Abstract
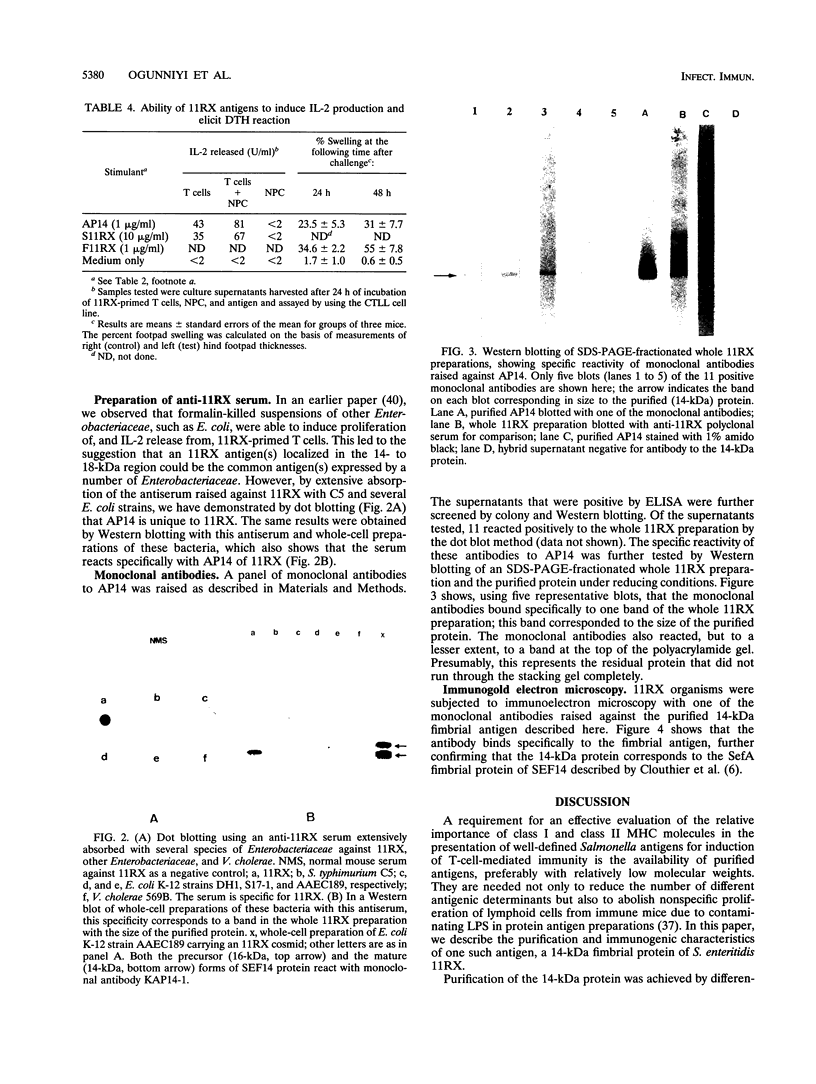
Our previous work, using proteins fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to define antigens of Salmonella enteritidis 11RX able to stimulate T cells from S. enteritidis 11RX-primed (BALB/c x C57BL/6)F1 mice, had indicated the presence of a major antigenic determinant of 14 to 18 kDa (H.-M. Vordermeier and I. Kotlarski, Immunol. Cell. Biol. 68:299-305, 1990). The 14-kDa size is similar to that of the monomeric units of one of the fimbrial structures, SEF14, produced by a human enteropathogen, S. enteritidis 27655 (J. Feutrier, W. W. Kay, and T. J. Trust, J. Bacteriol. 168:221-227, 1986). Here we present data which indicate that S. enteritidis 11RX also produces this protein and that it is able to elicit delayed-type hypersensitivity reactions in S. enteritidis 11RX-primed animals and to stimulate in vitro proliferation of, and cytokine release from, T cells obtained from these animals, implying that this fimbrial protein is likely to be an important immunogen of S. enteritidis. The protein was purified to homogeneity and is free from contamination with lipopolysaccharide. Standard immunoblot analysis with unabsorbed S. enteritidis 11RX antiserum and antiserum absorbed with Salmonella typhimurium C5 and various strains of Escherichia coli, as well as a panel of anti-14-kDa-protein monoclonal antibodies, suggests that this fimbrial protein is not the common antigen expressed by a number of organisms belonging to the family Enterobacteriaceae. Immunogold electron microscopy with one of these monoclonal antibodies confirms that the 14-kDa protein and SEF14 are identical.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attridge S. R., Kotlarski I. In vitro lymphokine release by lymphocytes from mice infected with Salmonella. Aust J Exp Biol Med Sci. 1985 Oct;63(Pt 5):489–502. doi: 10.1038/icb.1985.53. [DOI] [PubMed] [Google Scholar]
- Attridge S. R., Kotlarski I. Local transfer of delayed-type hypersensitivity after Salmonella infection in mice. Infect Immun. 1985 Dec;50(3):807–812. doi: 10.1128/iai.50.3.807-812.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom W. H., Husson R. N., Young R. A., David J. R., Piessens W. F. In vivo and in vitro characterization of murine T-cell clones reactive to Mycobacterium tuberculosis. Infect Immun. 1987 Sep;55(9):2223–2229. doi: 10.1128/iai.55.9.2223-2229.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Carbone F. R., Moore M. W., Sheil J. M., Bevan M. J. Induction of cytotoxic T lymphocytes by primary in vitro stimulation with peptides. J Exp Med. 1988 Jun 1;167(6):1767–1779. doi: 10.1084/jem.167.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouthier S. C., Müller K. H., Doran J. L., Collinson S. K., Kay W. W. Characterization of three fimbrial genes, sefABC, of Salmonella enteritidis. J Bacteriol. 1993 May;175(9):2523–2533. doi: 10.1128/jb.175.9.2523-2533.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. L., Gangarosa E. J. Nontyphoid salmonellosis. South Med J. 1978 Dec;71(12):1540–1545. doi: 10.1097/00007611-197812000-00028. [DOI] [PubMed] [Google Scholar]
- Cooke E. M. Epidemiology of foodborne illness: UK. Lancet. 1990 Sep 29;336(8718):790–793. doi: 10.1016/0140-6736(90)93251-j. [DOI] [PubMed] [Google Scholar]
- Falo L. D., Jr, Sullivan K., Benacerraf B., Mescher M. F., Rock K. L. Analysis of antigen presentation by metabolically inactive accessory cells and their isolated membranes. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6647–6651. doi: 10.1073/pnas.82.19.6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feutrier J., Kay W. W., Trust T. J. Purification and characterization of fimbriae from Salmonella enteritidis. J Bacteriol. 1986 Oct;168(1):221–227. doi: 10.1128/jb.168.1.221-227.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Goodnough M. C., Johnson E. A. Control of Salmonella enteritidis infections in poultry by polymyxin B and trimethoprim. Appl Environ Microbiol. 1991 Mar;57(3):785–788. doi: 10.1128/aem.57.3.785-788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988 Jun;9(6):168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Hug E., Väth U., Müller I. Effective protection against Listeria monocytogenes and delayed-type hypersensitivity to listerial antigens depend on cooperation between specific L3T4+ and Lyt 2+ T cells. Infect Immun. 1985 Apr;48(1):263–266. doi: 10.1128/iai.48.1.263-266.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Virkola R., Westurlund B., Holthöfer H., Parkkinen J. Tissue tropism of Escherichia coli adhesins in human extraintestinal infections. Curr Top Microbiol Immunol. 1990;151:115–127. doi: 10.1007/978-3-642-74703-8_6. [DOI] [PubMed] [Google Scholar]
- Kotlarski I., Pope M., Doherty K., Attridge S. R. The in vitro proliferative response of lymphoid cells of mice infected with Salmonella enteritidis 11RX. Immunol Cell Biol. 1989 Feb;67(Pt 1):19–29. doi: 10.1038/icb.1989.3. [DOI] [PubMed] [Google Scholar]
- Krogfelt K. A. Bacterial adhesion: genetics, biogenesis, and role in pathogenesis of fimbrial adhesins of Escherichia coli. Rev Infect Dis. 1991 Jul-Aug;13(4):721–735. doi: 10.1093/clinids/13.4.721. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee S. P., Stoker N. G., Grant K. A., Handzel Z. T., Hussain R., McAdam K. P., Dockrell H. M. Cellular immune responses of leprosy contacts to fractionated Mycobacterium leprae antigens. Infect Immun. 1989 Aug;57(8):2475–2480. doi: 10.1128/iai.57.8.2475-2480.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M. W., Carbone F. R., Bevan M. J. Introduction of soluble protein into the class I pathway of antigen processing and presentation. Cell. 1988 Sep 9;54(6):777–785. doi: 10.1016/s0092-8674(88)91043-4. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K. H., Collinson S. K., Trust T. J., Kay W. W. Type 1 fimbriae of Salmonella enteritidis. J Bacteriol. 1991 Aug;173(15):4765–4772. doi: 10.1128/jb.173.15.4765-4772.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl P., Lintermans P., Marin M., Couturier M. Epidemiological study of Salmonella enteritidis strains of animal origin in Belgium. Epidemiol Infect. 1991 Feb;106(1):11–16. doi: 10.1017/s0950268800056399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera M. J., Rivera N., Castillo J., Rubio M. C., Gómez-Lus R. Molecular and epidemiological study of Salmonella clinical isolates. J Clin Microbiol. 1991 May;29(5):927–932. doi: 10.1128/jcm.29.5.927-932.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue D. C., Tauxe R. V., Rowe B. International increase in Salmonella enteritidis: a new pandemic? Epidemiol Infect. 1990 Aug;105(1):21–27. doi: 10.1017/s0950268800047609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd E. Epidemiology of foodborne illness: North America. Lancet. 1990 Sep 29;336(8718):788–790. doi: 10.1016/0140-6736(90)93250-s. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Turcotte C., Woodward M. J. Cloning, DNA nucleotide sequence and distribution of the gene encoding the SEF14 fimbrial antigen of Salmonella enteritidis. J Gen Microbiol. 1993 Jul;139(7):1477–1485. doi: 10.1099/00221287-139-7-1477. [DOI] [PubMed] [Google Scholar]
- USHIBA D., SAITO K., AKIYAMA T., NAKANO M., SUGIYAMA T., SHIRONO S. Studies on experimental typhoid: bacterial multiplication and host cell response after infection with Salmonella enteritidis in mice immunized with live and killed vaccines. Jpn J Microbiol. 1959 Apr;3:231–242. doi: 10.1111/j.1348-0421.1959.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Villarreal B., Mastroeni P., de Hormaeche R. D., Hormaeche C. E. Proliferative and T-cell specific interleukin (IL-2/IL-4) production responses in spleen cells from mice vaccinated with aroA live attenuated Salmonella vaccines. Microb Pathog. 1992 Oct;13(4):305–315. doi: 10.1016/0882-4010(92)90040-u. [DOI] [PubMed] [Google Scholar]
- Vordermeier H. M., Kotlarski I. Identification of antigens which stimulate T lymphocytes of Salmonella enteritidis 11RX immunized mice. Immunol Cell Biol. 1990 Oct;68(Pt 5):299–305. doi: 10.1038/icb.1990.41. [DOI] [PubMed] [Google Scholar]
- Vordermeier H. M., Kotlarski I. Partial purification and characterization of low molecular weight antigens of Salmonella enteritidis 11RX. Immunol Cell Biol. 1990 Oct;68(Pt 5):307–316. doi: 10.1038/icb.1990.42. [DOI] [PubMed] [Google Scholar]
- von Boehmer H., Kisielow P., Kishi H., Scott B., Borgulya P., Teh H. S. The expression of CD4 and CD8 accessory molecules on mature T cells is not random but correlates with the specificity of the alpha beta receptor for antigen. Immunol Rev. 1989 Jun;109:143–151. doi: 10.1111/j.1600-065x.1989.tb00023.x. [DOI] [PubMed] [Google Scholar]