Abstract
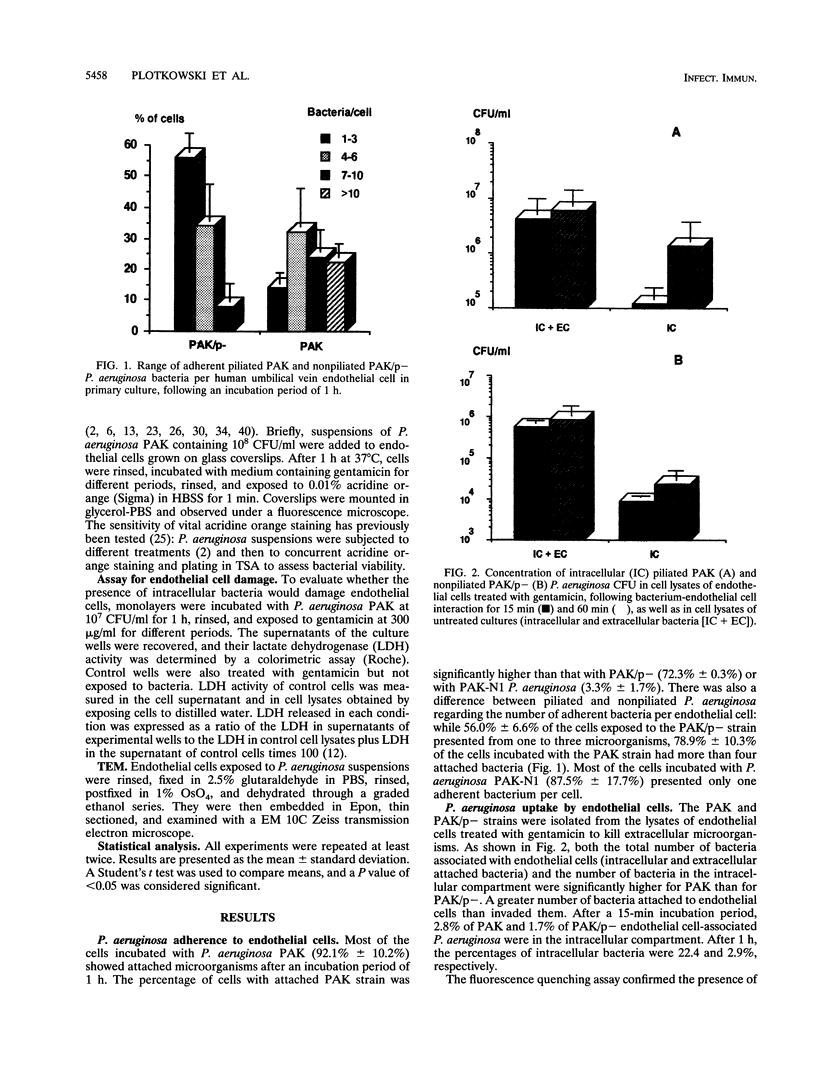
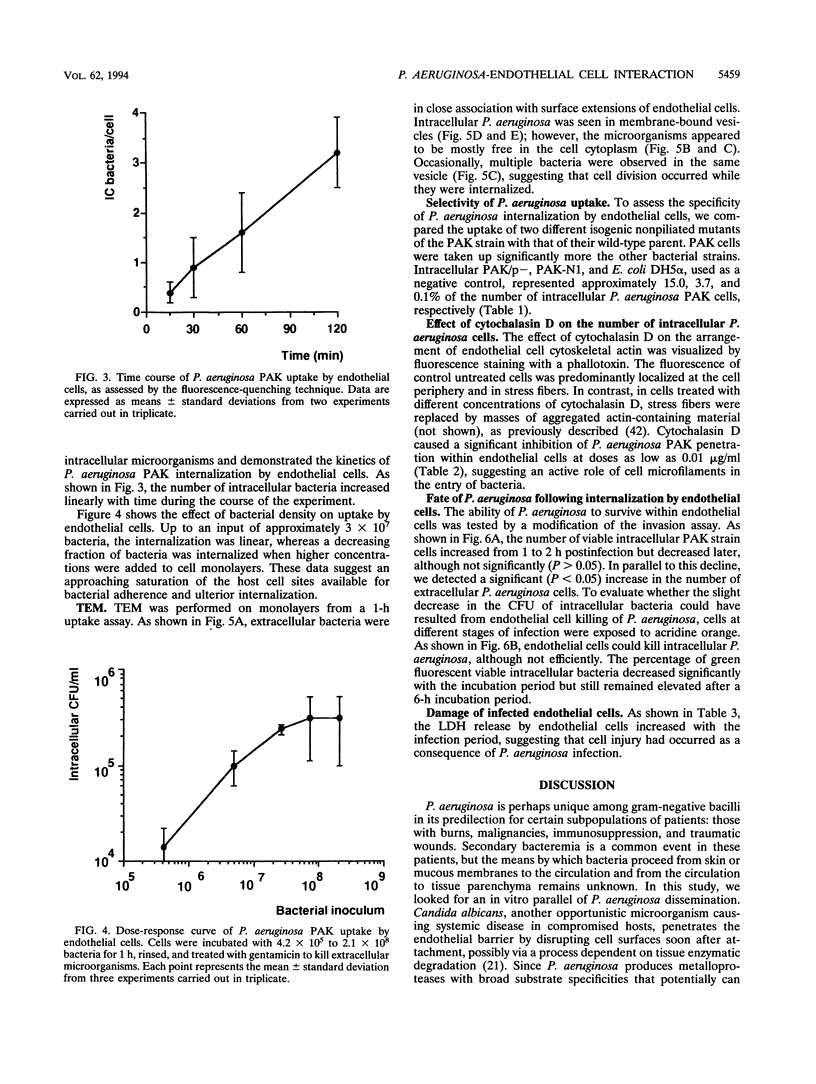
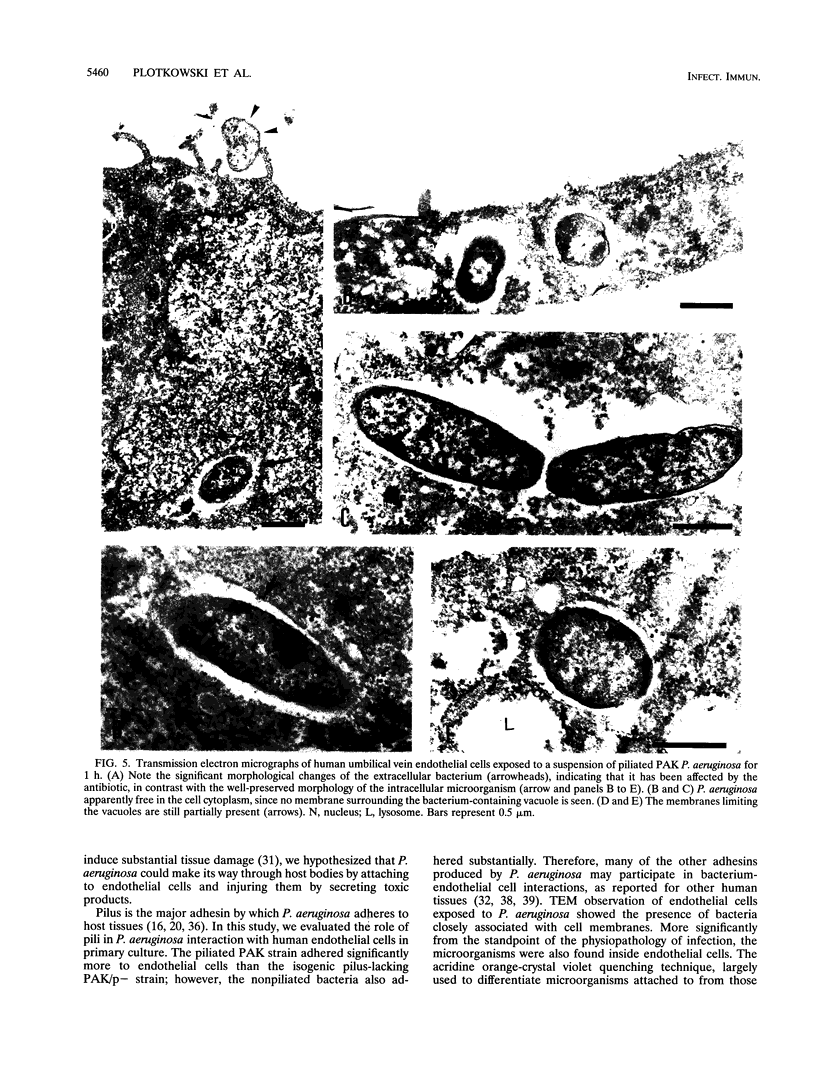
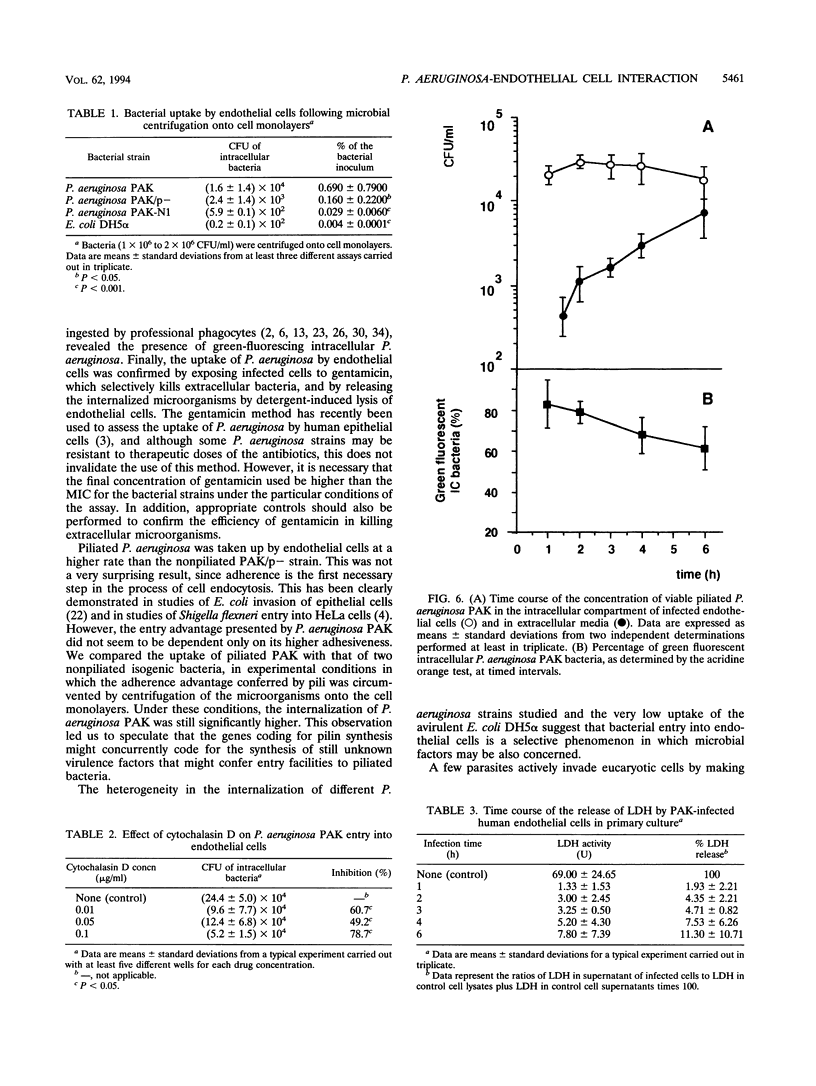
The pathogenesis of Pseudomonas aeruginosa disseminated infections depends on bacterial interaction with blood vessels. We have hypothesized that in order to traverse the endothelial barrier, bacteria would have to adhere to and damage endothelial cells. To test this hypothesis, we studied the adherence to human endothelial cells in primary culture of the piliated P. aeruginosa strain PAK and of two isogenic nonpiliated strains: PAK/p-, which carries a mutation in the pilin structural gene, and PAK-N1, a mutant defective in the regulatory rpoN gene. PAK adhered significantly more than did the pilus-lacking strains. P. aeruginosa was also taken up by endothelial cells, as determined by quantitative bacteriologic assays and by transmission electron microscopy. This internalization of P. aeruginosa seems to be a selective process, since the piliated strain was taken up significantly more than the nonpiliated bacteria and the avirulent Escherichia coli DH5 alpha, even following bacterial centrifugation onto the cell monolayers. A significant fraction of the internalized P. aeruginosa PAK was recovered in a viable form after 6 h of residence within endothelial cells. Progressive endothelial cell damage resulted from PAK intracellular harboring, as indicated by the release of lactate dehydrogenase. An increasing concentration of PAK cells was recovered from the extracellular medium with time, suggesting that ingested bacteria were released from endothelial cells and multiplied freely. We speculate that in vivo the ability of some P. aeruginosa strains to resist intracellular residence would afford protection from host defenses and antibiotics and that the release of viable bacteria into bloodstream may represent a central feature of the pathogenesis of bacteremia in compromised patients.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellinati-Pires R., Melki S. E., Colletto G. M., Carneiro-Sampaio M. M. Evaluation of a fluorochrome assay for assessing the bactericidal activity of neutrophils in human phagocyte dysfunctions. J Immunol Methods. 1989 May 12;119(2):189–196. doi: 10.1016/0022-1759(89)90395-5. [DOI] [PubMed] [Google Scholar]
- Chi E., Mehl T., Nunn D., Lory S. Interaction of Pseudomonas aeruginosa with A549 pneumocyte cells. Infect Immun. 1991 Mar;59(3):822–828. doi: 10.1128/iai.59.3.822-828.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerc P., Sansonetti P. J. Entry of Shigella flexneri into HeLa cells: evidence for directed phagocytosis involving actin polymerization and myosin accumulation. Infect Immun. 1987 Nov;55(11):2681–2688. doi: 10.1128/iai.55.11.2681-2688.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock L. E., Thomas D. D. Penetration of endothelial cell monolayers by Borrelia burgdorferi. Infect Immun. 1989 May;57(5):1626–1628. doi: 10.1128/iai.57.5.1626-1628.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig P., Todd T., Sastry P. A., Lee K. K., Hodges R. S., Paranchych W., Irvin R. T. Role of pili in adhesion of Pseudomonas aeruginosa to human respiratory epithelial cells. Infect Immun. 1988 Jun;56(6):1641–1646. doi: 10.1128/iai.56.6.1641-1646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flick M. R., Cluff L. E. Pseudomonas bacteremia. Review of 108 cases. Am J Med. 1976 Apr;60(4):501–508. doi: 10.1016/0002-9343(76)90716-6. [DOI] [PubMed] [Google Scholar]
- Gallagher P. G., Watanakunakorn C. Pseudomonas bacteremia in a community teaching hospital, 1980-1984. Rev Infect Dis. 1989 Nov-Dec;11(6):846–852. doi: 10.1093/clinids/11.6.846. [DOI] [PubMed] [Google Scholar]
- Geelen S., Bhattacharyya C., Tuomanen E. The cell wall mediates pneumococcal attachment to and cytopathology in human endothelial cells. Infect Immun. 1993 Apr;61(4):1538–1543. doi: 10.1128/iai.61.4.1538-1543.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson R. L., Lee M. K., Soderland C., Chi E. Y., Rubens C. E. Group B streptococci invade endothelial cells: type III capsular polysaccharide attenuates invasion. Infect Immun. 1993 Feb;61(2):478–485. doi: 10.1128/iai.61.2.478-485.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill R. J., Vann J. M., Proctor R. A. Phagocytosis of Staphylococcus aureus by cultured bovine aortic endothelial cells: model for postadherence events in endovascular infections. Infect Immun. 1986 Dec;54(3):833–836. doi: 10.1128/iai.54.3.833-836.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Young M. R. Interference with normal phagosome-lysosome fusion in macrophages, using ingested yeast cells and suramin. Nature. 1975 Jul 3;256(5512):47–49. doi: 10.1038/256047a0. [DOI] [PubMed] [Google Scholar]
- Irvin R. T., Doig P., Lee K. K., Sastry P. A., Paranchych W., Todd T., Hodges R. S. Characterization of the Pseudomonas aeruginosa pilus adhesin: confirmation that the pilin structural protein subunit contains a human epithelial cell-binding domain. Infect Immun. 1989 Dec;57(12):3720–3726. doi: 10.1128/iai.57.12.3720-3726.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto K. S., Lory S. Formation of pilin in Pseudomonas aeruginosa requires the alternative sigma factor (RpoN) of RNA polymerase. Proc Natl Acad Sci U S A. 1989 Mar;86(6):1954–1957. doi: 10.1073/pnas.86.6.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Hoyer L. W., Nachman R. L. Synthesis of von Willebrand factor by cultured human endothelial cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1906–1909. doi: 10.1073/pnas.71.5.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly N. M., Kluftinger J. L., Pasloske B. L., Paranchych W., Hancock R. E. Pseudomonas aeruginosa pili as ligands for nonopsonic phagocytosis by fibronectin-stimulated macrophages. Infect Immun. 1989 Dec;57(12):3841–3845. doi: 10.1128/iai.57.12.3841-3845.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz S. A., Drutz D. J., Harrison J. L., Huppert M. Adherence and penetration of vascular endothelium by Candida yeasts. Infect Immun. 1983 Oct;42(1):374–384. doi: 10.1128/iai.42.1.374-384.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korth M. J., Lara J. C., Moseley S. L. Epithelial cell invasion by bovine septicemic Escherichia coli. Infect Immun. 1994 Jan;62(1):41–47. doi: 10.1128/iai.62.1.41-47.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg D. P., Helmke R. J., German V. F., Mangos J. A. Resistance of mucoid Pseudomonas aeruginosa to nonopsonic phagocytosis by alveolar macrophages in vitro. Infect Immun. 1988 Dec;56(12):3173–3179. doi: 10.1128/iai.56.12.3173-3179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallolas J., Gatell J. M., Miró J. M., Marco F., Soriano E. Epidemiologic characteristics and factors influencing the outcome of Pseudomonas aeruginosa bacteremia. Rev Infect Dis. 1990 Jul-Aug;12(4):718–719. doi: 10.1093/clinids/12.4.718. [DOI] [PubMed] [Google Scholar]
- Miliotis M. D., Koornhof H. J., Phillips J. I. Invasive potential of noncytotoxic enteropathogenic Escherichia coli in an in vitro Henle 407 cell model. Infect Immun. 1989 Jul;57(7):1928–1935. doi: 10.1128/iai.57.7.1928-1935.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. Comparative biology of intracellular parasitism. Microbiol Rev. 1985 Sep;49(3):298–337. doi: 10.1128/mr.49.3.298-337.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngeleka M., Martineau-Doizé B., Fairbrother J. M. Septicemia-inducing Escherichia coli O115:K"V165"F165(1) resists killing by porcine polymorphonuclear leukocytes in vitro: role of F165(1) fimbriae and K"V165" O-antigen capsule. Infect Immun. 1994 Feb;62(2):398–404. doi: 10.1128/iai.62.2.398-404.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S. K., Yurberg E. R., Hatcher V. B., Levitt M. A., Lowy F. D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985 Oct;50(1):218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis C. G., Kniker W. T. Assessment of blood leukocyte microbial killing by using a new fluorochrome microassay. J Reticuloendothel Soc. 1979 Aug;26(2):155–170. [PubMed] [Google Scholar]
- Plotkowski M. C., Bajolet-Laudinat O., Puchelle E. Cellular and molecular mechanisms of bacterial adhesion to respiratory mucosa. Eur Respir J. 1993 Jun;6(6):903–916. [PubMed] [Google Scholar]
- Prince A. Adhesins and receptors of Pseudomonas aeruginosa associated with infection of the respiratory tract. Microb Pathog. 1992 Oct;13(4):251–260. doi: 10.1016/0882-4010(92)90035-m. [DOI] [PubMed] [Google Scholar]
- Pruzanski W., Saito S., Nitzan D. W. The influence of lysostaphin on phagocytosis, intracellular bactericidal activity, and chemotaxis of human polymorphonuclear cells. J Lab Clin Med. 1983 Aug;102(2):298–305. [PubMed] [Google Scholar]
- Ramphal R., Koo L., Ishimoto K. S., Totten P. A., Lara J. C., Lory S. Adhesion of Pseudomonas aeruginosa pilin-deficient mutants to mucin. Infect Immun. 1991 Apr;59(4):1307–1311. doi: 10.1128/iai.59.4.1307-1311.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M., Reen D. J. Characterization of antibody-mediated inhibition of Pseudomonas aeruginosa adhesion to epithelial cells. Infect Immun. 1992 Aug;60(8):3332–3338. doi: 10.1128/iai.60.8.3332-3338.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. A., Ramphal R., Lory S. Genetic analysis of Pseudomonas aeruginosa adherence: distinct genetic loci control attachment to epithelial cells and mucins. Infect Immun. 1992 Sep;60(9):3771–3779. doi: 10.1128/iai.60.9.3771-3779.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. L., Rommel F. A rapid micro method for the simultaneous determination of phagocytic-microbiocidal activity of human peripheral blood leukocytes in vitro. J Immunol Methods. 1977;17(3-4):241–247. doi: 10.1016/0022-1759(77)90106-5. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Wright S. D., Silverstein S. C., Mah B. Functional characterization of macrophage receptors for in vitro phagocytosis of unopsonized Pseudomonas aeruginosa. J Clin Invest. 1988 Sep;82(3):872–879. doi: 10.1172/JCI113692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannenbaum J., Brett J. G. Evidence for regulation of actin synthesis in cytochalasin D-treated HEp-2 cells. Exp Cell Res. 1985 Oct;160(2):435–448. doi: 10.1016/0014-4827(85)90191-0. [DOI] [PubMed] [Google Scholar]
- Vann J. M., Proctor R. A. Ingestion of Staphylococcus aureus by bovine endothelial cells results in time- and inoculum-dependent damage to endothelial cell monolayers. Infect Immun. 1987 Sep;55(9):2155–2163. doi: 10.1128/iai.55.9.2155-2163.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virji M., Kayhty H., Ferguson D. J., Alexandrescu C., Moxon E. R. Interactions of Haemophilus influenzae with cultured human endothelial cells. Microb Pathog. 1991 Mar;10(3):231–245. doi: 10.1016/0882-4010(91)90057-h. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Straus D. C., Johanson W. G., Jr, Berry V. K., Bass J. A. Role of pili in adherence of Pseudomonas aeruginosa to mammalian buccal epithelial cells. Infect Immun. 1980 Sep;29(3):1146–1151. doi: 10.1128/iai.29.3.1146-1151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulf E., Deboben A., Bautz F. A., Faulstich H., Wieland T. Fluorescent phallotoxin, a tool for the visualization of cellular actin. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4498–4502. doi: 10.1073/pnas.76.9.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler E. J., Douglas H. Pseudomonas aeruginosa vasculitis and bacteremia following conjunctivitis: a simple model of fatal pseudomonas infection in neutropenia. J Infect Dis. 1979 Mar;139(3):288–296. doi: 10.1093/infdis/139.3.288. [DOI] [PubMed] [Google Scholar]
- de Chastellier C., Berche P. Fate of Listeria monocytogenes in murine macrophages: evidence for simultaneous killing and survival of intracellular bacteria. Infect Immun. 1994 Feb;62(2):543–553. doi: 10.1128/iai.62.2.543-553.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]