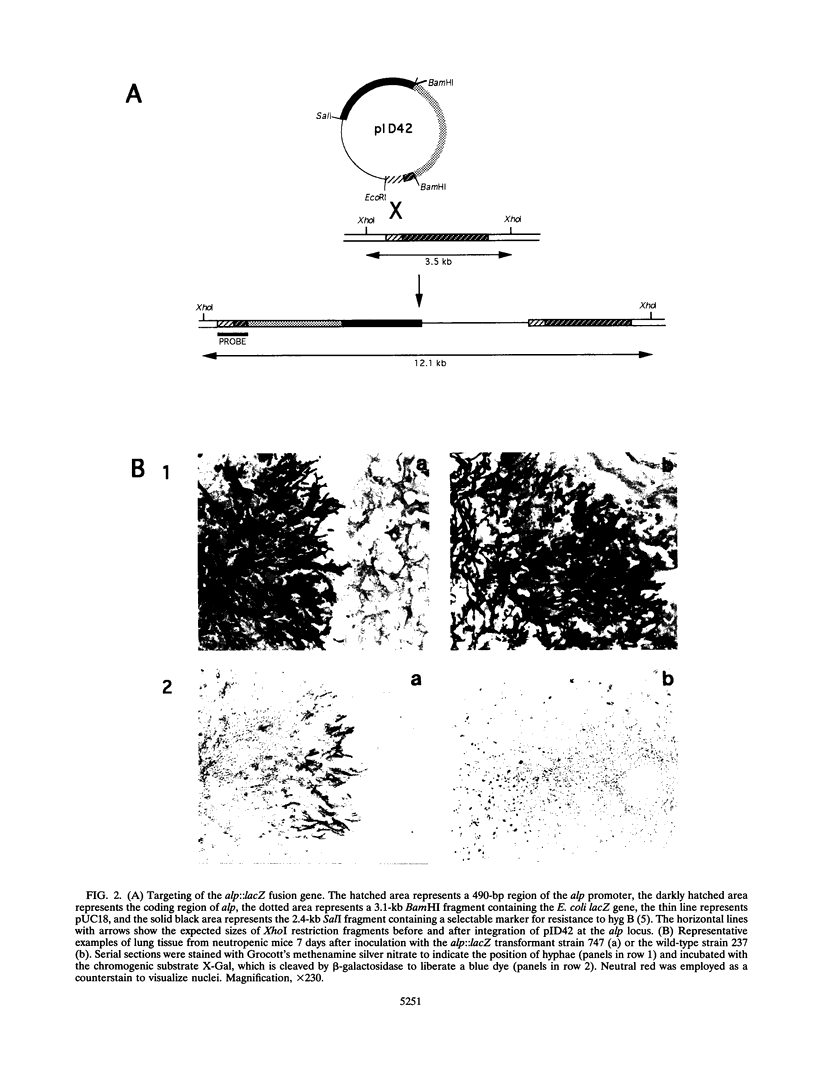
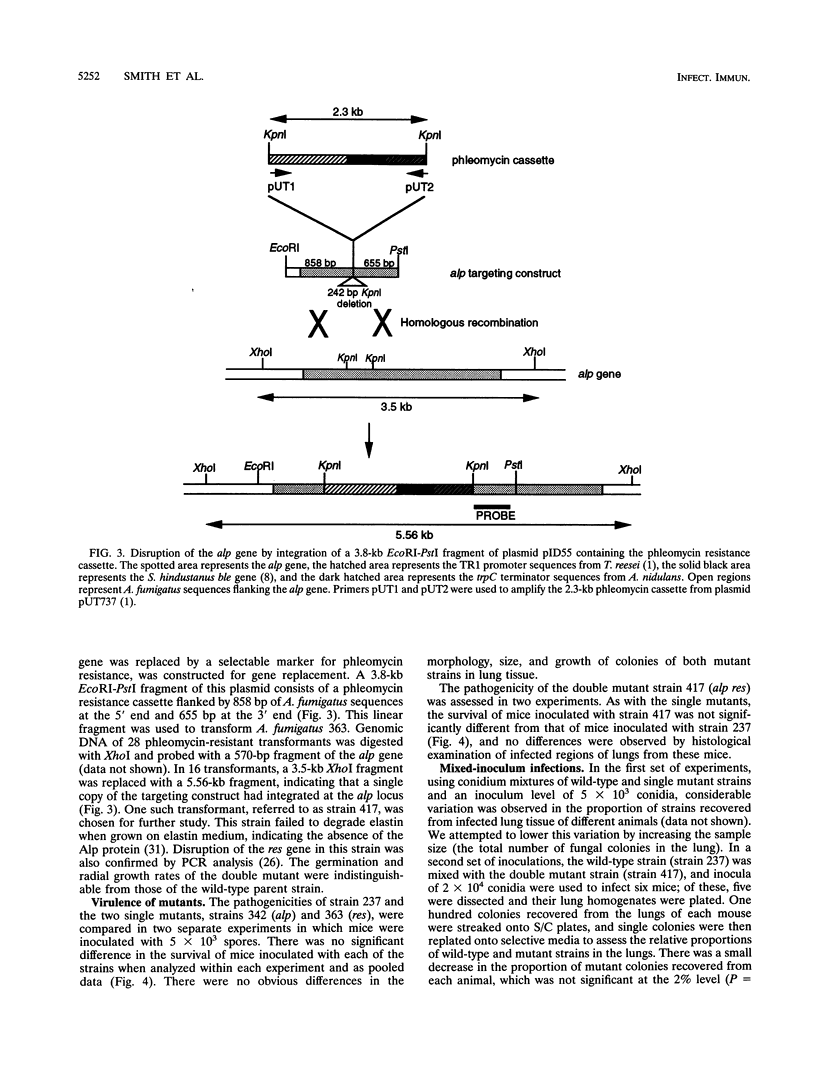
Abstract
To investigate the pathogenicity of Aspergillus fumigatus mutants lacking putative virulence factors, we have developed a new murine model of invasive pulmonary aspergillosis based on neutropenia, the major factor predisposing patients to this infection. Mice were treated with cyclophosphamide and inoculated by the intranasal route with 5 x 10(3) conidia, a significant reduction from inoculum levels used in previous models. Evidence for the production of the extracellular alkaline protease (Alp) in lung tissue was obtained by using a fungal transformant harboring an alp::lacZ reporter gene fusion. The pathogenicities of single mutant strains lacking either Alp or the ribotoxin restrictocin and of a double mutant strain lacking both proteins were assessed in this infection model. There were no significant differences between the mutant and the wild-type strains in terms of mortality or histological-features. Inoculations with mixtures of conidia showed that the double mutant strain is slightly less virulent than the wild-type strain. We conclude that Alp and restrictocin are not important virulence determinants in pulmonary infection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calmels T., Parriche M., Durand H., Tiraby G. High efficiency transformation of Tolypocladium geodes conidiospores to phleomycin resistance. Curr Genet. 1991 Sep;20(4):309–314. doi: 10.1007/BF00318520. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Cove D. J. The induction and repression of nitrate reductase in the fungus Aspergillus nidulans. Biochim Biophys Acta. 1966 Jan 11;113(1):51–56. doi: 10.1016/s0926-6593(66)80120-0. [DOI] [PubMed] [Google Scholar]
- Cullen D., Leong S. A., Wilson L. J., Henner D. J. Transformation of Aspergillus nidulans with the hygromycin-resistance gene, hph. Gene. 1987;57(1):21–26. doi: 10.1016/0378-1119(87)90172-7. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Dixon D. M., Polak A., Walsh T. J. Fungus dose-dependent primary pulmonary aspergillosis in immunosuppressed mice. Infect Immun. 1989 May;57(5):1452–1456. doi: 10.1128/iai.57.5.1452-1456.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drocourt D., Calmels T., Reynes J. P., Baron M., Tiraby G. Cassettes of the Streptoalloteichus hindustanus ble gene for transformation of lower and higher eukaryotes to phleomycin resistance. Nucleic Acids Res. 1990 Jul 11;18(13):4009–4009. doi: 10.1093/nar/18.13.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein D. J., Biddinger P. W., Rhodes J. C. Experimental murine invasive pulmonary aspergillosis. Am J Clin Pathol. 1990 Apr;93(4):510–515. doi: 10.1093/ajcp/93.4.510. [DOI] [PubMed] [Google Scholar]
- Jaton-Ogay K., Suter M., Crameri R., Falchetto R., Fatih A., Monod M. Nucleotide sequence of a genomic and a cDNA clone encoding an extracellular alkaline protease of Aspergillus fumigatus. FEMS Microbiol Lett. 1992 Apr 15;71(2):163–168. doi: 10.1016/0378-1097(92)90506-j. [DOI] [PubMed] [Google Scholar]
- Kolattukudy P. E., Lee J. D., Rogers L. M., Zimmerman P., Ceselski S., Fox B., Stein B., Copelan E. A. Evidence for possible involvement of an elastolytic serine protease in aspergillosis. Infect Immun. 1993 Jun;61(6):2357–2368. doi: 10.1128/iai.61.6.2357-2368.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kothary M. H., Chase T., Jr, Macmillan J. D. Correlation of elastase production by some strains of Aspergillus fumigatus with ability to cause pulmonary invasive aspergillosis in mice. Infect Immun. 1984 Jan;43(1):320–325. doi: 10.1128/iai.43.1.320-325.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamy B., Moutaouakil M., Latge J. P., Davies J. Secretion of a potential virulence factor, a fungal ribonucleotoxin, during human aspergillosis infections. Mol Microbiol. 1991 Jul;5(7):1811–1815. doi: 10.1111/j.1365-2958.1991.tb01930.x. [DOI] [PubMed] [Google Scholar]
- Latgé J. P., Moutaouakil M., Debeaupuis J. P., Bouchara J. P., Haynes K., Prévost M. C. The 18-kilodalton antigen secreted by Aspergillus fumigatus. Infect Immun. 1991 Aug;59(8):2586–2594. doi: 10.1128/iai.59.8.2586-2594.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monod M., Paris S., Sarfati J., Jaton-Ogay K., Ave P., Latgé J. P. Virulence of alkaline protease-deficient mutants of Aspergillus fumigatus. FEMS Microbiol Lett. 1993 Jan 1;106(1):39–46. doi: 10.1111/j.1574-6968.1993.tb05932.x. [DOI] [PubMed] [Google Scholar]
- Paris S., Monod M., Diaquin M., Lamy B., Arruda L. K., Punt P. J., Latgé J. P. A transformant of Aspergillus fumigatus deficient in the antigenic cytotoxin ASPFI. FEMS Microbiol Lett. 1993 Jul 15;111(1):31–36. doi: 10.1111/j.1574-6968.1993.tb06357.x. [DOI] [PubMed] [Google Scholar]
- Polak-Wyss A. Protective effect of human granulocyte colony-stimulating factor (hG-CSF) on Cryptococcus and Aspergillus infections in normal and immunosuppressed mice. Mycoses. 1991 May-Jun;34(5-6):205–215. doi: 10.1111/j.1439-0507.1991.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Powell W. A., Kistler H. C. In vivo rearrangement of foreign DNA by Fusarium oxysporum produces linear self-replicating plasmids. J Bacteriol. 1990 Jun;172(6):3163–3171. doi: 10.1128/jb.172.6.3163-3171.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard U., Büttner S., Eiffert H., Staib F., Rüchel R. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J Med Microbiol. 1990 Dec;33(4):243–251. doi: 10.1099/00222615-33-4-243. [DOI] [PubMed] [Google Scholar]
- Rhame F. S. Prevention of nosocomial aspergillosis. J Hosp Infect. 1991 Jun;18 (Suppl A):466–472. doi: 10.1016/0195-6701(91)90058-g. [DOI] [PubMed] [Google Scholar]
- Roilides E., Uhlig K., Venzon D., Pizzo P. A., Walsh T. J. Prevention of corticosteroid-induced suppression of human polymorphonuclear leukocyte-induced damage of Aspergillus fumigatus hyphae by granulocyte colony-stimulating factor and gamma interferon. Infect Immun. 1993 Nov;61(11):4870–4877. doi: 10.1128/iai.61.11.4870-4877.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabetta J. R., Miniter P., Andriole V. T. The diagnosis of invasive aspergillosis by an enzyme-linked immunosorbent assay for circulating antigen. J Infect Dis. 1985 Nov;152(5):946–953. doi: 10.1093/infdis/152.5.946. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Braude A. Selective protection against conidia by mononuclear and against mycelia by polymorphonuclear phagocytes in resistance to Aspergillus. Observations on these two lines of defense in vivo and in vitro with human and mouse phagocytes. J Clin Invest. 1982 Mar;69(3):617–631. doi: 10.1172/JCI110489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. M., Davies J. E., Holden D. W. Construction and pathogenicity of Aspergillus fumigatus mutants that do not produce the ribotoxin restrictocin. Mol Microbiol. 1993 Sep;9(5):1071–1077. doi: 10.1111/j.1365-2958.1993.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Spreadbury C. L., Krausz T., Pervez S., Cohen J. Invasive aspergillosis: clinical and pathological features of a new animal model. J Med Vet Mycol. 1989;27(1):5–15. doi: 10.1080/02681218980000021. [DOI] [PubMed] [Google Scholar]
- Streifel A. J., Lauer J. L., Vesley D., Juni B., Rhame F. S. Aspergillus fumigatus and other thermotolerant fungi generated by hospital building demolition. Appl Environ Microbiol. 1983 Aug;46(2):375–378. doi: 10.1128/aem.46.2.375-378.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Cohen J., Holden D. W. An Aspergillus fumigatus alkaline protease mutant constructed by gene disruption is deficient in extracellular elastase activity. Mol Microbiol. 1992 Jun;6(12):1663–1671. doi: 10.1111/j.1365-2958.1992.tb00891.x. [DOI] [PubMed] [Google Scholar]
- Tang C. M., Cohen J., Krausz T., Van Noorden S., Holden D. W. The alkaline protease of Aspergillus fumigatus is not a virulence determinant in two murine models of invasive pulmonary aspergillosis. Infect Immun. 1993 May;61(5):1650–1656. doi: 10.1128/iai.61.5.1650-1656.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang C. M., Smith J. M., Arst H. N., Jr, Holden D. W. Virulence studies of Aspergillus nidulans mutants requiring lysine or p-aminobenzoic acid in invasive pulmonary aspergillosis. Infect Immun. 1994 Dec;62(12):5255–5260. doi: 10.1128/iai.62.12.5255-5260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Waldorf A. R., Levitz S. M., Diamond R. D. In vivo bronchoalveolar macrophage defense against Rhizopus oryzae and Aspergillus fumigatus. J Infect Dis. 1984 Nov;150(5):752–760. doi: 10.1093/infdis/150.5.752. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Weiner M. H., Drutz D. J. Immunologic studies of disseminated infection with Aspergillus fumigatus in the nude mouse. J Infect Dis. 1981 May;143(5):726–733. doi: 10.1093/infdis/143.5.726. [DOI] [PubMed] [Google Scholar]
- van Gorcom R. F., Pouwels P. H., Goosen T., Visser J., van den Broek H. W., Hamer J. E., Timberlake W. E., van den Hondel C. A. Expression of an Escherichia coli beta-galactosidase fusion gene in Aspergillus nidulans. Gene. 1985;40(1):99–106. doi: 10.1016/0378-1119(85)90028-9. [DOI] [PubMed] [Google Scholar]