Abstract
Purpose
β2-Microglobulin (β2M) has been shown to promote osteomimicry and the proliferation of human prostate cancer cells. The objective of this study is to determine the mechanism by which targeting β2M using anti-β2M antibody inhibited growth and induced apoptosis in prostate cancer cells.
Experimental Design
Polyclonal and monoclonal β2M antibodies were used to interrupt β2M signaling in human prostate cancer cell lines and the growth of prostate tumors in mice. The effects of the β2M antibody on a survival factor, androgen receptor (AR), and its target gene, prostate-specific antigen (PSA) expression, were investigated in cultured cells and in tumor xenografts.
Results
The β2M antibody inhibited growth and promoted apoptosis in both AR-positive and PSA-positive, and AR-negative and PSA-negative, prostate cancer cells via the down-regulation of the AR in AR-positive prostate cancer cells and directly caused apoptosis in AR-negative prostate cancer cells in vitro and in tumor xenografts. The β2M antibody had no effect on AR expression or the growth of normal prostate cells.
Conclusions
β2M downstream signaling regulates AR and PSA expression directly in AR-positive prostate cancer cells. In both AR-positive and AR-negative prostate cancer cells, interrupting β2M signaling with the β2M antibody inhibited cancer cell growth and induced its apoptosis. The β2M antibody is a novel and promising therapeutic agent for the treatment of human prostate cancers.
β2-Microglobulin (β2M) is produced by all nucleated cells as a 119-amino-acid residue protein and, after processing, is secreted in a 99-amino-acid form (11,800 Da; refs. 1, 2). The most common known function of β2M, a light-chain antigen-presenting molecule, is to serve as a coreceptor for the presentation of the MHC class I in nucleated cells for cytotoxic T-cell recognition (3). However, cancer cells frequently down-regulate the expression of MHC class I to evade recognition by the immune system (4–7), presumably allowing the secretion of free β2M into circulation or in the tumor microenvironment. Our laboratory first identified β2M, an active component secreted by prostate cancer, and prostate and bone stromal cells, as a major growth factor and signaling molecule (8). β2M conferred osteomimicry, the ability of cancer cells to mimic gene expression by bone cells, in prostate cancer cells through the activation of a cyclic AMP (cAMP) –dependent protein kinase A (PKA) and cAMP-responsive element binding (CREB) protein signaling pathway (9). The use of a sequence-specific small interfering RNA (siRNA) targeting β2M and its signaling resulted in extensive prostate cancer cell death in vitro and greatly promoted prostate tumor regression in immunocom-promised mice (8). We also showed that interrupting β2M signaling similarly blocked human renal cell carcinoma growth (10). β2M has recently been shown to be a useful biomarker for advanced human prostate cancer (11). β2M seems to be a downstream androgen target gene, more specific than prostate-specific antigen (PSA), under the control of the androgen receptor (AR), in a human LNCaP prostate cancer cell line (11).
Anti–β2M antibody is a potent interrupter of β2M-mediated signaling (8, 12). The β2M antibody was shown to be a highly cytotoxic reagent against the growth of solid tumors like renal cell carcinoma (13) as well as liquid tumors, such as leukemia, lymphoma, and multiple myeloma (12). We showed here that the β2M antibody inhibited the expression of a survival factor, AR, and its target gene, PSA, in AR-positive and PSA-positive human prostate cancer cell lines, including androgen-dependent LNCaP and androgen-independent C4-2B cells (14), and in androgen-independent C4-2 tumor xenograft models. The β2M antibody also suppressed growth and induced apoptosis in both AR-positive and PSA-positive, and AR-negative and PSA-negative human prostate cancer cells and in xenograft tumors in mice. Moreover, our studies showed that the β2M antibody induced prostate cancer cell death through an activation of a caspase-9–mediated apoptotic cascade pathway without affecting normal or nontumorigenic prostatic epithelial and stromal cells. These results support the idea that targeting β2M signaling via the external application of the β2M antibody can profoundly alter intracellular cell signaling networks, including, but not limited to, the AR downstream signaling axis. Effective β2M antibody–mediated targeting of the growth of both AR-positive and PSA-positive, and AR-negative and PSA-negative human prostate cancer cells may prove to be an attractive and safe therapeutic approach for the treatment of human prostate cancer and its lethal progression.
Materials and Methods
Cell lines, cell culture, and β2M antibody
The human prostate cancer cell line LNCaP (androgen dependent), the LNCaP lineage–derived bone metastatic subline C4-2B (androgen independent; ref. 14), DU-145 (brain metastatic, androgen independent), PC3 (bone metastatic, androgen independent), and ARCaP (ascites-fluid–derived, androgen repressive; refs. 15, 16) were cultured in T-medium (Invitrogen) supplemented with 5% fetal bovine serum, 100 IU/mL penicillin, and 100 μg/mL streptomycin. A human normal/nontumorigenic prostatic epithelial cell line, RWPE-1 (American Type Culture Collection), was cultured in keratinocyte serum-free medium supplemented with 5 ng/mL human recombinant epidermal growth factor and 0.05 mg/mL bovine pituitary extract (Invitrogen). These prostate cancer and normal cell lines were maintained in 5% CO2 at 37°C. The anti-β2M antibody, a polyclonal antibody, was obtained from Santa Cruz Biotechnology, Inc. (sc-15366), for in vitro cell culture studies and in vivo animal experiments. We also tested the β2M monoclonal antibody (Santa Cruz Biotechnology; sc-13565) and found it to have similar inhibitory effects on the growth of human prostate cancer cells in vitro (data not shown).
Reverse transcription-PCR
LNCaP and C4-2B cells were plated on six-well dishes at 3 × 105 cells per well and grown to 70% confluence in T-medium with 5% fetal bovine serum. The cells were gently washed with PBS and incubated in T-medium plus 5% dextran-coated, charcoal-treated fetal bovine serum for overnight incubation. The cells were then treated with 0, 1, 5, or 10 μg/mL of β2M antibody; the β2M antibody was preincubated for 30 min with the same amounts of purified human β2M protein (Sigma) or 10 μg/mL of isotype control IgG for 24 h. The total RNA was isolated from these treated cells using a RNeasy Mini Kit (Qiagen) and subjected to reverse transcription according to the manufacturer's instructions (Invitrogen). The primer sequences used for PCR analysis were AR [5′-ATGGCTGT-CATTCAGTACTCCTGGA-3′ (forward) and 5′-AGATGGGCTT-GACTTTCCCAGAAAG-3′ (reverse)], PSA [5′-ATGTGGGTCCCGGTT-GTCTTCCTCACCCTGTC-3′ (forward) and 5′-TCAGGGGTTGGCCAC-GATGGTGTCCTTGATC-3′ (reverse)], and glyceraldehyde-3-phosphate dehydrogenase [5′-ACCACAGTCCATGCCATCA-3′ (forward) and 5′-TCCACCACCCTGTTGCTGT-3′ (reverse)], respectively. The thermal profiles for AR, PSA, and glyceraldehyde-3-phosphate dehydrogenase cDNA amplification are 25 cycles, starting with denaturation for 1 min at 94°C, followed by 1 min of annealing at 61 °C (for AR), 55°C (for PSA), and 60°C (for glyceraldehyde-3-phosphate dehydrogenase), and 1 min of extension at 72°C. The reverse transcription-PCR products were analyzed by agarose gel electrophoresis.
Western blot analysis and ELISA
Cell lysates were prepared from β2M monoclonal antibody–treated or IgG-treated prostate cells using a lysis buffer [50 mmol/L Tris (pH 8), 150 mmol/L NaCl, 0.02% NaN3, 0.1% SDS, 1% NP40, and 0.5% sodium deoxycholate] containing 1 mmol/L phenylmethylsulfonyl fluoride and a protease inhibitor cocktail (Roche Applied Science). The protein concentration was determined by the Bradford assay using the Coomassie Plus Protein Reagent (Pierce). Western blot was done with the Novex system (Invitrogen) as described previously (8, 10). The primary antibodies anti-AR (1:500 dilution) and PSA (1:1,000 dilution; Santa Cruz Biotechnology); anti–caspase-9, caspase-3, and poly(ADP)ribose poly-merase (PARP; 1:1,000 dilution; Cell Signaling Technology); and the secondary antibodies that were conjugated with horseradish peroxidase (1:5,000 dilution; GE Healthcare) were used. The detection of protein bands was done with the use of enhanced chemiluminescence Western Blotting Detection Reagents (GE Healthcare). The soluble PSA levels were determined by microparticle ELISA with the Abbott IMx machine (Abbott Laboratories).
Cell proliferation assay
LNCaP (6,000 cells per well), C4-2B (6,000 cells per well), DU-145 (3,000 cells per well), PC3 (3,000 cells per well), ARCaP (5,000 cells per well), and RWPE-1 (6,000 cells per well) cells were plated on 96-well plates and treated with the β2M antibody or control IgG for a 3-d incubation. The cell numbers were measured every 24 h by mitochondrial 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS), assay with the use of the CellTiter 96 AQueous One Solution Cell Proliferation Assay (Promega) according to the manufacturer's instructions.
Sub-G1 DNA content measurement
LNCaP and C4-2B prostate cancer cells were plated on six-well plates at 3 × 105 per well in T-medium containing 5% dextran-coated, charcoal-treated fetal bovine serum and exposed to 10 μg/mL of β2M monoclonal antibody or control IgG for 48-h incubation. The treated cells were collected by trypsinization and fixed in 70% ice-cold ethanol, incubated with RNase A (100 μg/mL; Sigma) for 30 min, and stained with propidium iodide (25 μg/mL; Chemicon) for 30 min. The cell cycle was determined by a FACScan flow cytometer and CellQuest software (Becton Dickinson Labware) for analysis of sub-G1 DNA content.
In vivo animal experiments
All the animal experiments were approved and done in accordance with institutional guidelines. The mice were maintained at the Animal Research Facility in Emory University. To test the antitumor efficacy and AR expression regulated by the β2M antibody in vivo, 4-wk-old male athymic nu/nu mice (National Cancer Institute) were inoculated s.c. with C4-2 or PC3 prostate cancer cells with 2 × 106 cells per mouse. After 3 wk (PC3 tumor) or 4 wk (C4-2 tumor) of inoculation, 10 μg of β2M monoclonal antibody mixed with Surgifoam (Ethicon Inc.) to keep and slow release the β2M antibody around the tumors were given by intratumoral implantation, one shot per mouse. The control group mice received equal doses of isotype IgG or placebo (saline) implanted the same way as the β2M antibody. After 1 wk of treatment, tumor tissues were harvested from the euthanized mice and fixed in 10% formalin, dehydrated in ethanol, embedded in paraffin, and sectioned in slides. The blank tissue slides were subjected to immunohistochemical staining with anti-AR antibody (Santa Cruz Biotechnology) and M30 CytoDeath marker (DiaPharma Group, Inc.), and detected by the Dako Autostainer Plus system (Dako Corp.). For quantification of AR and M30 CytoDeath staining, 100 cells at five randomly selected areas were counted and the positive-staining cells were recorded.
Statistical analysis
Statistical analyses were done as described previously (9). Student's t test and two-tailed distribution were applied in the analysis of statistical significance.
Results
β2M antibody decreased AR and PSA expression in human prostate cancer cells
We previously showed that β2M is a novel signaling and growth-regulating molecule capable of promoting cell proliferation and survival in human prostate and renal cancer cells (8, 10). Interrupting β2M and its downstream signaling by β2M siRNA induced cell death in both human prostate and renal carcinoma models (8, 13). Because the downstream targets for β2M signaling interruption are not completely clear in human prostate cancer cells, we conducted a cDNA microarray study (17) comparing β2M siRNA stably transfected AR-positive and PSA-positive C4-2B prostate cancer cells with their scramble stably transfected control clones. The results of these studies showed a 4-fold and 16-fold decreased expression of AR and PSA mRNA, respectively, in C4-2B cells, and these data were confirmed by reverse transcription-PCR and Western blot.5 To test the hypothesis that blocking β2M-mediated signaling pathways may affect AR gene expression and transactivation, which are involved in prostate cancer cell growth, survival, and progression, we tested the effect of a new reagent, β2M polyclonal antibody, on AR and PSA expression in AR-positive and PSA-positive LNCaP (androgen dependent) and C4-2B (androgen independent) cells. Consistent with cDNA microarray data, interrupting β2M by the β2M antibody decreased endogenous AR and PSA mRNA expression as determined by reverse transcription-PCR (Fig. 1A). The inhibitory effect of the β2M antibody (0-10 μg/mL) was concentration dependent, and the addition of purified β2M protein rescued the decreased AR and PSA mRNA expression that had been inhibited by the β2M antibody in LNCaP and C4-2B cells. Isotype-matched control IgG (10 μg/mL) did not suppress AR and PSA mRNA expression. In parallel, the β2M antibody (0-10 μg/mL) also inhibited AR and PSA protein levels in a concentration-dependent manner as analyzed by Western blot (Fig. 1B), and this inhibition can also be rescued by the addition of purified β2M protein to the cultured LNCaP and C4-2B cells. The control IgG did not change AR and PSA protein expression. Consistent with the blockade of AR expression, we found that secreted soluble PSA levels, assayed by ELISA, were also decreased by the β2M antibody, but not the control IgG, in LNCaP and C4-2B cells (Fig. 1C). These results indicate that the β2M antibody diminished AR and PSA mRNA and protein expression in both androgen-dependent and androgen-independent human prostate cancer cells.
Fig. 1.
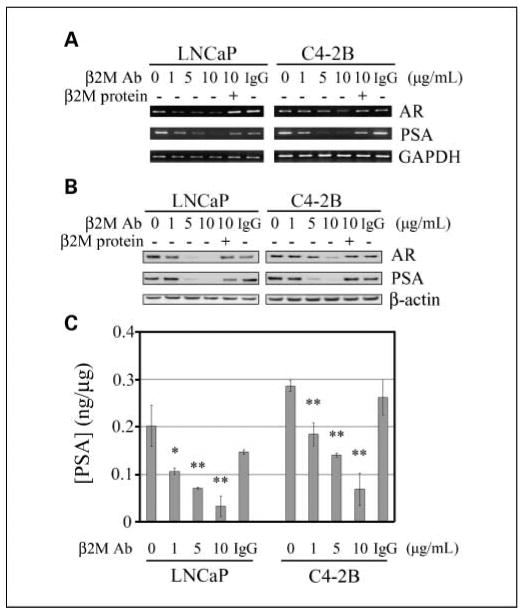
β2M antibody inhibited AR and PSA mRNA and protein expression in human prostate cancer cells. A, β2M antibody (β2M Ab) decreased AR and PSA mRNA expression in a dose-dependent manner (0-10 μg/mL, 24-h treatment) in both LNCaP (androgen dependent) and C4-2B (androgen independent) prostate cancer cell lines detected by reverse transcription-PCR. The inhibitory effect was restored by the preincubation of the β2M antibody with purified β2M protein. Isotype control IgG (10 μg/mL) did not affect AR and PSA mRNA expression. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. B, β2M antibody inhibited AR and PSA protein expression in a dose-dependent pattern (0-10 μg/mL, 24-h treatment) in LNCaP and C4-2B cells assayed by Western blot. The inhibitory effect was abrogated by the preincubation of β2M antibody with β2M protein. Control IgG (10 μg/mL) did not change AR and PSA protein expression. β-Actin was used as an internal loading control. C, secreted soluble PSA levels were also decreased by the β2M antibody (0-10 μg/mL), but not the control IgG, in a concentration-dependent inhibition in LNCaP and C4-2B cells determined by ELISA. The concentrations of PSA (ng) were normalized by total proteins (μg). *, P < 0.05; **; P < 0.005, significant differences from the β2M-antibody – untreated group. Columns, mean; bars, SD.
β2M antibody inhibited cell proliferation in human prostate cancer cell lines
Because β2M stimulated prostate and renal cancer cell growth through the promotion of cAMP/PKA/CREB signaling pathway and the activation of cyclins and cell cycle progression (8, 10), we investigated the possibility that interrupting the β2M-mediated signaling axis may be cytotoxic to prostate cancer cells. When the LNCaP and C4-2B cells were exposed to the β2M antibody (0-20 μg/mL) for a 2-day incubation, the growth of these two prostate cancer cell lines was inhibited in a concentration-dependent manner, with an IC50 of 10.3 and 7.4 μg/mL, respectively (Fig. 2A). The purified β2M protein was shown to rescue the β2M antibody–induced inhibition of prostate cancer cell proliferation, whereas the control IgG did not affect the growth of the LNCaP and C4-2B cells (Fig. 2A). Because of the AR heterogeneity in human prostate cancer cells (18), we compared the effects of the β2M antibody on the cell proliferation of AR-positive (LNCaP, C4-2B, and ARCaP) and AR-negative (PC3 and DU-145) human prostate cancer cell lines. Figure 2B shows that the β2M antibody (10 μg/mL) inhibited the proliferation of these prostate cancer cells at day 3 by 57% (LNCaP), 82% (C4-2B), 91% (DU-145), 93% (PC3), and 94% (ARCaP). These data suggest that the β2M antibody significantly inhibited cell proliferation in a broad range of human prostate cancer cell lines.
Fig. 2.
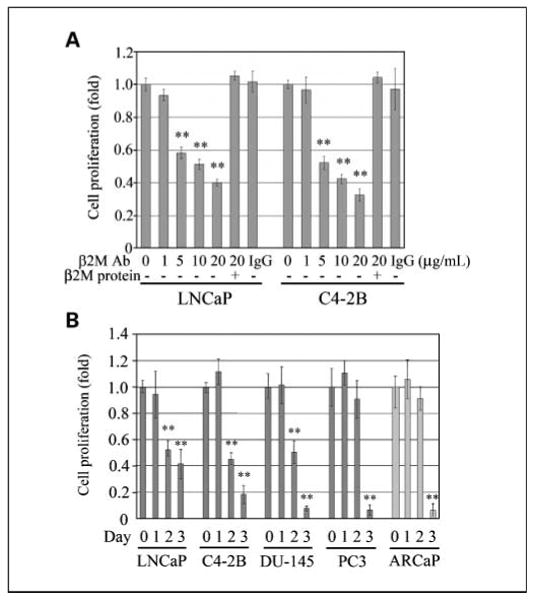
β2M antibody inhibited the growth of prostate cancer cell lines. A, β2M antibody significantly affected the cell proliferation of LNCaP and C4-2B prostate cancer cells, with a dose-dependent inhibition (0-20 μg/mL) after 2-d incubation determined by mitochondrial MTS assay (Promega). Purified β2M protein rescued the inhibitory effect on cell growth regulated by the β2M antibody. IgG (20 μg/mL) did not decrease the growth of LNCaP and C4-2B cells. The relative fold was assigned as 1.0 in the absence of β2M antibody treatment. **, P < 0.005, significant differences from the β2M-antibody – untreated group. Columns, mean of five replicate experiments; bars, SD. B, β2M antibody (10 μg/mL) inhibited the cell proliferation of a broad range of human prostate cancer cell lines, LNCaP, C4-2B, DU-145, PC3, and ARCaP, during 3-d treatment.The cell numbers were measured daily with a mitochondrial MTS method. The relative fold was assigned as 1.0 at day 0 for each prostate cancer cell line. **, P < 0.005, significant differences from day 0 for each cell line. Columns, mean of four or five replicate experiments; bars, SD.
β2M antibody induced apoptotic death and inhibited AR expression of prostate cancer cells in vitro and in mouse xenograft models
To determine the molecular mechanism by which the β2M antibody inhibited the growth of prostate cancer cells, we first examined apoptotic death in LNCaP and C4-2B cells, including sub-G1 DNA content analysis and activation of caspase (19) and PARP expression. The results of flow cytometric analysis revealed that the β2M antibody greatly increased sub-G1 DNA contents in LNCaP (% sub-G1 = 82.49) and C4-2B (% sub-G1 = 79.45) cells compared with the control IgG-treated LNCaP (% sub-G1 = 0.86) and C4-2B (% sub-G1 = 0.54) cells (Fig. 3A). Western blot analysis of caspases showed that cleaved caspase-9, caspase-3, and PARP, a downstream factor of caspases, were increased by exposing the LNCaP and C4-2B cells to the β2M antibody, but not the control IgG, for a 48-h incubation (Fig. 3B). The induction of cleaved caspases and PARP was attenuated by the preincubation of the β2M antibody with purified β2M protein. In addition, cell death induced by the β2M antibody was also confirmed at the level of light microscopy in LNCaP and C4-2B cells (Fig. 3C).
Fig. 3.
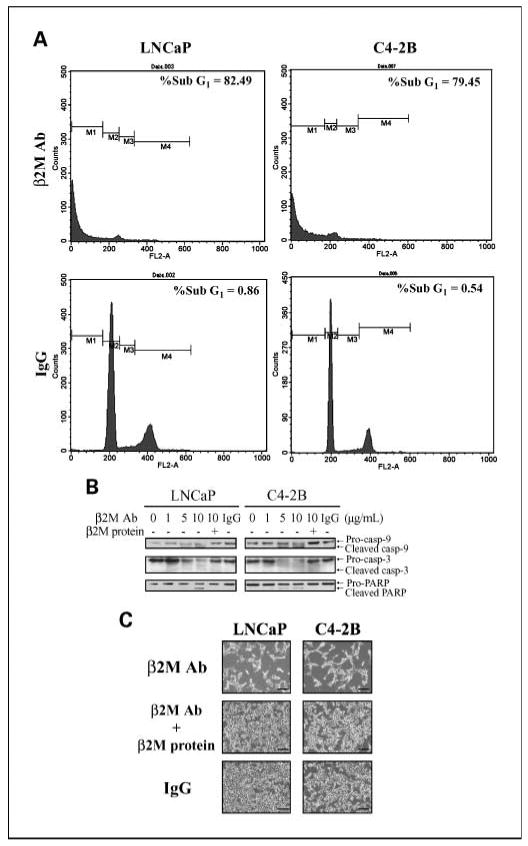
β2M antibody induced the cell death of prostate cancer cells through an apoptotic cascade pathway. A, LNCaP and C4-2B cells were exposed to either the β2M antibody or isotype control IgG (10 μg/mL) for 48-h incubation and subjected to cell cycle analysis determined by flow cytometry. Both LNCaP and C4-2B cells treated with the β2M antibody showed a marked increase in the sub-G1DNA contents compared with IgG-treated cells. B, β2M antibody (0-10 μg/mL, 48-h treatment) activated the expression of cleaved caspase-9, caspase-3, and PARP proteins in a dose-dependent pattern in LNCaP and C4-2B cells as assayed by Western blot. β2M protein rescued the apoptotic effect of the β2M antibody. Control IgG (10 μg/mL) did not activate cleaved caspase and PARP expression. C, LNCaP and C4-2B cells were treated with the β2M antibody; the β2M antibody was preincubated with β2M protein or control IgG (10 μg/mL) for 48 h and examined by light microscopy. Bar, 250 μm.
Next, we examined the effects of the β2M antibody on cell death and/or the status of AR in preexisting C4-2 (AR positive) and PC3 (AR negative) prostate tumors grown in mice as subcutaneous xenografts, with the antibody delivered as Surgifoam implants, and isotype-matched IgG and saline delivered similarly as controls. After 1-week treatment, tumor tissues were harvested from the euthanized mice and subjected to immunohistochemical staining of the AR and a commercially available cell death marker, M30 CytoDeath. Figure 4A and B shows that the β2M antibody dramatically inhibited AR expression in C4-2 tumors and induced cell death in both C4-2 and PC3 tumors in mice compared with the IgG-treated and saline-treated controls. The cell numbers of positive AR staining in the β2M-antibody–treated C4-2 tumor xenografts were greatly decreased from 81 ± 6 per 100 cells (IgG controls) and 76 ± 4 per 100 cells (saline controls) to 10 ± 3 per 100 cells. Markedly increased prostate cancer death from the β2M antibody was observed in both C4-2 (the positive M30 CytoDeath staining cells were 36 ± 8 cells per 100 cells) and PC3 (55 ± 15 cells per 100 cells) tumor specimens compared with the IgG-treated (C4-2, 9 ± 2 cells per 100 cells; PC3, 16 ± 3 cells per 100 cells) and saline-treated (C4-2, 10 ± 3 cells per 100 cells; PC3, 13 ± 2 cells per 100 cells) control groups.
Fig. 4.
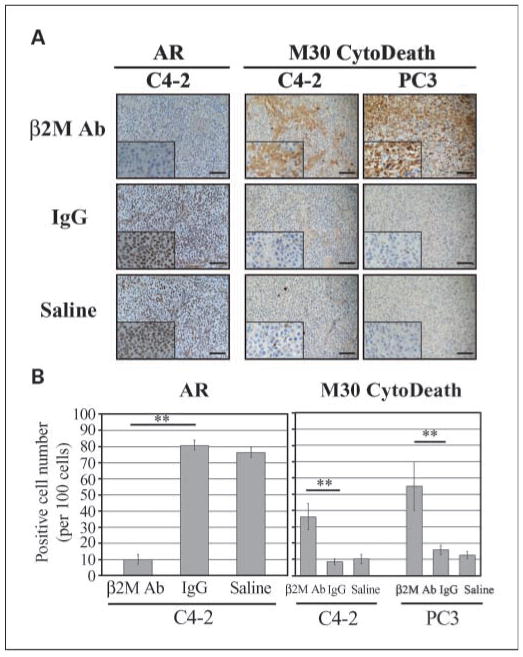
β2M antibody decreased AR expression and induced the cell death of subcutaneous C4-2 and PC3 prostate tumor growth in athymic nu/nu mice. A, immunohistochemical analysis showed dramatic down-regulation of AR expression in β2M antibody – treated subcutaneous C4-2 tumor mouse xenografts (n = 4) but not in the control IgG-treated (n = 4) or saline-treated (n = 4) C4-2 tumor-bearing mice. The β2M antibody also markedly induced apoptotic death in both subcutaneous C4-2 (n = 4) and PC3 (n = 4) prostate tumors in xenograft mice assayed by M30 CytoDeath marker staining. Bar, 100 μm. B, quantification of the positive AR and M30 CytoDeath marker staining cells in C4-2 and PC3 tumor specimens from the immunohistochemical analysis (A). One hundred cells at five randomly selected areas were counted. **, P < 0.005, significant differences from the control IgG group. Columns, mean; bars, SD.
We further investigated whether the β2M antibody may be a safe reagent to selectively kill cancer but not normal or nontumorigenic immortalized cell lines. A human nontumorigenic prostatic epithelial cell line, RWPE-1, was exposed to the β2M antibody and the control IgG. In contrast to human prostate cancer cells, the β2M antibody did not inhibit RWPE-1 cell growth (Fig. 5A), did not decrease its endogenous AR expression (Fig. 5B), and did not activate apoptotic marker expression as assayed by Western blot (Fig. 5B). While the β2M antibody showed low cytotoxicity in RWPE-1 cells, it also did not affect the growth of P69, a SV40-immortalized human normal prostatic epithelial cell line (20), and human normal prostatic stromal cells (data not shown).
Fig. 5.
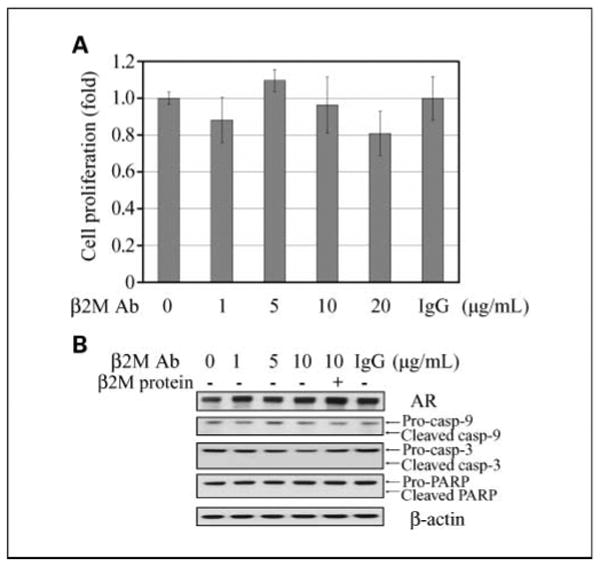
β2M antibody did not affect cell proliferation and endogenous AR expression; it also did not induce apoptotic death in human normal/nontumorigenic prostatic epithelial cells. A, β2M antibody (0-20 μg/mL, 3-d incubation) did not significantly affect cell proliferation of human normal prostatic epithelial cells, RWPE-1, as determined by mitochondrial MTS assay. Control IgG (20 μg/mL) also did not affect the growth of RWPE-1cells. The relative fold was assigned as 1.0 in the absence of β2M antibody treatment. Columns, mean of five replicate experiments; bars, SD. B, β2M antibody (0-10 μg/mL, 24-h treatment) did not inhibit AR nor activate cleaved caspase-9, caspase-3, and PARP protein expression in RWPE-1cells assayed by Western blot. Control IgG (10 μg/mL) also did not affect AR, cleaved caspase, or PARP protein expression.
In summary, our results collectively indicate that the β2M antibody effectively induced human prostate cancer, but not normal prostate, cell apoptosis in culture. The β2M antibody induced cell death in prostate tumor xenografts in mice regardless of their AR status. The β2M antibody was also shown to down-regulate AR and PSA expression in AR-positive and PSA-positive human prostate cancer cells grown in culture and as subcutaneous xenografts in mice.
Discussion
Prostate cancer progression from an androgen-dependent to an androgen-independent state symbolizes its hormone-refractory status and occurs in patients clinically. Because there is currently no effective therapy for the management of hormone-refractory prostate cancer, we undertook the investigation of the molecular mechanisms and effects of a recently identified novel molecular target, β2M, using β2M antibody as a single agent in experimental models of human prostate cancer. Our results showed that the β2M antibody exerted growth inhibitory and apoptotic action in AR-positive and PSA-positive human prostate cancer cells. The β2M antibody was also shown to induce similar apoptotic death in AR-negative and PSA-negative, and androgen-unresponsive human prostate cancer cells. Because aberrant androgen signaling mediated by the AR, a ligand-activated transcription factor and a survival factor, plays a key role in regulating prostate cancer growth and survival even in cells that are considered as androgen refractory (21, 22), we investigated the effects of the β2M antibody on the AR-signaling axis based on a cDNA microarray study, in which targeting β2M was shown to markedly down-regulate AR and PSA in AR-positive human prostate cancer cells C4-2B. Our results confirmed that the β2M antibody blocked AR signaling and PSA production in a series of AR-positive and PSA-positive, and lineage-related LNCaP (androgen dependent), C4-2 (androgen independent), and C4-2B (androgen independent) cells in a β2M-dependent manner (i.e., β2M protein could rescue the inhibitory effects of the β2M antibody).
We previously reported that a small protein, β2M, which was considered as a “housekeeping” gene product (23), was a key growth and signaling molecule regulating osteomimicry and promoting growth and survival in prostate cancer cells (8, 9). Targeting β2M and its signaling by β2M siRNA greatly induced prostate cancer cell death both in cultured cells and in mice with preestablished human prostate tumors (8). In the present study, we used the β2M antibody to block β2M-related signaling pathways, hoping to induce apoptosis in prostate tumors and rationalize the exploration of the β2M antibody as a novel agent for clinical trial in men with hormone-refractory cancer. We showed that the β2M antibody as a single agent significantly inhibited AR and PSA mRNA and protein expression in both LNCaP and C4-2B cells and induced apoptotic cell death in prostate tumor cells in vitro and in mouse xenografts (C4-2 and PC3 tumors) in vivo regardless of their AR status. The selective ability of the β2M antibody to block prostate tumor growth without affecting normal or nontumorigenic cells, including human normal prostatic epithelial and stromal cells, and normal hematopoietic cells in vitro or other normal tissues in vivo (12), suggests that the β2M antibody is a cancer-specific targeting agent that can be applied in the treatment of human prostate cancers. This conclusion is supported by previous studies in which immune intact mice with β2M knockdown survived and developed mild degrees of iron overload and arthritis without compromising their life expectancy (24–26). In addition, during a 10-week observation period, we have not noted any toxicity in mice treated intratumorally with the β2M antibody as evaluated by their body weights and physical appearance (data not shown). This observation is concurred by the early report of Yang et al. (12) although additional work is warranted to test the potential cytotoxicity of this antibody in immune intact hosts. We envision, nevertheless, that the β2M antibody can be applied in a cyclic manner to patients with prostate cancer, allowing the immune system to return to normal function during the off-cycle of the β2M antibody application.
Other than the blockade of the β2M antibody on AR survival factor expression, the detailed molecular mechanisms by which the β2M antibody induced prostate cancer apoptosis are unclear. We previously showed that β2M promoted the expression of cell cycle markers, cyclin D1 and cyclin A, and cell growth in prostate cancer cells through the activation of a cAMP/PKA/CREB signaling pathway (8). We also showed that β2M stimulated renal cancer cell proliferation via the induction of phosphatidylinositol 3-kinase (PI3K)/Akt, mitogen-activated protein kinase (MAPK), and cAMP/PKA/CREB pathways (10). This pleiotropic cell signaling network activated by β2M is likely to be the target for the β2M antibody. It has been amply documented that the activation of AR, PI3K/Akt, and MAPK pathways are important features contributing to uncontrolled prostate cancer cell growth and survival (22, 27, 28). Indeed, we observed that the β2M antibody blocked not only the AR (Fig. 1A and B) but also the cell signaling network mediated by PI3K/Akt and MAPK pathways in LNCaP and C4-2B cells (Supplementary Fig. S1). These results are consistent with previous presentations that blocking β2M-mediated signaling pathways can interrupt the PI3K/Akt and MAPK signaling pathways and induce c-Jun-NH2-kinase phosphorylation, resulting in the activation of a caspase-9 –dependent apoptotic cascade in human renal cell carcinoma (13) and hematologic cancer cells (12). The constitutive activation of a PI3K/Akt signaling pathway has been shown in prostate cancer cell lines by the inactivation of the PTEN tumor suppressor (29). Because the PI3K/Akt signaling pathway has been reported to mediate AR mRNA and protein expression through AR promoter regulation (30), we anticipated that the β2M antibody inhibition of the PI3K/Akt and MAPK signaling pathways would cause growth retardation, apoptosis, and down-regulation of AR expression and activity in AR-positive and PSA-positive LNCaP/C4-2/C4-2B cells. Likewise, because of the blockade of these critical signaling pathways, we also expected diminished growth and induced apoptosis in AR-negative prostate cancer cells in vitro and in vivo. These results could have significant clinical implications. For example, the β2M antibody could be superior to other antiandrogenic therapies with actions that rely on intrinsic AR expression by prostate cancer cells. The β2M antibody could be used either as a single reagent or in combination with other therapeutic modalities for the treatment of both hormone-dependent and hormone-refractory prostate cancers because these have been shown to exhibit marked heterogeneity of AR expression (31). This approach is promising, considering recent success in the development of therapeutic antibodies (32), such as trastuzumab, a HER2/erbB2 antibody for breast cancers; bevacizumab, a vascular endothelial growth factor antibody; and cetuximab, an epidermal growth factor receptor antibody for metastatic colon cancers.
In summary, our investigation revealed for the first time that (a) the β2M antibody inhibited the expression of the AR and PSA in both androgen-dependent and androgen-independent AR-positive and PSA-positive human prostate cancer cells; (b) the β2M antibody has a broad spectrum of growth-inhibitory effects in both AR-positive and AR-negative prostate cancer cells; and (c) although the β2M antibody has been shown to be a potent pleiotropic signaling and growth inhibitor and to induce programmed cell death through a caspase-9 –dependent pathway in prostate cancer cells, this antibody exhibited low cytotoxicity in human normal prostatic epithelial and stromal cells, which make it an attractive and safe therapeutic agent for future clinical application to treat prostate cancer and its progression.
Supplementary Material
Acknowledgments
Grant support: NIH grant P01CA98912 (L.W.K. Chung) and U54 CA119338 and Department of Defense grants PC040260 (L.W.K. Chung), W81XWH-07-1-0172, andW81XWH-08-1-0321and PC073356 (W-C. Huang).
We thank Gary Mawyer for editing the manuscript, our colleagues for helpful discussions, and Dr. Peter S. Nelson and colleagues (Fred Hutchinson Cancer Research Center, Seattle,WA) for performing the cDNA microarray in this study.
Footnotes
Unpublished data.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest: No potential conflicts of interest were disclosed.
References
- 1.Gussow D, Rein R, Ginjaar I, et al. The human β 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987;139:3132–8. [PubMed] [Google Scholar]
- 2.Cunningham BA, Wang JL, Berggard I, Peterson PA. The complete amino acid sequence of β2-microglobulin. Biochemistry. 1973;12:4811–22. doi: 10.1021/bi00748a001. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen LO, Hansen AS, Olsen AC, Gerwien J, Nissen MH, Buus S. The interaction between β2-microglobulin (β2m) and purified class-I major histocompatibility (MHC) antigen. Scand J Immunol. 1994;39:64–72. doi: 10.1111/j.1365-3083.1994.tb03341.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdul M, Hoosein N. Changes in β-2 microglobulin expression in prostate cancer. Urol Oncol. 2000;5:168–72. doi: 10.1016/s1078-1439(00)00063-6. [DOI] [PubMed] [Google Scholar]
- 5.Vitale M, Rezzani R, Rodella L, et al. HLA class I antigen and transporter associated with antigen processing (TAP1 and TAP2) down-regulation in high-grade primary breast carcinoma lesions. Cancer Res. 1998;58:737–42. [PubMed] [Google Scholar]
- 6.Korkolopoulou P, Kaklamanis L, Pezzella F, Harris AL, Gatter KC. Loss of antigen-presenting molecules (MHC class I and TAP-1) in lung cancer. Br J Cancer. 1996;73:148–53. doi: 10.1038/bjc.1996.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vegh Z, Wang P, Vanky F, Klein E. Selectively down-regulated expression of major histocompatibility complex class I alleles in human solid tumors. Cancer Res. 1993;53:2416–20. [PubMed] [Google Scholar]
- 8.Huang WC, Wu D, Xie Z, et al. β2-Microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–16. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 9.Huang WC, Xie Z, Konaka H, Sodek J, Zhau HE, Chung LW. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Res. 2005;65:2303–13. doi: 10.1158/0008-5472.CAN-04-3448. [DOI] [PubMed] [Google Scholar]
- 10.Nomura T, Huang WC, Zhau HE, et al. β2-Microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12:7294–305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 11.Gross M, Top I, Laux I, et al. {β}-2-Microglobulin is an androgen-regulated secreted protein elevated in serum of patients with advanced prostate cancer. Clin Cancer Res. 2007;13:1979–86. doi: 10.1158/1078-0432.CCR-06-1156. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Qian J, Wezeman M, et al. Targeting β2-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell. 2006;10:295–307. doi: 10.1016/j.ccr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 13.Nomura T, Huang WC, Seo S, Zhau HE, Mimata H, Chung LW. Targeting β2-microglobulin mediated signaling as a novel therapeutic approach for human renal cell carcinoma. J Urol. 2007;178:292–300. doi: 10.1016/j.juro.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Thalmann GN, Sikes RA, Wu TT, et al. LNCaP progression model of human prostate cancer: androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Wang R, Xie ZH, et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate. 2006;66:1664–73. doi: 10.1002/pros.20488. [DOI] [PubMed] [Google Scholar]
- 16.Zhau HY, Chang SM, Chen BQ, et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci U S A. 1996;93:15152–7. doi: 10.1073/pnas.93.26.15152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson PS, Clegg N, Arnold H, et al. The program of androgen-responsive genes in neoplastic prostate epithelium. Proc Natl Acad Sci U S A. 2002;99:11890–5. doi: 10.1073/pnas.182376299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruizeveld de Winter JA, Janssen PJ, Sleddens HM, et al. Androgen receptor status in localized and locally progressive hormone refractory human prostate cancer. Am J Pathol. 1994;144:735–46. [PMC free article] [PubMed] [Google Scholar]
- 19.Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–6. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 20.Bae VL, Jackson-Cook CK, Brothman AR, Maygarden SJ, Ware JL. Tumorigenicity of SV40 Tantigen immortalized human prostate epithelial cells: association with decreased epidermal growth factor receptor (EGFR) expression. Int J Cancer. 1994;58:721–9. doi: 10.1002/ijc.2910580517. [DOI] [PubMed] [Google Scholar]
- 21.Dehm SM, Tindall DJ. Regulation of androgen receptor signaling in prostate cancer. Expert Rev Anticancer Ther. 2005;5:63–74. doi: 10.1586/14737140.5.1.63. [DOI] [PubMed] [Google Scholar]
- 22.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25:276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Zakrajsek BA. Effect of experimental treatment on housekeeping gene expression: validation by real-time, quantitative RT-PCR. J Biochem Biophys Methods. 2000;46:69–81. doi: 10.1016/s0165-022x(00)00129-9. [DOI] [PubMed] [Google Scholar]
- 24.Muckenthaler MU, Rodrigues P, Macedo MG, et al. Molecular analysis of iron overload in β2-microglobulin-deficient mice. Blood Cells Mol Dis. 2004;33:125–31. doi: 10.1016/j.bcmd.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Kingsbury DJ, Mear JP, Witte DP, Taurog JD, Roopenian DC, Colbert RA. Development of spontaneous arthritis in β2-microglobulin-deficient mice without expression of HLA-B27: association with deficiency of endogenous major histocompatibility complex class I expression. Arthritis Rheum. 2000;43:2290–6. doi: 10.1002/1529-0131(200010)43:10<2290::AID-ANR17>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 26.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in β2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248:1227–30. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 27.Uzgare AR, Isaacs JT. Enhanced redundancy in Akt and mitogen-activated protein kinase-induced survival of malignant versus normal prostate epithelial cells. Cancer Res. 2004;64:6190–9. doi: 10.1158/0008-5472.CAN-04-0968. [DOI] [PubMed] [Google Scholar]
- 28.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 29.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–3. [PubMed] [Google Scholar]
- 30.Yang L, Xie S, Jamaluddin MS, et al. Induction of androgen receptor expression by phosphatidylinositol 3-kinase/Akt downstream substrate, FOXO3a, and their roles in apoptosis of LNCaP prostate cancer cells. J Biol Chem. 2005;280:33558–65. doi: 10.1074/jbc.M504461200. [DOI] [PubMed] [Google Scholar]
- 31.Magi-Galluzzi C, Xu X, Hlatky L, et al. Heterogeneity of androgen receptor content in advanced prostate cancer. Mod Pathol. 1997;10:839–45. [PubMed] [Google Scholar]
- 32.Lin MZ, Teitell MA, Schiller GJ. The evolution of antibodies into versatile tumor-targeting agents. Clin Cancer Res. 2005;11:129–38. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


