Abstract
Leptin, the product of the Lep gene, reports levels of adiposity to the hypothalamus and other regulatory cells, including pituitary somatotropes, which secrete GH. Leptin deficiency is associated with a decline in somatotrope numbers and function, suggesting that leptin may be important in their maintenance. This hypothesis was tested in a new animal model in which exon 17 of the leptin receptor (Lepr) protein was selectively deleted in somatotropes by Cre-loxP technology. Organ genotyping confirmed the recombination of the floxed LepR allele only in the pituitary. Deletion mutant mice showed a 72% reduction in pituitary cells bearing leptin receptor (LEPR)-b, a 43% reduction in LEPR proteins and a 60% reduction in percentages of immunopositive GH cells, which correlated with reduced serum GH. In mutants, LEPR expression by other pituitary cells was like that of normal animals. Leptin stimulated phosphorylated Signal transducer and activator of transcription 3 expression in somatotropes from normal animals but not from mutants. Pituitary weights, cell numbers, IGF-I, and the timing of puberty were not different from control values. Growth curves were normal during the first 3 months. Deletion mutant mice became approximately 30–46% heavier than controls with age, which was attributed to an increase in fat mass. Serum leptin levels were either normal in younger animals or reflected the level of obesity in older animals. The specific ablation of the Lepr exon 17 gene in somatotropes resulted in GH deficiency with a consequential reduction in lipolytic activity normally maintained by GH and increased adiposity.
Deleting the signaling portion of leptin receptors selectively in somatotropes by Cre-LoxP technology in mice causes growth hormone deficiency, increases in fat mass, and adult onset obesity.
Leptin, a product of the Lep gene in adipocytes (1), is an important cytokine-like regulator that signals to the brain the systemic fat mass to appropriately regulate appetite and food intake and energy expenditure. Leptin inhibits appetite by stimulating anorexigenic neurons and inhibiting (orexigenic) neurons (2,3,4,5,6,7,8). The level of circulating leptin is directly proportional to the level of adiposity, and central and peripheral administration of leptin stimulates a profound decrease in appetite and increase in energy expenditure. Leptin may play broader roles in metabolic regulation (9,10,11,12,13).
There is also evidence that leptin is involved in neuroendocrine circuits that regulate the pituitary (14,15,16,17,18,19,20). The importance of leptin to the pituitary is highlighted by the phenotype of rodents with mutations in leptin or the leptin receptor (Lepr) gene (21,22). These animals show reduced numbers of somatotropes and gonadotropes as well as infertility, hyperphagia, obesity, and reduced energy expenditure (21,22,23,24). Infertility is also seen in obese humans bearing an inactivating mutation in LEP (25,26,27). Humans lacking the full-signaling LEPR (exon 16) are both infertile and have impaired growth (28). It is not clear whether the changes in the pituitary are directly caused by the lack of leptin or the metabolic disease and infertility because steroid stimulation is needed for the development and maintenance of somatotropes and gonadotropes (29,30,31).
LEPR is related to the class 1 cytokine receptor superfamily (32,33,34,35,36). Only the long form of the leptin receptor (LEPRb) has a single-pass transmembrane domain and a 302-amino acid cytosolic domain that binds and activates the Janus kinase (JAK)/signal transducer and activator of transcription (STAT) pathway (34). Subsets of all pituitary cells express LEPRb (21,37,38,39,40,41,42,43,44,45).
The relationship between leptin and pituitary somatotropes is particularly interesting because GH has a profound influence on body composition by direct interactions with adipocytes through GH receptors (46,47,48). GH is lipolytic, thereby decreasing adiposity and reducing leptin production (33,34,35). Whereas acromegalics have reduced body fat and lower leptin (39,40), GH-deficient humans have higher adiposity and leptin (49,50).
Most somatotropes express receptors for leptin (21,37,38,39,40,41,42,43,44,45), and studies have shown that leptin stimulates GH secretion (16,45,47,51,52,53,54,55,56,57,58,59,60,61). Shimon et al. (45) reported that cultured human fetal pituitary cells expressed LEPR and leptin stimulation of GH secretion can be seen as early as 8 wk of life (45). Recently Luque et al. (56) reported an in vivo study in which leptin restored GH secretion and pituitary GH mRNA in ob/ob mice that were infused with exogenous leptin for 7 d. In addition, leptin caused an increase in pituitary GHRH receptors. No changes were noted in hypothalamic GHRH mRNA levels themselves, which suggested that GHRH did not mediate the restoration. Recent studies from our laboratory reported that exposure to 10–100 pg/ml leptin, in vitro increased the number of GH-immunopositive cells, reduced by 24 h of fasting (62). Collectively these studies suggest that leptin may function directly to regulate or maintain somatotropes. To test this hypothesis, mice with somatotrope-specific knockout of LEPR JAK-STAT function were generated by crossing mice carrying the Cre-recombinase transgene driven by the rat GH promoter (rGHp-Cre) (63) with mice in which exon 17 of Lepr is flanked by loxP sequences (floxed). Exon 17 of Lepr codes for the region containing the JAK binding site in the cytoplasmic domain of LEPR (64). Deletion of this exon by Cre-recombinase results in the production of a truncated protein that would not activate the JAK/STAT pathways. The phenotype of the somatotrope-specific Lepr knockouts showed reduced GH and increased fat mass, highlighting the importance of leptin in the direct regulation of somatotrope function and suggesting that somatotropes themselves act as metabolic sensors, to maintain body composition.
Materials and Methods
Production of deletion mutant mice
All animal care protocols were approved by the Institutional Animal Care and Use committee. Mice in which Cre-recombinase is targeted to somatotropes were developed by Luque et al. (65). The Cre-recombinase (Cre) was targeted to GH cells with a construct that included the 310-bp 5′ end of the initiation codon of the rat GH gene (rGHp). Mice bearing loxP sequences flanking exon 17 of Lepr were characterized and used in recent studies to successfully ablate exon 17 of Lepr in neurons (66,67,68).
Cre+/− (100% C57BL/6) mice were crossbred with Leprfl/fl mice [100% Friend leukemia virus/NIH Jackson (FVB/NJ)] to generate Cre+/−, Leprfl/wt mice (F1 generation). The double-heterozygote offspring (50% C57BL/6, 50%FVB/NJ) were then cross-bred with Leprfl/fl mice (100% FVB/NJ) to produce Leprfl/fl mice with and without Cre (F2 generation; 75% FVB/NJ 25% C57BL/6). Cre-positive and -negative Leprfl/fl mice from this generation were then inbred to produce additional mice for study (F3 and F4 generation; 75% FVB/NJ, 25% C57BL/6).
Genomic DNA was extracted from tail snips from weanling mice (d 21) or adults after application of topical anesthetic cream. The Cre transgene was detected by primers that amplify a band of 166 bp (65); sense sequence is 5′-CGT ACT GAC GGT GGG AGA AT-3′ and antisense sequence is 5′-CCC GGC AAA ACA GGT AGT TA-3′. The PCR conditions were as follows: I step, one cycle at 95 C for 10 min; II step, 30–33 cycles a: 95 C for 1 min; 63 C for 1 min and 72 C for 1 min; and III step, one cycle at 72C for 10 min.
Three primers were used to detect the wild-type and intact floxed Lepr allele as well as Lepr with deleted exon 17 (LeprΔΔ): 1) mLepr65A (5′-AGA ATG AAA AAG TTG TTT TGG-3′), 2) mLepr-105 (5′-ACA GGC TTG AGA ACA TGA ACA-3′), and 3) mLepr-106 (5′-GTC TGA TTT GAT AGA TGG TCT T-3′). This set of primers detected wild-type Lepr as a band at 180 bp, floxed Lepr at 249 bp, and LeprΔ at 228 bp. PCR conditions began with a 1-min denaturation at 95 C followed by 35–40 cycles at 94, 55, and 72 C for 30 sec each.
Detection of growth and pubertal changes
After weaning, experimental mice were housed four to five mice per cage and fed Teklad 8640 diet (Harlan, Houston TX; crude protein 22%; crude fat 5%; crude fiber 4.5%) and water ad libitum. Mice were weighed at weaning (d 21) and twice a week until 55 d old and every 4 wk thereafter up to 12–13 months of age.
Nose to anus length was recorded using fine calipers at d 21. To determine normal growth and development of genitalia, balanopreputial separation (distance between anus and penis) was determined in males and vaginal opening was detected in females. The animals were checked every day for signs of puberty, including testicular descent (normally at 25 d of age) and vaginal opening (normally from 25–30 d of age).
Dual-energy x-ray absorptiometry (DEXA) analysis of body composition
DEXA analysis (whole body) was performed on 3.5- and 10-month-old control and deletion mutant mice to determine changes, if any in fat and lean mass (four to five mice per group). The mice were weighed and anesthetized (0.14 ml per 100 g body weight; 61.5 mg ketamine per 7.7 mg xylazine per milliliter) and scanned using a Piximus 2 DEXA (Lunar Corp., Madison, WI), specially designed for small-animal densitometry.
Collection of animals, pituitaries, sera, and immunoassays
Mice were anesthetized with isoflurane and decapitated; trunk blood was collected for hormone determinations. The mouse/rat GH enzyme immunoassay (EIA) kit was obtained from Linco/Millipore Corp. (St. Charles, MO; EZRMGH-45k). The sensitivity of this EIA was 0.07 ng/ml. Mouse IGF-I kits were from R&D Systems (Minneapolis, MN; DY791) and detected 50–10,000 pg/ml. Insulin was assayed by kits obtained from ALPCO Diagnostics (Salem, NH; 08-10-1145-01), which detected 0.15–5.5 ng/ml. Blood glucose was assayed by a glucometer on freshly collected blood.
A subset of pituitaries was removed, fixed in 4% paraformaldehyde and paraffin embedded, sectioned, and immunolabeled for rat GH, as in previous publications (69,70,71). Other pituitaries were dispersed into single cells and used for the studies of responses to leptin and the immunolabeling as described below. In addition, the pituitary and other organs (cerebrum, cerebellum, hypothalamus, heart, lung, liver spleen, stomach, pancreas, muscle, adipose tissue, adrenal, kidney, gonads, and uterus) were collected from adult and 21-d-old mice and frozen for genotyping and the determination of recombination of the floxed Lepr gene.
Pituitary cell dispersion and leptin stimulation
The dispersion protocol was optimized for mouse pituitaries from that previously reported for rats (30). We reduced the concentration of trypsin from 5 to 1 mg/ml and incubated the pituitary pieces for 20 min at 37 C. After centrifugation for 7 min (208 × g), the supernatant was replaced with dispersion solution, and the cells were gently dispersed through an 18-gauge needle 20–40 times. Cells were grown on poly-d-lysine coated coverslips in 24-well trays in DMEM containing insulin, sodium selenite, and transferrin (Sigma-Aldrich, St. Louis, MO). To test the responses to leptin, cells were grown for 2 or 15 h and then stimulated for 15–30 min with 50–150 nm mouse leptin diluted in DMEM containing protease inhibitors cocktail (Sigma-Aldrich). Then the cells were fixed with 2% glutaraldehyde and washed in phosphate buffer and kept at 4 C until their use in immunolabeling experiments.
Immunolabeling protocols
The immunolabeling protocols for GH, bovine LH-β (LHβ; from J. G. Pierce, University of California, Los Angeles, CA), human FSH-β, human TSHβ, rat prolactin (PRL), and 17-39 ACTH (from our laboratory) (69,70,71) and leptin (62,72,73) have been published. Controls involved the addition of competing antigens to the antisera. The LEPR was detected with antisera to LEPRb from α Diagnostics International Services (OBR13S; San Antonio, TX), which also provided the antigen for absorption (ObR13-P) tests, which neutralized immunolabeling. Optimal labeling was seen with 1:10,000 to 1:20,000 dilutions of anti-LEPR or LEPRb. Dual labeling for LEPR and pituitary hormones was done with the following: 1:200,000 antibovine LHβ, 1:220,000 antirat GH; 1:50,000 anti-FSHβ, 1:40,000 anti-TSHβ, 1:40,000 antirat PRL, 1:40,000 anti-17-39ACTH. Antisera to GH, FSH, TSH, and PRL were from the Hormone Distribution Program (Torrance, CA).
The antisera to phosphorylated (p) STAT3 was from Cell Signaling Technology (Beverly, MA), raised against synthetic phosphopeptide corresponding to residues surrounding tyrosine 705 of mouse STAT3. This is a monoclonal rabbit antibody, which does not have cross-reactivity with non-pSTAT3 or other phosphoproteins. Tests showed an optimal dilution of 1:10,000–1:15,000. Analysis of changes in immunolabeling patterns were done by counting immunolabeled cells in at least 10 fields from two different coverslips/group, sampling at least 60 fields/group.
ELISAs and pituitary protein extraction
Pituitary and hypothalamic proteins were extracted from three groups of adult (3–4 months) controls and deletion mutants for EIA for LEPR proteins. Freshly collected pituitaries and hypothalami were cut into small chunks and then spun to remove supernatant media and debris. Lysis buffer cocktail [RIPA buffer (radioimmunoprecipitation assay ; Sigma-Aldrich, R0278), and 1:1000 protease inhibitor cocktail (Sigma-Aldrich; P8340)] was added (100 μl per pituitary), followed by 10 min incubation on ice. After centrifugation for 10 min at 4 C, the supernatant was collected and protein concentration assayed. LEPR proteins were then detected by EIA (R&D Systems; Duoset, DY497). The detection and capture antibodies in this kit recognize a murine myeloma line protein that contains the extracellular domain of the LEPR.
Statistical analysis
Averages are of at least three separate experiments, or three to four different animals. One-way ANOVA was used to detect differences in a given set of experimental groups, followed by the Fisher least significant differences (LSD) post hoc test or Student’s t test. P < 0.05 was considered to be significant. A post hoc power analysis has been done to establish the number of replicates needed for these experiments as described in previous studies (62).
Results
The mice bearing the Cre-recombinase transgene and one (heterozygote) or two (homozygote) floxed alleles of Lepr exon 17 were fertile. The analysis of 12 F1 generation and eight F2 generation litters showed that numbers of pups/litter (8,9,10,11,12,13,14) were as expected from a strain that was at least 50% FVB/NJ. Survival rates were 95–97%. Litters from the F3 generation deletion mutant dams had on average only six pups per litter, whereas those from Cre-negative dams had normal numbers of pups (8,9,10,11,12,13,14). All surviving mice in the litters from deletion mutant dams had milk spots and they grew normally until they were weaned. Growth and assay data reported here are from 376 mice (F1-F4 generations) and in vitro studies are from 20 F6-F7 generation mice.
Reduction in LEPRs in mutant pituitaries
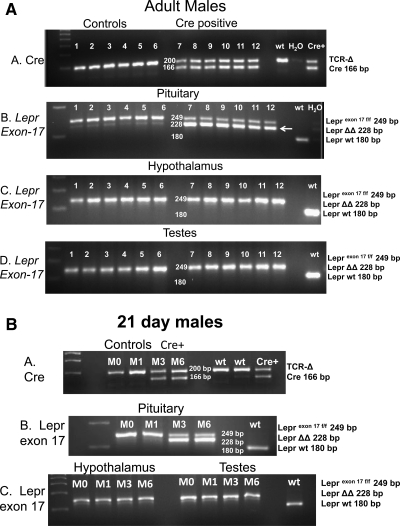
Organ genotyping confirmed recombination of the floxed Lepr gene only in the pituitaries of Cre-positive adult and 21-d-old mice (Fig. 1, A and B, show results from adult (Fig. 1A) and 21-d-old (Fig. 1B) males. In both figures, gel A shows the detection of the presence of Cre-recombinase as a 166-bp band, which is not found in the wild-type (wt) DNA. Positive controls included detection of T cell receptor-Δ, a housekeeping gene, and the use of DNA from a Cre+ animal.
Figure 1.
A and B, Genotyping of representative organs from adult (A) or 21-d (B) male deletion mutant or littermate control mice. Gel A in both figures shows the presence of the Cre-recombinase transgene in the mutant mice as a band at 166 bp. TCR-δ (TCR-Δ) is the T cell receptor-δ housekeeping gene (200 bp). Littermate controls and the wt animal have only TCR-Δ. Also included is DNA from a Cre+ animal. Gel B in both figures shows floxed exon 17 of the leptin receptor gene in the pituitaries of these mice. Both alleles are floxed, so the product appears as a 249-bp band in all control and mutant mice. A wt control mouse shows only the 180-bp wild-type Lepr. Gel B also shows the presence of truncated Lepr (with exon 17 deleted) as a 228-bp band (LeprΔΔ), only in the pituitaries of the adult and 21-d Cre+ males (arrow). Gels C and D show genotyping of hypothalami and testes from these same mice, which have only the 249-bp floxed Lepr exon 17 band.
Gel B in both figures shows the detection of Lepr exon 17 in the pituitaries. The excised Lepr exon 17 is seen as an additional 228-bp band (LeprΔΔ) in the pituitaries of the adult and 21-d Cre positive mice. Gels B, C, and D show only the 249-bp band for Lepr alleles in Cre-negative pituitaries and gonads and hypothalami. Similar results were seen in the 13 additional organs and when female organs were tested (data not shown).
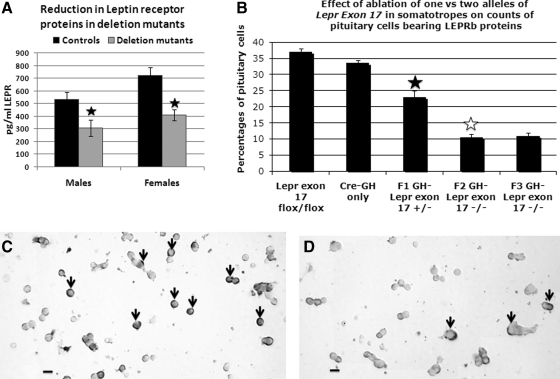
The EIA data from whole pituitary extracts showed a significant 42–43% decrease in LEPR proteins in pituitaries of both male and female deletion mutant mice (Fig. 2A). LEPR in hypothalami of deletion mutants did not differ from controls (data not shown).
Figure 2.
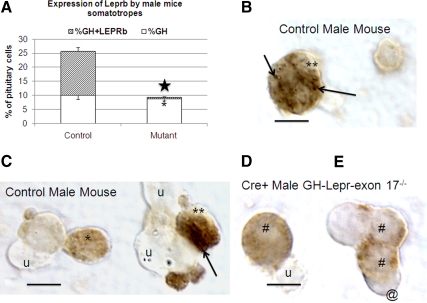
Reduction of LEPR in deletion mutant mice. A, EIA shows a significant (filled star) 42–43% reduction in LEPR proteins in deletion mutant male (P < 0.03) or female (P < 0.001) pituitaries. B, Percentages of cells from male mice immunolabeled for LEPRb controls including mice bearing only two floxed alleles of Lepr or only the Cre-recombinase transgene (Cre only) are not different from one another (P = 0.051). There is a significant decline in percentages of pituitary cells bearing LEPRb in heterozygous mice bearing one floxed and one wild-type allele of Lepr (P < 0.001, filled star) compared with either control group. In F2 and F3 homozygous mice, there is a further reduction in LEPRb target cells (F2 vs. F1, P < 0.001; F3 vs. F1, P < 0.002, open star). C and D, Photograph showing immunolabeling for LEPRb in fields from control (C) or deletion mutant (D) F3 generation mice including the reduction in labeled cells in the deletion mutants. The labeling for LEPRb is typically concentrated in a ring or in patches around the periphery of the target cells. Bar, 20 μm.
Because the antibody used in the EIA recognizes all LEPR isoforms and thus may underrepresent changes in LEPRb proteins, pituitary cells from control and deletion mutants were immunolabeled with an antibody that only recognizes the portion of the LEPRb that was deleted. The percentages of cells immunolabeled for LEPRb were 33–37% in controls (littermates bearing two floxed alleles of Lepr exon 17 and no Cre-recombinase or mice bearing only the rCre-GHp transgene) (Fig. 2B). Figure 2C depicts control cells immunolabeled for LEPRb. The immunolabeling is typically concentrated at the cell periphery and is eliminated by preincubation of the LEPR antiserum with 100 ng/ml LEPR antigen overnight, confirming specificity of the signal.
Hetero- and homozygote deletion mutants showed a graded reduction in LEPRb-immunolabeled cells (Fig. 2B). F1 generation heterozygous deletion mutant male mice had a 40% reduction in LEPRb cells (P < 0.001), whereas homozygous deletion mutant mice (from F2 and F3 generations) showed further reductions (up to 72%) in LEPRb target cells (F2 vs. F1, P < 0.001; F3 vs. F1, P < 0.002; Fig. 2D). The percentages of LEPRb protein-bearing cells were also reduced significantly (P < 0.001) in female mice, from 29 ± 4% in control to 8 ± 0.7% in F3 generation homozygote deletion mutants. Figure 2D illustrates the significant reduction in labeling in the field from the deletion mutants.
GH expression is reduced in deletion mutant mice
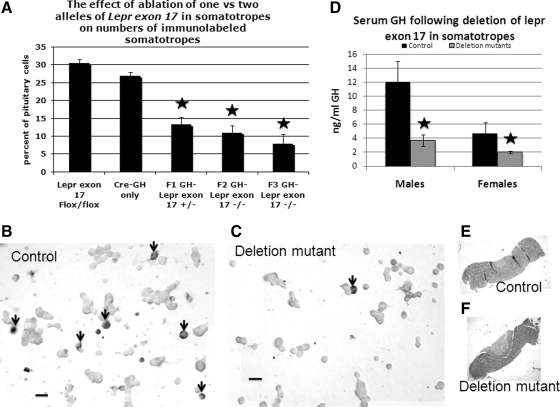
Parallel wells from the same pituitary cell populations used to detect LEPRb were immumolabeled for GH (Fig. 3) to determine whether the loss in LEPRs altered GH protein expression. There was a significant decrease in GH immunopositive cells in both heterozygous and homozygous deletion mutant male mice, compared with controls (P < 0.001). The values from the deletion mutant groups are not different from one another. The proportion of GH-immunopositive cells was also similarly reduced in deletion mutant female mice from 28 ± 1% in controls (F3) to 10 ± 1% in homozygous F2 or F3 generation deletion mutants (P < 0.001). The micrograph in Fig. 3B shows numerous, small to medium size, densely labeled cells (arrows), typical of somatotropes from control mice, whereas Fig. 3C illustrates the reduced numbers of GH-immunopositive cells in a field from homozygous deletion mutants. Despite the reduction in GH-immunopositive cells, there were no changes in overall numbers of pituitary cells when counts of dispersed cell populations from control and deletion mutant mice were compared (data not shown). Also, there were no changes in pituitary size or morphology in the deletion mutants as shown by paraffin sections in Fig. 3, E and F.
Figure 3.
Reduction in GH cells in deletion mutant mice. A, Control mice have 28–33% GH cells and heterozygous (F1 generation) and homozygous (F2 and F3 generation) deletion mutants have significantly reduced percentages of somatotropes (as detected by content of GH proteins) (P < 0.001). The values from the three groups of mutant mice are not different from one another. B, Illustration of one of the fields from a control mouse immunolabeled for GH showing numerous black-labeled small or medium sized GH cells (arrows). C, Cells from a homozygous deletion mutant mouse, showing the significantly reduced numbers of black-labeled GH cells (arrow). D, Serum GH EIA in male and female mice. At least three different groups were averaged, each of which had three to five mice per experimental group. F2 and F3 generation deletion mutant mice had significantly reduced GH in males (P = 0.02) and females (P = 0.05). Star, Significantly different from control values. E and F, Paraffin sections of whole-mouse pituitaries from control (D) or deletion mutant (E) mice showing no gross changes in size or morphology in the mutants.
To correlate the reduction in GH immunolabeled cells with GH secretion, sera from adult F3 generation mice were assayed by EIA for GH and IGF-I sampling three groups, each containing three to five animals per group. Serum GH was reduced by 72% in the homozygous deletion mutant male mice (P = 0.02) and 50% in deletion mutant females (P = 0.05) (Fig. 3D). No significant changes were seen in serum IGF-I or insulin in either sex (data not shown).
Selective deletion of LEPR in somatotropes
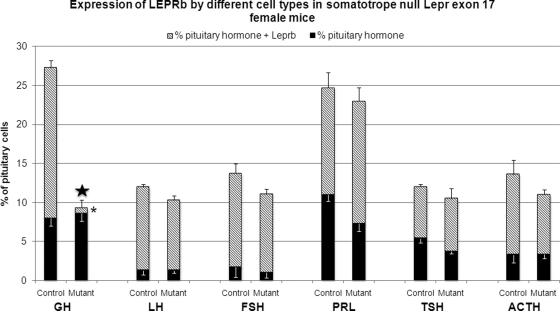
Dual labeling for LEPRb and GH, LH, FSH, PRL, TSH, and ACTH was done to detect the selectivity of the reduction in LEPRb and its impact on the other cell types. The stacked bars in Fig. 4 show the total percentage of each cell type in the pituitaries of control and mutant diestrous female mice; significant changes (a reduction) are seen only in the GH cell population. Similarly, the only changes in LEPRb expression (shown by the hatched portion of each bar) are seen among somatotropes. More than 80% of LH or FSH gonadotropes and more than 50% of PRL, TSH, and ACTH cells express LEPRb. The ablation of Lepr exon 17 in somatotropes did not affect their numbers or their expression of LEPRb. Figure 5A shows the counts of cells dual labeled for LEPRb and GH in the male, showing the same reduction in somatotropes and expression of LEPRb seen in the females. In ongoing studies of other cell types in the male, no changes in numbers or expression of LEPRb are seen (data not shown).
Figure 4.
Selective reduction in LEPRb proteins in somatotropes. Counts of cells labeled for LEPRb and/or the pituitary hormone. Each stacked bar represents the percentages of each cell type in the pituitary population and only percentages of GH cells are reduced in the deletion mutant population. (P < 0.001) (star). There are no significant changes in the percentages of any other cell type. The hatched regions in each bar, representing the proportion of each cell type that expresses LEPRb, show a significant reduction in expression of LEPRb only by somatotropes (P < 0.001, asterisk) in deletion mutants but no change in expression of leptin receptors by other cell types.
Figure 5.
Reduction in LEPRb selectively in male mice somatotropes. A, The expression of LEPRb by somatotropes is reduced significantly in the mutant (asterisk) along with a significant reduction overall in cells with GH antigens (P < 0.001, star). B and C, Illustration of dual-labeled somatotropes from control fields showing the label for LEPRb as black patches (arrows) in GH cells (double asterisks). GH cell in C (single asterisk) shows only one patch of label for LEPRb in that focal plane. D and E, Illustration of GH cells from deletion mutant mice (# sign), The GH cells show no black patches of label for LEPRb. The pale orange label for GH can still be seen. Cell labeled with @ is labeled for LEPRb but not GH. u, Unlabeled cells. Bar, 10 μm.
Figure 5, B and C, show photographs of dual-labeled cells in control male mice in which immunolabeling for GH was orange and that for LEPRb was black. GH-positive cells have black spots indicative of LEPRb labeling (double asterisks). A single asterisk points to GH cells that do not show the LEPRb labeling. Cells with GH label and no black spots are evident in the field from the deletion mutant in Fig. 5, D and E (# sign), whereas a GH-negative cell retained LEPRb labeling (@ sign).
Somatotropes from deletion mutant animals do not respond to leptin
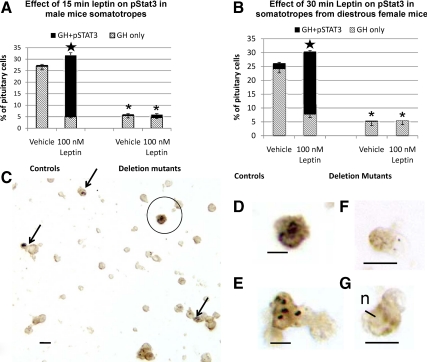
Plated cultures of anterior pituitary cells from F6 and F7 generation mice were exposed to 50–150 nm leptin for 15–30 min, fixed, and then immunolabeled for pSTAT3. Tests showed that optimal labeling was detected after 15 or 30 min in 100–150 nm leptin. When dual-labeled fields from control animals were counted, there was virtually no pSTAT3 expression in somatotropes exposed to the vehicle control (shown as hatched regions in each bar graph, Fig. 6, A and B). After 15 min in 100 nm leptin, there was a significant increase in expression of pSTAT3 to 66 ± 11% of male mice somatotropes (P < 0.001) along with an overall increase in the percentages of cells with GH antigens (P = 0.042). Similar results were seen in control females; however, the pSTAT3 expression rose to 45 ± 7% of somatotropes (data not shown). When control female cells were exposed to 150 nm leptin for 30 min; however, the pSTAT3 expression rose to 75 ± 3% of somatotropes, and the overall percentage of somatotropes was increased significantly (P = 0.03) (Fig. 6B). In contrast, cells from these F6 and F7 generation deletion mutant mice showed significantly fewer somatotropes overall (P < 0.001) and no significant changes in GH or pSTAT3 expression in response to leptin (Fig. 6, A and B). Figure 6, C–E illustrate the dual labeling for pSTAT3, which is mostly punctate, black and over nuclei and GH, which is orange-amber. Figure 6C illustrates a field from a control, leptin-treated culture and Fig. 6, D and E, show higher magnifications of the pSTAT3 labeling in GH cells. Figure 6, F and E, illustrate two GH cells from mutants, which show no evidence of labeling for pSTAT3.
Figure 6.
Somatotropes from mutant mice fail to respond to leptin. A and B, Freshly dispersed (A) or overnight cultures (B) of pituitary cells from male (A) or female (B) mice were treated with leptin, and the cells were dual labeled for pSTAT3 and GH. Counts in control animals showed that leptin stimulated a significant increase in the percentages of GH cells (P < 0.04) and in the percentage of GH cells expressing pSTAT3 (P < 0.001) after 15 min (A) or 30 min (B) (star). In contrast, somatotropes in deletion mutants from both groups show significantly reduced numbers of GH cells (P < 0.001, asterisks), little or no expression of pSTAT3, and no responses to leptin. C–E, Fields showing dual labeling for pSTAT3 (black) as punctate on nuclei (arrows) and that for GH as orange. One of the pSTAT3-expressing somatotropes in C is circled and magnified in D. F and G, Two somatotropes from deletion mutants showing orange label for GH but no labeling for pSTAT3.
Growth and timing of puberty is normal in 21- to 55-d-old mutant mice, but obesity develops with age
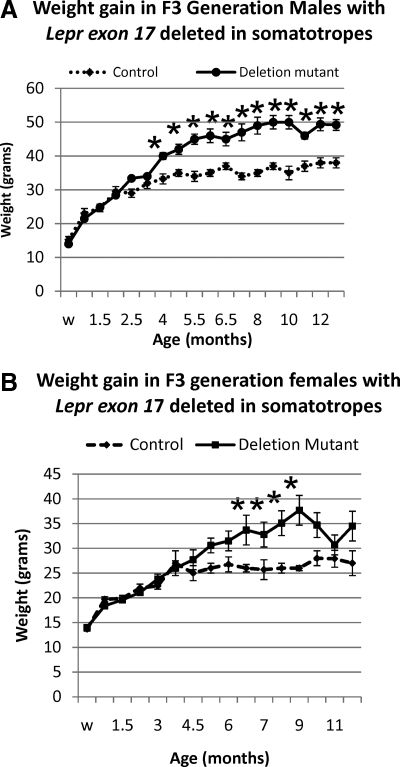
Despite the reduction in circulating GH, growth rates of deletion mutants were indistinguishable from that of their control littermates up to 3 months (Fig. 7, A and B) and similar to charts published for FVB/NJ and C57Bl/6 strains by Jackson Laboratories (Bar Harbor, ME). The timing of puberty was also normal in deletion mutant mice. Mean body weights of male (n = 7–10) and female (n = 7–14) homozygous deletion mutants began to diverge from controls (n = 7–10) at 4 and 6.5 months (Fig. 7, A and B), respectively, with male deletion mutants being 31% and female deletion mutation being 46% heavier than controls by 9 months of age. By 4 months of age, deletion mutant, homozygous males weighed significantly more than their age-matched controls and this significant difference continued throughout their life (differences at 4–7 and 11 months, P < 0.001; at 8–9 months, P = 0.007; 10 months, P = 0.005; 12 and 13 months, P = 0.002; Student’s t test). Females in the entire group did not exhibit significant weight gains over age-matched controls until they are 6.5 months old (P = 0.017). However, individual females did show a weight gain. The differences for the overall group become significant from 7 to 9 months of age (7 months, P = 0.02; 8 months, P = 0.015; 9 months, P = 0.038) after which the females in this F3 generation lost weight and the average weights were not different from those of the age-matched controls. The weight gain prompted tests of serum glucose and insulin, and both were not different from control mice.
Figure 7.
Weight gain in deletion mutant mice. A and B, Weanling (w; 21 d) and young growing mice show no differences when deletion mutant and littermate controls are compared. By 4 months of age, deletion mutant, homozygous males weighed significantly more than their age-matched controls, and this significant difference continued throughout their life (differences at 4–7 and 11 months, P < 0.001; at 8–9 months, P = 0.007; 10 months, P = 0.005; 12 and 13 months, P = 0.002; Student’s t test). Females do not exhibit significant weight gains over age-matched controls until they are 6.5 months old (P = 0.017). The differences are significant from 7 to 9 months of age (7 months, P = 0.02; 8 months, P = 0.015; 9 months, P = 0.038) after which the females in this F3 generation lost weight and the average weights were not different from those of the age-matched controls. Star, Significantly different from controls.
DEXA analysis of body composition
A subset of the male and female mice from the group in Fig. 7, A and B, was analyzed by DEXA at 3.5 and 10 months of age to correlate the weight changes with changes in percent body fat and lean mass. Table 1 shows a significant increase in weight in 3.5-month-old males and 3.5- and 10-month-old females. [Note: this subset of five deletion mutant females was heavier than the control group (P < 0.001), in contrast with the averaged data from Fig. 7B, which sampled 10–14 mice].
Table 1.
DEXA analysis of body composition
| Lean mass | Fat mass | Total | Weight | |
|---|---|---|---|---|
| Males | ||||
| 3.5-month controls | 21.4 ± 0.8 | 4.3 ± 0.2 | 26.5 ± 0.6 | 30 ± 0.8 |
| 3.5-month deletion mutants | 23.9 ± 0.9 | 9.75 ± 1a | 34.4 ± 5a | 41.5 ± 3a |
| 10-month controls | 24.5 ± 2 | 12.9 ± 3a | 37.4 ± 5a | 46 ± 3.6a |
| 10-month deletion mutants | 25.5 ± 0.6 | 8.5 ± 1a | 34 ± 1.5a | 39 ± 1.6a |
| Females | ||||
| 3.5-month controls | 15.5 ± 0.3 | 2.9 ± 0.3 | 18.4 ± 0.3 | 22 ± 0.3 |
| 3.5-month deletion mutants | 17 ± 0.3 | 4.7 ± 0.6a | 21.9 ± 0.9a | 25.6 ± 1a |
| 10-month controls | 19.5 ± 0.7 | 7.2 ± 1a | 26.8 ± 1.6a | 30.8 ± 1.5a |
| 10-month deletion mutants | 23.4 ± 4b | 17.4 ± 1b | 40.9 ± 5b | 44 ± 5b |
Values are average grams ± sem (n = 4–5).
Significantly different from 3.5-month-old controls in the same gender.
Significantly higher than all other groups in the same gender.
The increases in weight were correlated with significant increases in grams of body fat in the 3.5-month-old males (P = 0.009) and the 3.5- (P < 0.007) and 10 (P < 0.001)-month-old females (Table 1). There were no differences in lean body mass among the four groups of males. There was a significant increase in lean body mass in the 10-month-old females when compared with all other groups (P = 0.013).
The analysis of serum leptin and insulin was performed in five groups of 3- to 4-month F3 generation animals. There were no significant differences between control and mutant groups. However, leptin levels rose with obesity in the older males and females. Serum insulin and blood glucose also remained at normal levels for all young adult groups tested, even if they contained obese animals (data not shown).
Discussion
Leptin reduces fat stores by acting centrally to decrease appetite and increase metabolic rate (1,15,16,74), and GH acts on adipocytes to break down fat (48,75). Leptin may also feed back to directly regulate somatotrope functions because rodents lacking LEPRs or leptin have low numbers of somatotropes (47,76). Luque et al. (56) restored GH secretion by infusing ob/ob mice (lacking leptin) with exogenous leptin for 7 d. Leptin also restored GH pulses in fasted rats (52,77) and restored numbers of GH cells that had been reduced by fasting (62). In the present study, 15 min of leptin significantly increased numbers of immunopositive GH cells in vitro. Collectively these data suggest that leptin may be important in the maintenance of somatotropes, acting directly at the level of the pituitary.
We tested this hypothesis by developing a mouse model in which the signaling portion of the leptin receptor (LEPRb) was ablated selectively in somatotropes by Cre-loxP technology. Genotyping detected the presence of the deleted alleles (LeprΔΔ) only in the pituitaries of mice bearing Cre-recombinase as early as 21 d of age. The same Cre-positive mice showed no evidence of deleted Lepr exon 17 in 15 different organs. These findings confirm the selectivity of Cre-recombinase expression, originally reported by Luque et al. (65), who also showed that Cre-recombinase acted in embryonic pituitaries.
The pituitaries of the deletion mutants showed a dramatic reduction in pituitary LEPR and LEPRb isoform proteins. The antisera used in the immunolabeling specifically detected the long cytoplasmic domain in LEPRb. After deletion, LeprΔΔ would be expected to produce a truncated protein missing some of this cytoplasmic domain (64).
Dual immunolabeling showed a significant reduction in somatotropes with LEPRb to less than 1% of total pituitary cells, which was compounded by a reduction in immunopositive somatotropes. Thus, the overall losses in LEPRb protein-bearing cells are not unexpected because our counts have shown that in normal populations, somatotropes represent 41–60% of leptin target cells. Dual labeling for all other pituitary hormones (Fig. 4) showed no impact of the deletion of lepr exon 17 in somatotropes on the overall expression of their hormone or of LEPRb.
LEPR function was also abolished in somatotropes from mutant pituitaries, as tested by the detection of pSTAT3 immunocytochemically in somatotropes after 15 or 30 min stimulation with leptin. In contrast leptin stimulated 66–75% of somatotropes to express pSTAT3, and it brought out significantly more cells with GH antigens. This suggests a role for leptin in the expression of GH proteins, acting via the JAK/STAT pathway. These studies thus complemented and validated the studies of LEPRb, showing that the deletion of Lepr exon 17 removed a functional receptor in somatotropes.
The percentages of immunolabeled GH cells in heterozygote animals (with only one deleted allele of Lepr exon 17) and in homozygote animals, bearing two deleted alleles, were significantly reduced. The loss in immunopositive GH cells was correlated with a reduction in serum GH in homozygous deletion mutant males and females (Fig. 3). These animals were young adults (3–4 months of age), and many had not shown the obesity phenotype seen by weight gain, indicating that the reduction in serum GH preceded the abnormal weight increases. Interestingly, the reduction in serum GH was not associated with a reduction in serum IGF-I or changes in serum insulin, glucose, or leptin. This may explain why the deletion mutant mice gained weight and grew normally during the juvenile and peripubertal period. Hoffman et al. (78) reported that IGF-I was normal in cases of humans with adult-onset GH deficiency (AO-GHD). In addition, Mukherjee and Shalet (79) suggested that normal levels of IGF-I do not exclude a diagnosis of GH deficiency in humans because the IGF-I status is dependent on multiple factors including age and gender.
The weight gain and obese phenotype was initially discovered in the 3- to 4-month-old males because breeding groups were set up with males weighing 45–50 g. The homozygous deletion mutant males in the study reached maximal weights from 48 to 60 g by 9 months of age, and one reached a weight of 74 g. Female deletion mutants did not show the same rate of weight gain during the early adult period, although individuals did begin to gain weight as early as 3.5 months of age (Table 1).
DEXA analysis of younger (3.5 months old) deletion mutant males and females revealed that the increase in weight was due to an increase in fat mass without a significant change in lean mass (Table 1). The male deletion mutants stopped growing after 10 months, and there was no change in their body composition, whereas the control males got fatter. In contrast, the female mutants ultimately gained a lot more weight than the controls, which included increases in both lean and fat mass (Table 1). Thus, there is a sex difference, which means that it is likely that there are sex specific mechanisms regulating body composition. Ongoing studies with a complete lab animal monitoring system will determine whether hyperphagia or metabolic factors are involved. Similarly, ongoing studies will address possible skeletal differences, although the initial tests show no changes in bone (tibia) length or overall body length (data not shown).
These changes are consistent with the phenotype of AO-GHD patients, which shows an increase in body mass index and fat mass (75,80,81). GH therapy administered to AO-GHD patients produces lowered fat mass and a reduction in free leptin (75). This highlights the importance of the leptin-GH axis in the maintenance of optimal body composition.
In summary, these studies have demonstrated that deleting the JAK binding site in LEPR of somatotropes did not interfere with roles played by GH in longitudinal growth, early weight gain, or the timing of puberty. However, the percentages of immunopositive GH cells and serum GH in adult deletion mutant pituitaries are reduced. The studies of somatotrope responses to leptin mostly report that leptin stimulates GH secretion in vitro, (16,45,47,51,52,53,54,55,56,57,58,59,60,61) or restores somatotrope function in vivo (56).
In our newly developed deletion mutant animal model, the somatotropes from deletion mutant mice cannot receive leptin signals mediated by the JAK binding site in the LEPR, and there is no expression of pSTAT3. The resulting reduction in immunolabeled GH cells and GH support our hypothesis that leptin plays an important role in the direct maintenance of GH stores in this pituitary cell population. Thus, collectively the evidence suggests that leptin levels may inform somatotropes of fat stores so they can respond by secreting GH to control lipolysis and adiposity. This highlights the importance of somatotropes as metabolic sensors in the body, sensing levels of fat stores to optimize body composition.
Acknowledgments
We thank Dr. A. Parlow and the Hormone Distribution Program (Torrance, CA) for the antisera to rat pituitary hormone. We also thank Douglas Kessel, Sumiya Basunia, and Farhan Syed for excellent technical assistance.
Footnotes
This work was supported by bridging funds from the University of Arkansas for Medical Sciences Research Council and Grants R03 HD059066 and 1R01HD059056 (to G.V.C.) and core facilities funded by Grants NCRR P20 RR020146 and P30 NS047546 at the University of Arkansas for Medical Sciences and Grant P01 DK26687 (to S.C.); Carl L. Nelson Chair in Orthopaedic Creativity (to L.J.S.); Grants RYC-2007-00186 and BFU2008-01136/BFI (to R.L.); Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Merit Award R01 DK-30677; and a University of Illinois at Chicago Campus Research Board grant (to R.D.K.).
Presented in platform session at the 92nd Annual Meetings of The Endocrine Society, June 2010.
Disclosure Summary: The authors have no conflicts to disclose.
Abbreviations: AO-GHD, Adult-onset GH deficiency; DEXA, dual-energy x-ray absorptiometry; EIA, enzyme immunoassay; FVB/NJ, Friend leukemia virus/NIH Jackson; JAK, Janus kinase; LEPR, leptin receptor; p, phosphorylated; PRL, prolactin; rGHp, rat GH promoter; STAT, signal transducer and activator of transcription; wt, wild type.
First Published Online November 17, 2010
References
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Chua Jr SC, Leibel RL, Hirsch J 1991 Food deprivation and age modulate neuropeptide gene expression in the murine hypothalamus and adrenal gland. Brain Res Mol Brain Res 9:95–101 [DOI] [PubMed] [Google Scholar]
- Ebihara K, Ogawa Y, Katsuura G, Numata Y, Masuzaki H, Satoh N, Tamaki M, Yoshioka T, Hayase M, Matsuoka N, Aizawa-Abe M, Yoshimasa Y, Nakao K 1999 Involvement of agouti-related protein, an endogenous antagonist of hypothalamic melanocortin receptor, in leptin action. Diabetes 48:2028–2033 [DOI] [PubMed] [Google Scholar]
- Korner J, Savontaus E, Chua Jr SC, Leibel RL, Wardlaw SL 2001 Leptin regulation of Agrp and Npy mRNA in the rat hypothalamus. J Neuroendocrinol 13:959–966 [DOI] [PubMed] [Google Scholar]
- Mizuno T, Bergen H, Kleopoulos S, Bauman WA, Mobbs CV 1996 Effects of nutritional status and aging on leptin gene expression in mice: importance of glucose. Horm Metab Res 28:679–684 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Bergen H, Funabashi T, Kleopoulos SP, Zhong YG, Bauman WA, Mobbs CV 1996 Obese gene expression: reduction by fasting and stimulation by insulin and glucose in lean mice, and persistent elevation in acquired (diet-induced) and genetic (yellow agouti) obesity. Proc Natl Acad Sci USA 93:3434–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno TM, Makimura H, Silverstein J, Roberts JL, Lopingco T, Mobbs CV 1999 Fasting regulates hypothalamic neuropeptide Y, agouti-related peptide, and proopiomelanocortin in diabetic mice independent of changes in leptin or insulin. Endocrinology 140:4551–4557 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Mobbs CV 1999 Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140:814–817 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Blum RM, Wade GN 2000 Metabolic control of food intake and estrous cycles in Syrian hamsters. I. Plasma insulin and leptin. Am J Physiol Regul Integr Comp Physiol 278:R476–R485 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Buckley CA, Blum RM, Zhou D, Szymanski L, Day DE, Bartness TJ 2002 Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. Eur J Neurosci 16:377–379 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Goldman MD, Tang S, Bean B, Ji H, Friedman MI 1998 Leptin indirectly affects estrous cycles by increasing metabolic fuel oxidation. Horm Behav 33:217–228 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Zhou D 1999 Interactive effects of central leptin and peripheral fuel oxidation on estrous cyclicity. Am J Physiol 277:R1020–R1024 [DOI] [PubMed] [Google Scholar]
- Schneider JE, Wade GN 1989 Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science 244:1326– 1328 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Flier JS 1998 Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J Clin Invest 101:1020–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS 1996 Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252 [DOI] [PubMed] [Google Scholar]
- Casanueva FF, Dieguez C 1999 Neuroendocrine regulation and actions of leptin. Front Neuroendocrinol 20:317–363 [DOI] [PubMed] [Google Scholar]
- Chan JL, Heist K, DePaoli AM, Veldhuis JD, Mantzoros CS 2003 The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111:1409–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn PD, Cunningham MJ, Pau KY, Spies HG, Clifton DK, Steiner RA 1998 The stimulatory effect of leptin on the neuroendocrine reproductive axis of the monkey. Endocrinology 139:4652–4662 [DOI] [PubMed] [Google Scholar]
- Pralong FP, Gaillard RC 2001 Neuroendocrine effects of leptin. Pituitary 4:25–32 [DOI] [PubMed] [Google Scholar]
- Zamorano PL, Mahesh VB, De Sevilla LM, Chorich LP, Bhat GK, Brann DW 1997 Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology 65:223–228 [DOI] [PubMed] [Google Scholar]
- Lloyd RV, Jin L, Tsumanuma I, Vidal S, Kovacs K, Horvath E, Scheithauer BW, Couce ME, Burguera B 2001 Leptin and leptin receptor in anterior pituitary function. Pituitary 4:33–47 [DOI] [PubMed] [Google Scholar]
- Urbanski HF 2001 Leptin and puberty. Trends Endocrinol Metab 12:428–429 [DOI] [PubMed] [Google Scholar]
- Mann DR, Plant TM 2002 Leptin and pubertal development. Semin Reprod Med 20:93–102 [DOI] [PubMed] [Google Scholar]
- Yura S, Ogawa Y, Sagawa N, Masuzaki H, Itoh H, Ebihara K, Aizawa-Abe M, Fujii S, Nakao K 2000 Accelerated puberty and late-onset hypothalamic hypogonadism in female transgenic skinny mice overexpressing leptin. J Clin Invest 105:749–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S 1999 Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341:879–884 [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O'Rahilly S 1997 Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature 387:903–908 [DOI] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD 1998 A leptin missense mutation associated with hypogonadism and morbid obesity. Nat Genet 18:213–215 [DOI] [PubMed] [Google Scholar]
- Clément K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, Gourmelen M, Dina C, Chambaz J, Lacorte JM, Basdevant A, Bougnères P, Lebouc Y, Froguel P, Guy-Grand B 1998 A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature 392:398–401 [DOI] [PubMed] [Google Scholar]
- Childs GV, Iruthayanathan M, Akhter N, Johnson BW 2006 Estrogen mediated cross talk between the ovary and pituitary somatotrope. Pre-ovulatory support for reproductive activity. Mol Cell Endocrinol 247:60–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs GV, Iruthayanathan M, Akhter N, Unabia G, Whitehead-Johnson B 2005 Bipotential effects of estrogen on growth hormone synthesis and storage in vitro. Endocrinology 146:1780–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iruthayanathan M, Zhou YH, Childs GV 2005 Dehydroepiandrosterone restoration of growth hormone gene expression in aging female rats, in vivo and in vitro: evidence for actions via estrogen receptors. Endocrinology 146:5176–5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frühbeck G 2006 Intracellular signalling pathways activated by leptin. Biochem J 393:7–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghilardi N, Ziegler S, Wiestner A, Stoffel R, Heim MH, Skoda RC 1996 Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA 93:6231–6235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton NM, Emilsson V, Liu YL, Cawthorne MA 1998 Leptin action in intestinal cells. J Biol Chem 273:26194–26201 [DOI] [PubMed] [Google Scholar]
- Vaisse C, Halaas JL, Horvath CM, Darnell Jr JE, Stoffel M, Friedman JM 1996 Leptin activation of Stat3 in the hypothalamus of wild-type and ob/ob mice but not db/db mice. Nat Genet 14:95–97 [DOI] [PubMed] [Google Scholar]
- Schulz LC, Widmaier EP 2006 Leptin receptors. In: Castracane VD, Henson MC, eds. Leptin. Chap 2. New York: Springer Science; 11–33 [Google Scholar]
- Iqbal J, Pompolo S, Considine RV, Clarke IJ 2000 Localization of leptin receptor-like immunoreactivity in the corticotropes, somatotropes, and gonadotropes in the ovine anterior pituitary. Endocrinology 141:1515–1520 [DOI] [PubMed] [Google Scholar]
- Lin J, Barb CR, Matteri RL, Kraeling RR, Chen X, Meinersmann RJ, Rampacek GB 2000 Long form leptin receptor mRNA expression in the brain, pituitary, and other tissues in the pig. Domest Anim Endocrinol 19:53–61 [DOI] [PubMed] [Google Scholar]
- Jin L, Burguera BG, Couce ME, Scheithauer BW, Lamsan J, Eberhardt NL, Kulig E, Lloyd RV 1999 Leptin and leptin receptor expression in normal and neoplastic human pituitary: evidence of a regulatory role for leptin on pituitary cell proliferation. J Clin Endocrinol Metab 84:2903–2911 [DOI] [PubMed] [Google Scholar]
- Jin L, Zhang S, Burguera BG, Couce ME, Osamura RY, Kulig E, Lloyd RV 2000 Leptin and leptin receptor expression in rat and mouse pituitary cells. Endocrinology 141:333–339 [DOI] [PubMed] [Google Scholar]
- Sone M, Nagata H, Takekoshi S, Osamura RY 2001 Expression and localization of leptin receptor in the normal rat pituitary gland. Cell Tissue Res 305:351–356 [DOI] [PubMed] [Google Scholar]
- Sone M, Osamura RY 2001 Leptin and the pituitary. Pituitary 4:15–23 [DOI] [PubMed] [Google Scholar]
- Cai A, Hyde JF 1999 The human growth hormone-releasing hormone transgenic mouse as a model of modest obesity: differential changes in leptin receptor (OBR) gene expression in the anterior pituitary and hypothalamus after fasting and OBR localization in somatotrophs. Endocrinology 140:3609–3614 [DOI] [PubMed] [Google Scholar]
- Cai A, Hyde JF 1998 Upregulation of leptin receptor gene expression in the anterior pituitary of human growth hormone-releasing hormone transgenic mice. Endocrinology 139:420–423 [DOI] [PubMed] [Google Scholar]
- Shimon I, Yan X, Magoffin DA, Friedman TC, Melmed S 1998 Intact leptin receptor is selectively expressed in human fetal pituitary and pituitary adenomas and signals human fetal pituitary growth hormone secretion. J Clin Endocrinol Metab 83:4059–4064 [DOI] [PubMed] [Google Scholar]
- Asada N, Takahashi Y, Honjo M 2000 Effects of 22K or 20K human growth hormone on lipolysis, leptin production in adipocytes in the presence and absence of human growth hormone binding protein. Horm Res 54:203–207 [DOI] [PubMed] [Google Scholar]
- Isozaki O, Tsushima T, Miyakawa M, Demura H, Seki H 1999 Interaction between leptin and growth hormone (GH)/IGF-I axis. Endocr J 46(Suppl):S17–S24 [DOI] [PubMed] [Google Scholar]
- Veldhuis JD, Roemmich JN, Richmond EJ, Rogol AD, Lovejoy JC, Sheffield-Moore M, Mauras N, Bowers CY 2005 Endocrine control of body composition in infancy, childhood, and puberty. Endocr Rev 26:114–146 [DOI] [PubMed] [Google Scholar]
- Casanueva F, Dieguez C 1998 Interactions between body composition, leptin and growth hormone status. In: Shalet SM ed. Growth hormone in adults. London: Bailliere Tindall; 297–314 [DOI] [PubMed] [Google Scholar]
- Marzullo P, Buckway C, Pratt KL, Colao A, Guevara-Aguirre J, Rosenfeld RG 2002 Leptin concentrations in GH deficiency: the effect of GH insensitivity. J Clin Endocrinol Metab 87:540–545 [DOI] [PubMed] [Google Scholar]
- Nagatani S, Zeng Y, Keisler DH, Foster DL, Jaffe CA 2000 Leptin regulates pulsatile luteinizing hormone and growth hormone secretion in the sheep. Endocrinology 141:3965–3975 [DOI] [PubMed] [Google Scholar]
- Pombo M, Pombo CM, Astorga R, Cordido F, Popovic V, Garcia-Mayor RV, Dieguez C, Casanueva FF 1999 Regulation of growth hormone secretion by signals produced by the adipose tissue. J Endocrinol Invest 22:22–26 [PubMed] [Google Scholar]
- Chen C, Roh SG, Nie GY, Loneragan K, Xu RW, Ruan M, Clarke LJ, Goding JW, Gertler A 2001 The in vitro effect of leptin on growth hormone secretion from primary cultured ovine somatotrophs. Endocrine 14:73–78 [DOI] [PubMed] [Google Scholar]
- Roh SG, Nie GY, Loneragan K, Gertler A, Chen C 2001 Direct modification of somatotrope function by long-term leptin treatment of primary cultured ovine pituitary cells. Endocrinology 142:5167–5171 [DOI] [PubMed] [Google Scholar]
- Saleri R, Giustina A, Tamanini C, Valle D, Burattin A, Wehrenberg WB, Baratta M 2004 Leptin stimulates growth hormone secretion via a direct pituitary effect combined with a decreased somatostatin tone in a median eminence-pituitary perifusion study. Neuroendocrinology 79:221–228 [DOI] [PubMed] [Google Scholar]
- Luque RM, Huang ZH, Shah B, Mazzone T, Kineman RD 2007 Effects of leptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am J Physiol Endocrinol Metab 292:E891–E899 [DOI] [PubMed] [Google Scholar]
- Baratta M, Saleri R, Mainardi GL, Valle D, Giustina A, Tamanini C 2002 Leptin regulates GH gene expression and secretion and nitric oxide production in pig pituitary cells. Endocrinology 143:551–557 [DOI] [PubMed] [Google Scholar]
- Giusti M, Bocca L, Florio T, Corsaro A, Spaziante R, Schettini G, Minuto F 2002 In vitro effect of human recombinant leptin and expression of leptin receptors on growth hormone-secreting human pituitary adenomas. Clin Endocrinol (Oxf) 57:449–455 [DOI] [PubMed] [Google Scholar]
- Mizuno I, Okimura Y, Takahashi Y, Kaji H, Abe H, Chihara K 1999 Leptin stimulates basal and GHRH-induced GH release from cultured rat anterior pituitary cells in vitro. Kobe J Med Sci 45:221–227 [PubMed] [Google Scholar]
- Saleri R, Grasselli F, Tamanini C 2005 Effects of different culture conditions and leptin on GH mRNA expression and GH secretion by pig pituitary cells. Horm Metab Res 37:214–219 [DOI] [PubMed] [Google Scholar]
- Zieba DA, Amstalden M, Morton S, Gallino JL, Edwards JF, Harms PG, Williams GL 2003 Effects of leptin on basal and GHRH-stimulated GH secretion from the bovine adenohypophysis are dependent upon nutritional status. J Endocrinol 178:83–89 [DOI] [PubMed] [Google Scholar]
- Crane C, Akhter N, Johnson BW, Iruthayanathan M, Syed F, Kudo A, Zhou YH, Childs GV 2007 Fasting and glucose effects on pituitary leptin expression: is leptin a local signal for nutrient status? J Histochem Cytochem 55:1059–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer RR, Mathews LS, Palmiter RD, Brinster RL 1988 Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Dev 2:453–461 [DOI] [PubMed] [Google Scholar]
- Chua Jr SC, Koutras IK, Han L, Liu SM, Kay J, Young SJ, Chung WK, Leibel RL 1997 Fine structure of the murine leptin receptor gene: splice site suppression is required to form two alternatively spliced transcripts. Genomics 45:264–270 [DOI] [PubMed] [Google Scholar]
- Luque RM, Amargo G, Ishii S, Lobe C, Franks R, Kiyokawa H, Kineman RD 2007 Reporter expression, induced by a growth hormone promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology 148:1946–1953 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua Jr SC, Elmquist JK, Lowell BB 2004 Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991 [DOI] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Liu H, Dragatsis I, Dietrich P, Ludwig T, Boozer CN, Chua Jr SC 2005 Neuronal deletion of Lepr elicits diabesity in mice without affecting cold tolerance or fertility. Am J Physiol Endocrinol Metab 289:E403–E411 [DOI] [PubMed] [Google Scholar]
- McMinn JE, Liu SM, Dragatsis I, Dietrich P, Ludwig T, Eiden S, Chua Jr SC 2004 An allelic series for the leptin receptor gene generated by CRE and FLP recombinase. Mamm Genome 15:677–685 [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Miller BT 1994 Cytochemical detection of gonadotropin-releasing hormone-binding sites on rat pituitary cells with luteinizing hormone, follicle-stimulating hormone, and growth hormone antigens during diestrous up-regulation. Endocrinology 134:1943–1951 [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Miller BT, Collins TJ 1999 Differential expression of gonadotropin and prolactin antigens by GHRH target cells from male and female rats. J Endocrinol 162:177–187 [DOI] [PubMed] [Google Scholar]
- Childs GV, Unabia G, Rougeau D 1994 Cells that express luteinizing hormone (LH) and follicle-stimulating hormone (FSH) β-subunit messenger ribonucleic acids during the estrous cycle: the major contributors contain LHβ, FSHβ, and/or growth hormone. Endocrinology 134:990–997 [DOI] [PubMed] [Google Scholar]
- Akhter N, Johnson BW, Crane C, Iruthayanathan M, Zhou YH, Kudo A, Childs GV 2007 Anterior pituitary leptin expression changes in different reproductive states: stimulation, in vitro, by gonadotropin releasing hormone (GnRH). J Histochem Cytochem 55:151–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDuffie IA, Akhter N, Childs GV 2004 Regulation of leptin mRNA and protein expression in pituitary somatotropes. J Histochem Cytochem 52:263–273 [DOI] [PubMed] [Google Scholar]
- Yu WH, Kimura M, Walczewska A, Karanth S, McCann SM 1997 Role of leptin in hypothalamic-pituitary function. Proc Natl Acad Sci USA 94:1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randeva HS, Murray RD, Lewandowski KC, O'Callaghan CJ, Horn R, O'Hare P, Brabant G, Hillhouse EW, Shalet SM 2002 Differential effects of GH replacement on the components of the leptin system in GH-deficient individuals. J Clin Endocrinol Metab 87:798–804 [DOI] [PubMed] [Google Scholar]
- Popovic V, Damjanovic S, Dieguez C, Casanueva FF 2001 Leptin and the pituitary. Pituitary 4:7–14 [DOI] [PubMed] [Google Scholar]
- Vuagnat BA, Pierroz DD, Lalaoui M, Englaro P, Pralong FP, Blum WF, Aubert ML 1998 Evidence for a leptin-neuropeptide Y axis for the regulation of growth hormone secretion in the rat. Neuroendocrinology 67:291–300 [DOI] [PubMed] [Google Scholar]
- Hoffman DM, O'Sullivan AJ, Baxter RC, Ho KK 1994 Diagnosis of growth-hormone deficiency in adults. Lancet 343:1064–1068 [DOI] [PubMed] [Google Scholar]
- Mukherjee A, Shalet SM 2009 The value of IGF1 estimation in adults with GH deficiency. Eur J Endocrinol 161(Suppl 1):S33–S39 [DOI] [PubMed] [Google Scholar]
- Abs R, Mattsson AF, Bengtsson BA, Feldt-Rasmussen U, Góth MI, Koltowska-Häggström M, Monson JP, Verhelst J, Wilton P 2005 Isolated growth hormone (GH) deficiency in adult patients: baseline clinical characteristics and responses to GH replacement in comparison with hypopituitary patients. A sub-analysis of the KIMS database. Growth Horm IGF Res 15:349–359 [DOI] [PubMed] [Google Scholar]
- Doga M, Bonadonna S, Gola M, Mazziotti G, Giustina A 2006 Growth hormone deficiency in the adult. Pituitary 9:305–311 [DOI] [PubMed] [Google Scholar]