Abstract
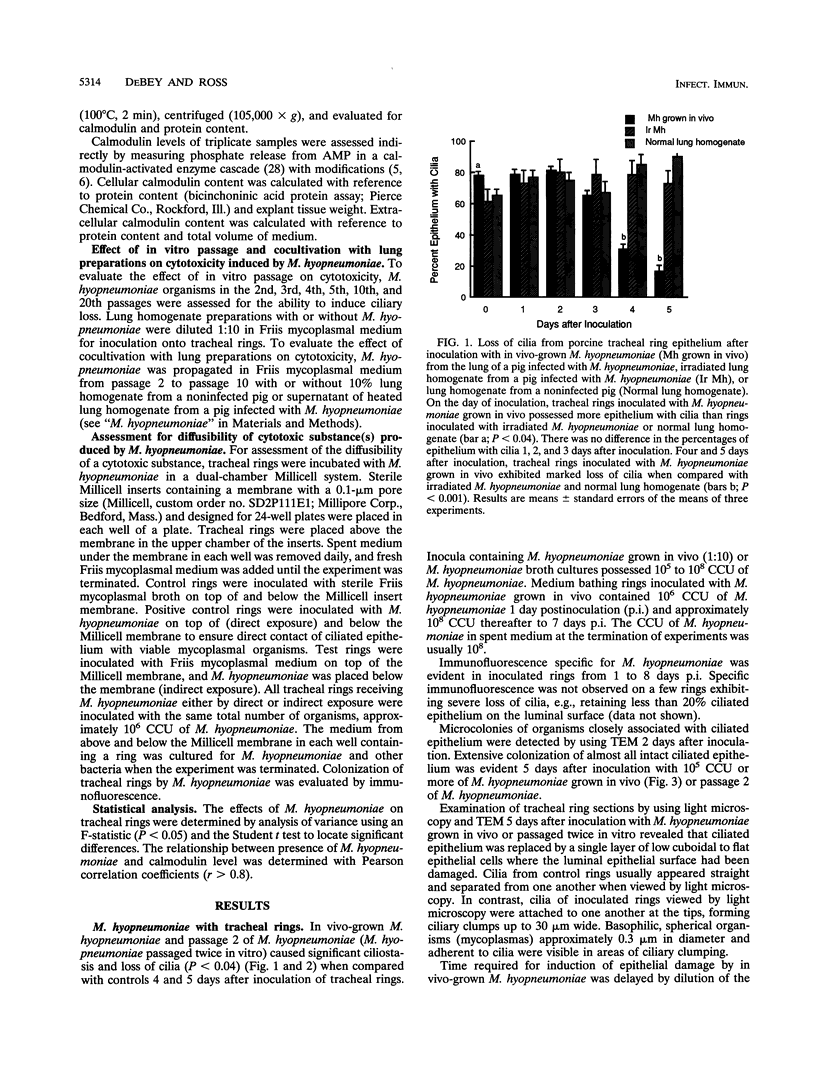
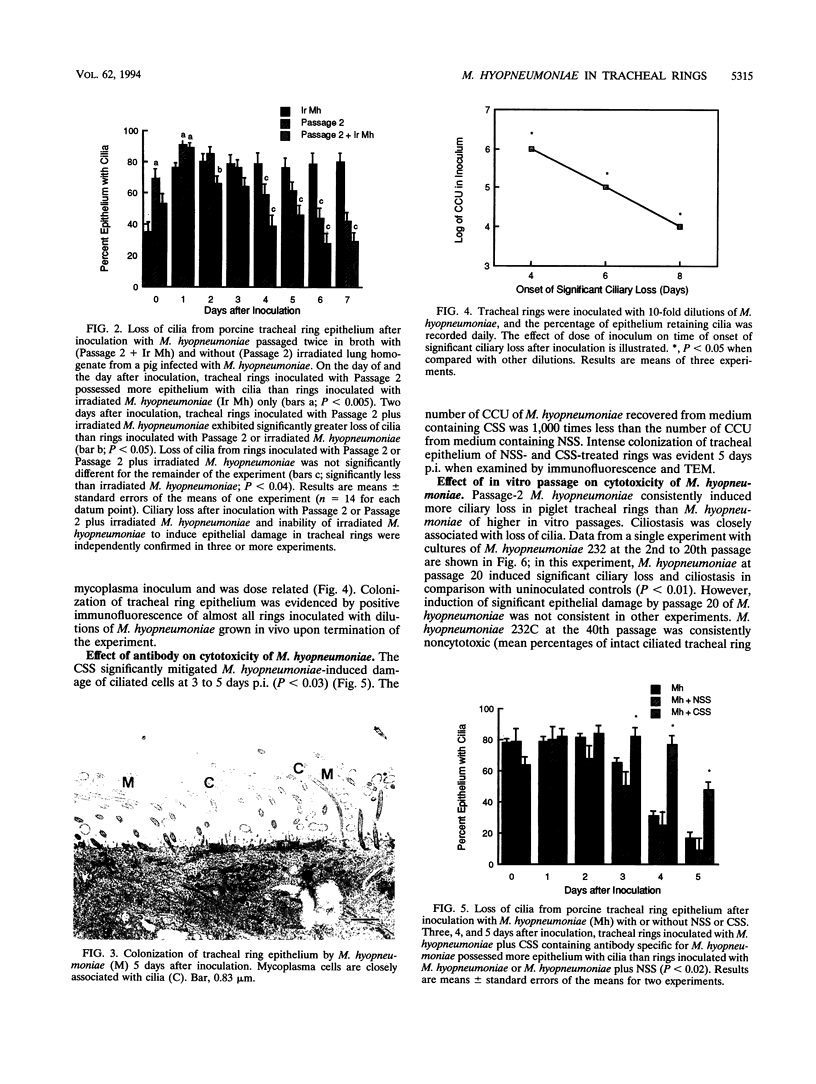
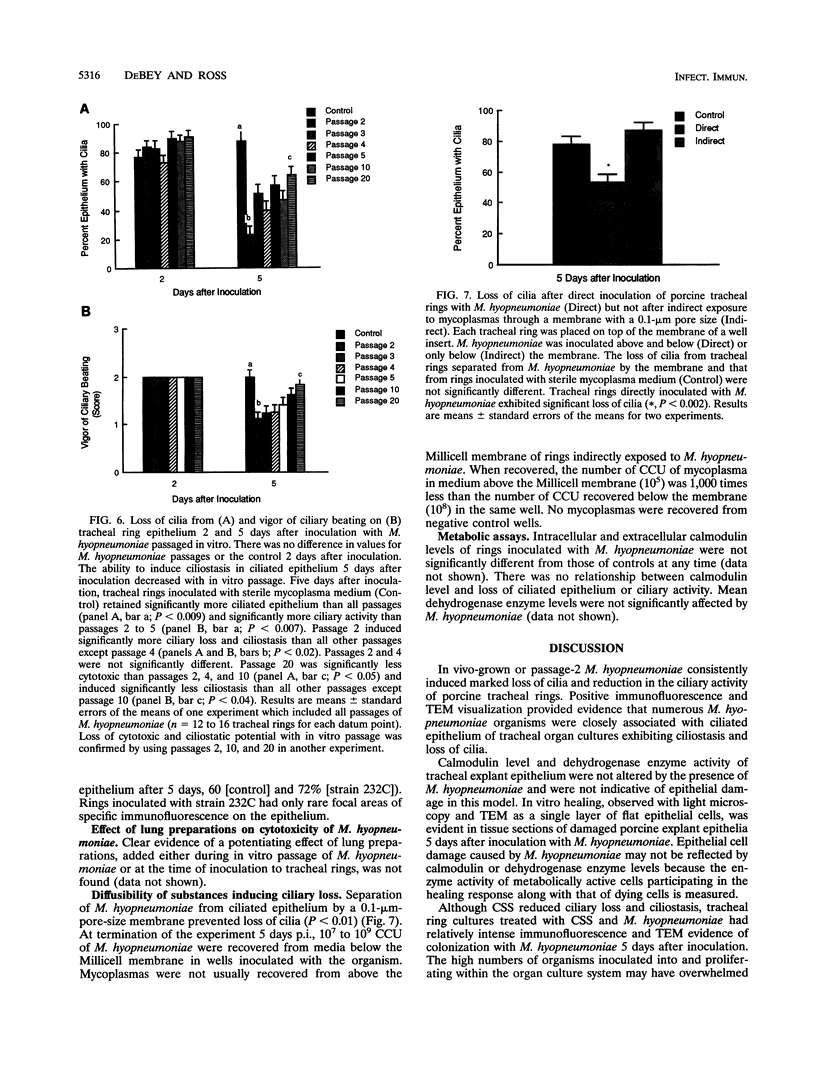
In vivo- and in vitro-grown Mycoplasma hyopneumoniae organisms were inoculated onto newborn piglet tracheal organ cultures to provide a model for interaction of this organism with ciliated respiratory epithelium. Ciliostasis and loss of cilia in tracheal rings were induced by M. hyopneumoniae grown in vivo and with low-passage cultures when grown in vitro. Levels of calmodulin or dehydrogenase enzymes in tracheal ring epithelium were not altered even though ciliostasis and loss of cilia induced by M. hyopneumoniae were extensive. The capacity for inducing epithelial damage diminished with in vitro passage of the organism. Attempts to induce higher-passage cultures to attach to cilia, cause ciliostasis, or cause ciliary damage by supplementation of mycoplasmal medium with porcine lung extract failed. Epithelial damage induced by M. hyopneumoniae in tracheal rings was averted by using porcine immune serum or by separating the organisms from ciliated epithelium with a 0.1-microns-pore-size membrane. Attachment, or at least close association, of M. hyopneumoniae to ciliated epithelium appeared to be necessary to induce ciliostasis and loss of cilia in this model.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackermann M. R., Debey M. C., Debey B. M. Bronchiolar metaplasia and Ulex europaeus agglutinin I (UEA-I) affinity in Mycoplasma hyopneumoniae-infected lungs of six pigs. Vet Pathol. 1991 Nov;28(6):533–535. doi: 10.1177/030098589102800611. [DOI] [PubMed] [Google Scholar]
- Aricó B., Miller J. F., Roy C., Stibitz S., Monack D., Falkow S., Gross R., Rappuoli R. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6671–6675. doi: 10.1073/pnas.86.17.6671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereiter M., Young T. F., Joo H. S., Ross R. F. Evaluation of the ELISA and comparison to the complement fixation test and radial immunodiffusion enzyme assay for detection of antibodies against Mycoplasma hyopneumoniae in swine serum. Vet Microbiol. 1990 Nov;25(2-3):177–192. doi: 10.1016/0378-1135(90)90075-7. [DOI] [PubMed] [Google Scholar]
- DeBey M. C., Jacobson C. D., Ross R. F. Histochemical and morphologic changes of porcine airway epithelial cells in response to infection with Mycoplasma hyopneumoniae. Am J Vet Res. 1992 Sep;53(9):1705–1710. [PubMed] [Google Scholar]
- Finlay B. B., Fry J., Rock E. P., Falkow S. Passage of Salmonella through polarized epithelial cells: role of the host and bacterium. J Cell Sci Suppl. 1989;11:99–107. doi: 10.1242/jcs.1989.supplement_11.8. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Heffron F., Falkow S. Epithelial cell surfaces induce Salmonella proteins required for bacterial adherence and invasion. Science. 1989 Feb 17;243(4893):940–943. doi: 10.1126/science.2919285. [DOI] [PubMed] [Google Scholar]
- Friis N. F. Some recommendations concerning primary isolation of Mycoplasma suipneumoniae and Mycoplasma flocculare a survey. Nord Vet Med. 1975 Jun;27(6):337–339. [PubMed] [Google Scholar]
- Gabridge M. G., Polisky R. B. Quantitative reduction of 2,3,4-triphenyl tetrazolium chloride by hamster trachea organ cultures: effects of Mycoplasma pneumoniae cells and membranes. Infect Immun. 1976 Jan;13(1):84–91. doi: 10.1128/iai.13.1.84-91.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary S. J., Walczak E. M. Cytopathic effect of whole cells and purified membranes of Mycoplasma hyopneumoniae. Infect Immun. 1983 Jul;41(1):132–136. doi: 10.1128/iai.41.1.132-136.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary S. J., Walczak E. M. Isolation of a cytopathic factor from Mycoplasma hyopneumoniae. Infect Immun. 1985 May;48(2):576–578. doi: 10.1128/iai.48.2.576-578.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker J., Bender L., Ott M., Wingender J., Lund B., Marre R., Goebel W. Deletions of chromosomal regions coding for fimbriae and hemolysins occur in vitro and in vivo in various extraintestinal Escherichia coli isolates. Microb Pathog. 1990 Mar;8(3):213–225. doi: 10.1016/0882-4010(90)90048-u. [DOI] [PubMed] [Google Scholar]
- Lee C. A., Falkow S. The ability of Salmonella to enter mammalian cells is affected by bacterial growth state. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4304–4308. doi: 10.1073/pnas.87.11.4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston C. W., Jr, Stair E. L., Underdahl N. R., Mebus C. A. Pathogenesis of mycoplasmal pneumonia in swine. Am J Vet Res. 1972 Nov;33(11):2249–2258. [PubMed] [Google Scholar]
- MARE C. J., SWITZER W. P. NEW SPECIES: MYCOPLASMA HYOPNEUMONIAE; A CAUSATIVE AGENT OF VIRUS PIG PNEUMONIA. Vet Med Small Anim Clin. 1965 Aug;60:841–846. [PubMed] [Google Scholar]
- Mebus C. A., Underdahl N. R. Scanning electron microscopy of trachea and bronchi from gnotobiotic pigs inoculated with Mycoplasma hyopneumoniae. Am J Vet Res. 1977 Aug;38(8):1249–1254. [PubMed] [Google Scholar]
- Pijoan C., Roberts D. H., Harding J. D. The effect of porcine mycoplasmas on pig tracheal organ cultures. J Appl Bacteriol. 1972 Sep;35(3):361–365. doi: 10.1111/j.1365-2672.1972.tb03710.x. [DOI] [PubMed] [Google Scholar]
- Rosengarten R., Kirchhoff H. Untersuchung über die cilienhemmende Aktivität verschiedener Mycoplasma hyorhinis-Stämme im Vergleich zu Acholeplasma- und anderen Mykoplasma-Spezies in Trachealorgankulturen. Zentralbl Veterinarmed B. 1981;28(1):27–45. [PubMed] [Google Scholar]
- Sharma R. K., Wang J. H. Preparation and assay of the Ca2+--dependent modulator protein. Adv Cyclic Nucleotide Res. 1979;10:187–198. [PubMed] [Google Scholar]
- Tajima M., Yagihashi T. Interaction of Mycoplasma hyopneumoniae with the porcine respiratory epithelium as observed by electron microscopy. Infect Immun. 1982 Sep;37(3):1162–1169. doi: 10.1128/iai.37.3.1162-1169.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington D. F., Fallon M. T., Watson H. L., Thorp R. K., Cassell G. H. Mycoplasma pulmonis V-1 surface protein variation: occurrence in vivo and association with lung lesions. Microb Pathog. 1989 Dec;7(6):429–436. doi: 10.1016/0882-4010(89)90023-5. [DOI] [PubMed] [Google Scholar]
- Underdahl N. R., Kennedy G. A., Ramos A. S., Jr Duration of Mycoplasma hyopneumoniae infection in gnotobiotic pigs. Can Vet J. 1980 Sep;21(9):258–261. [PMC free article] [PubMed] [Google Scholar]
- Williams P. P., Gallagher J. E. Cytopathogenicity of Mycoplasma hyopneumoniae in porcine tracheal ring and lung explant organ cultures alone and in combination with monolayer cultures of fetal lung fibroblasts. Infect Immun. 1978 May;20(2):495–502. doi: 10.1128/iai.20.2.495-502.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinski G. C., Ross R. F. Effect of growth in cell cultures and strain on virulence of Mycoplasma hyopneumoniae for swine. Am J Vet Res. 1990 Mar;51(3):344–348. [PubMed] [Google Scholar]



