitance to 10–30 pF) by applying a burst of suction. Whole-cell resistance was typically approximately 2.5 GΏ. Series resistance and capacitance were compensated using the amplifier circuitry. After obtaining the whole-cell configuration, a 5-min interval was allowed prior to recording to ensure the equilibration of the pipette saline with the cytosol. Currents were recorded with an A-M Systems (model 2400) patch-clamp amplifier at a sampling frequency of 10 kHz and passed to a digital converter (micro 1401) connected to a personal computer running Signal 4.0.3 (Cambridge Electrical Device), which was also used for offline analysis. Junction potentials were calculated using pCLAMP 9.0. For Ca2+, Mg2+, and Ba2+ extracellular salines, junction potential approached +4 mV and holding potential was adjusted accordingly. For all other salines, junction potential was less than 2 mV and no adjustments were made.
In all experiments, holding voltage was adjusted to −100 mV, consistent with the resting membrane potential of a plant cell under normal physiological conditions (Thuleau et al., 1994). To examine the I-V relationship of PrsS-induced current, we used a protocol that stepped the holding voltage to −180 mV, then applied a voltage ramp from −180 to +10 mV, over a period of 1 s, before returning to −100 mV (Fig. 1B). A 20-s interval was allowed between successive ramps. In some experiments, we also performed a stepped voltage protocol. The cell was held at 0 mV and stepped to −180 mV, then −170 mV , then sequentially at 10-mV intervals through to +10 mV, returning to 0 mV between each step (Fig. 2A). Data were sampled at 10 kHz and filtered at 5 kHz. I-V curves obtained by subtraction were constructed with averaged whole-cell current density (pA pF−1).
Data Analysis
To assess currents during ramp protocols, the current density (pA pF−1) was assessed at 11 points during each ramp (−180, −160, −140, −120, −100, −80, −60, −40, −20, 0, and 10 mV). The mean ± se at each voltage was calculated for plotting I-V relationships. Responses plotted (SI-induced current) were obtained by subtracting currents obtained under control conditions from those obtained during test conditions. Current density values shown (mean ± se) and statistical tests for current density are mostly shown at −100 mV. However, for much of the K+ data (with NH4+, Ba2+, TEA+, BAPTA, and GVV), we present data analysis at −180 mV, as it was apparent that there was little response at −100 mV. Statistical comparisons were performed using Student’s t test.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Further properties of the K+-permeable SI-induced conductance.
Supplemental Figure S2. The SI-stimulated Ca2+ channel is pH and ATP independent.
Supplementary Material
Acknowledgments
We are grateful to Dale Sanders, and also to the anonymous referees, for advice and useful comments relating to these studies. Work in the lab of V.E.F-T. is funded by the Biotechnology and Biological Sciences Research Council. Work in the lab of Y.G. was funded by the Biotechnology and Biological Sciences Research Council and the European Research Fund. Work in the lab of S.J.P. is funded by The Wellcome Trust, Infertility Research Trust, and Royal Society. J.W. was funded by a China Scholarship Council scholarship to perform research in Birmingham, UK.
References
- Amien S, Kliwer I, Márton ML, Debener T, Geiger D, Becker D, Dresselhaus T. (2010) Defensin-like ZmES4 mediates pollen tube burst in maize via opening of the potassium channel KZM1. PLoS Biol 8: e1000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Geiger D, Dunkel M, Roller A, Bertl A, Latz A, Carpaneto A, Dietrich P, Roelfsema MRG, Voelker C, et al. (2004) AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc Natl Acad Sci USA 101: 15621–15626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Franklin-Tong VE. (2007) Temporal and spatial activation of caspase-like enzymes induced by self-incompatibility in Papaver pollen. Proc Natl Acad Sci USA 104: 18327–18332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Poulter NS, Vatovec S, Franklin-Tong VE. (2008) Initiation of programmed cell death in self-incompatibility: role for cytoskeleton modifications and several caspase-like activities. Mol Plant 1: 879–887 [DOI] [PubMed] [Google Scholar]
- Bothwell JHF, Ng CK-Y. (2005) The evolution of Ca2+ signalling in photosynthetic eukaryotes. New Phytol 166: 21–38 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254 [DOI] [PubMed] [Google Scholar]
- Campanoni P, Blatt MR. (2007) Membrane trafficking and polar growth in root hairs and pollen tubes. J Exp Bot 58: 65–74 [DOI] [PubMed] [Google Scholar]
- Castle NA, Haylett DG, Jenkinson DH. (1989) Toxins in the characterization of potassium channels. Trends Neurosci 12: 59–65 [DOI] [PubMed] [Google Scholar]
- Cheung AY, Wu H-M. (2008) Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 59: 547–572 [DOI] [PubMed] [Google Scholar]
- Demidchik V, Maathuis FJM. (2007) Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol 175: 387–404 [DOI] [PubMed] [Google Scholar]
- Dennison KL, Spalding EP. (2000) Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol 124: 1511–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta R, Robinson KR. (2004) Identification and characterization of stretch-activated ion channels in pollen protoplasts. Plant Physiol 135: 1398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feijo JA, Malho R, Obermeyer G. (1995) Ion dynamics and its possible role during in-vitro pollen germination and tube growth. Protoplasma 187: 155–167 [Google Scholar]
- Foote HCC, Ride JP, Franklin-Tong VE, Walker EA, Lawrence MJ, Franklin FCH. (1994) Cloning and expression of a distinctive class of self-incompatibility (S) gene from Papaver rhoeas L. Proc Natl Acad Sci USA 91: 2265–2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin-Tong VE, Hackett G, Hepler PK. (1997) Ratio-imaging of [Ca2+]i in the self-incompatibility response in pollen tubes of Papaver rhoeas. Plant J 12: 1375–1386 [Google Scholar]
- Franklin-Tong VE, Holdaway-Clarke TL, Straatman KR, Kunkel JG, Hepler PK. (2002) Involvement of extracellular calcium influx in the self-incompatibility response of Papaver rhoeas. Plant J 29: 333–345 [DOI] [PubMed] [Google Scholar]
- Franklin-Tong VE, Ride JP, Franklin FCH. (1995) Recombinant stigmatic Self-Incompatibility-(S-) protein elicits a Ca2+ transient in pollen of Papaver rhoeas. Plant J 8: 299–307 [Google Scholar]
- Franklin-Tong VE, Ride JP, Read ND, Trewavas AJ, Franklin FCH. (1993) The self-incompatibility response in Papaver rhoeas is mediated by cytosolic-free calcium. Plant J 4: 163–177 [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF. (2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griessner M, Obermeyer G. (2003) Characterization of whole-cell K+ currents across the plasma membrane of pollen grain and tube protoplasts of Lilium longiflorum. J Membr Biol 193: 99–108 [DOI] [PubMed] [Google Scholar]
- Hamilton DWA, Hills A, Kohler B, Blatt MR. (2000) Ca2+ channels at the plasma membrane of stomatal guard cells are activated by hyperpolarization and abscisic acid. Proc Natl Acad Sci USA 97: 4967–4972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM, Brownlee C. (2004) The generation of Ca2+ signals in plants. Annu Rev Plant Biol 55: 401–427 [DOI] [PubMed] [Google Scholar]
- Higashiyama T. (2010) Peptide signaling in pollen-pistil interactions. Plant Cell Physiol 51: 177–189 [DOI] [PubMed] [Google Scholar]
- Kakeda K, Jordan ND, Conner A, Ride JP, Franklin-Tong VE, Franklin FCH. (1998) Identification of residues in a hydrophilic loop of the Papaver rhoeas S protein that play a crucial role in recognition of incompatible pollen. Plant Cell 10: 1723–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Piette BMAG, Deeks MJ, Franklin-Tong VE, Hussey PJ. (2010) A compartmental model analysis of integrative and self-regulatory ion dynamics in pollen tube growth. PLoS ONE 5: e13157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D, et al. (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijó JA. (2009) The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int J Dev Biol 53: 1609–1622 [DOI] [PubMed] [Google Scholar]
- Mouline K, Véry AA, Gaymard F, Boucherez J, Pilot G, Devic M, Bouchez D, Thibaud JB, Sentenac H. (2002) Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev 16: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeyer G, Blatt MR. (1995) Electrical properties of intact pollen grains of Lilium longiflorum: characteristics of the non-germinating pollen grain. J Exp Bot 46: 803–813 [Google Scholar]
- Palanivelu R, Preuss D. (2000) Pollen tube targeting and axon guidance: parallels in tip growth mechanisms. Trends Cell Biol 10: 517–524 [DOI] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI. (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Qi Z, Stephens NR, Spalding EP. (2006) Calcium entry mediated by GLR3.3, an Arabidopsis glutamate receptor with a broad agonist profile. Plant Physiol 142: 963–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu HY, Shang ZL, Zhang SL, Liu LM, Wu JY. (2007) Identification of hyperpolarization-activated calcium channels in apical pollen tubes of Pyrus pyrifolia. New Phytol 174: 524–536 [DOI] [PubMed] [Google Scholar]
- Rudd JJ, Franklin-Tong VE. (1999) Calcium signaling in plants. Cell Mol Life Sci 55: 214–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF. (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14: S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sather WA, McCleskey EW. (2003) Permeation and selectivity in calcium channels. Annu Rev Physiol 65: 133–159 [DOI] [PubMed] [Google Scholar]
- Schiøtt M, Romanowsky SM, Baekgaard L, Jakobsen MK, Palmgren MG, Harper JF. (2004) A plant plasma membrane Ca2+ pump is required for normal pollen tube growth and fertilization. Proc Natl Acad Sci USA 101: 9502–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Raschke K, Neher E. (1987) Voltage dependence of K channels in guard-cell protoplasts. Proc Natl Acad Sci USA 84: 4108–4112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang ZL, Ma LG, Zhang HL, He RR, Wang XC, Cui SJ, Sun DY. (2005) Ca2+ influx into lily pollen grains through a hyperpolarization-activated Ca2+-permeable channel which can be regulated by extracellular CaM. Plant Cell Physiol 46: 598–608 [DOI] [PubMed] [Google Scholar]
- Snowman BN, Kovar DR, Shevchenko G, Franklin-Tong VE, Staiger CJ. (2002) Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell 14: 2613–2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S, Isogai A. (2005) Self-incompatibility in plants. Annu Rev Plant Biol 56: 467–489 [DOI] [PubMed] [Google Scholar]
- Tapken D, Hollmann M. (2008) Arabidopsis thaliana glutamate receptor ion channel function demonstrated by ion pore transplantation. J Mol Biol 383: 36–48 [DOI] [PubMed] [Google Scholar]
- Thomas SG, Franklin-Tong VE. (2004) Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 429: 305–309 [DOI] [PubMed] [Google Scholar]
- Thuleau P, Ward JM, Ranjeva R, Schroeder JI. (1994) Voltage-dependent calcium-permeable channels in the plasma membrane of a higher plant cell. EMBO J 13: 2970–2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trussell LO, Jackson MB. (1987) Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci 7: 3306–3316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urquhart W, Gunawardena AH, Moeder W, Ali R, Berkowitz GA, Yoshioka K. (2007) The chimeric cyclic nucleotide-gated ion channel ATCNGC11/12 constitutively induces programmed cell death in a Ca2+ dependent manner. Plant Mol Biol 65: 747–761 [DOI] [PubMed] [Google Scholar]
- Verret F, Wheeler G, Taylor AR, Farnham G, Brownlee C. (2010) Calcium channels in photosynthetic eukaryotes: implications for evolution of calcium-based signalling. New Phytol 187: 23–43 [DOI] [PubMed] [Google Scholar]
- Véry AA, Davies JM. (2000) Hyperpolarization-activated calcium channels at the tip of Arabidopsis root hairs. Proc Natl Acad Sci USA 97: 9801–9806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Véry AA, Sentenac H. (2003) Molecular mechanisms and regulation of K+ transport in higher plants. Annu Rev Plant Biol 54: 575–603 [DOI] [PubMed] [Google Scholar]
- Wang YF, Fan LM, Zhang WZ, Zhang W, Wu WH. (2004) Ca2+-permeable channels in the plasma membrane of Arabidopsis pollen are regulated by actin microfilaments. Plant Physiol 136: 3892–3904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JM, Mäser P, Schroeder JI. (2009) Plant ion channels: gene families, physiology, and functional genomics analyses. Annu Rev Physiol 71: 59–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenseel MH, Jaffe LF. (1976) The major growth current through lily pollen tubes enters as K+ and leaves as H+. Planta 133: 1–7 [DOI] [PubMed] [Google Scholar]
- Wheeler MJ, de Graaf BHJ, Hadjiosif N, Perry RM, Poulter NS, Osman K, Vatovec S, Harper A, Franklin FCH, Franklin-Tong VE. (2009) Identification of the pollen self-incompatibility determinant in Papaver rhoeas. Nature 459: 992–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ, Broadley MR. (2003) Calcium in plants. Ann Bot (Lond) 92: 487–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman MR, Yount NY. (2007) Unifying themes in host defence effector polypeptides. Nat Rev Microbiol 5: 727–740 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.