Abstract
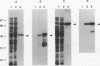
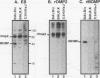
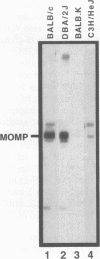
Chlamydia trachomatis is an important human pathogen. Research to develop a Chlamydia vaccine has focused on the major outer membrane protein (MOMP). Determinants of this protein elicit serovar-specific neutralizing antibodies which are thought to play a critical role in protective immunity. MOMP-specific antibody responses are highly variable in the polymorphic population. Genetic factors which might influence the MOMP-specific immune response are consequently of particular interest. The C. psittaci strain guinea pig inclusion conjunctivitis (GPIC) is a natural pathogen of the guinea pig that causes both ocular and genital tract infections that closely resemble those caused by C. trachomatis in humans. As such, it provides an excellent model for disease. In this report, we explore the influence of major histocompatibility complex-linked genes on the MOMP-specific antibody response in mice immunized with either whole GPIC elementary bodies or recombinant GPIC MOMP. Our results indicate that the MOMP-specific antibody response is major histocompatibility complex linked such that mice of the H-2d haplotype are high responders while mice of the H-2k haplotype are low responders. We demonstrate that MOMP-specific B cells are present in H-2k strains which are, however, deficient in MOMP-specific helper T cells. Although immunization of low-MOMP-responder strains with whole chlamydial elementary bodies induces high levels of immunoglobulin G antibody specific for Omp2, the cysteine-rich outer membrane protein, MOMP-specific B cells are unable to receive help from Omp2-specific T cells. The failure of intermolecular help from Omp2-specific T cells and related observations raise important issues regarding the processing and presentation of chlamydial antigens and the design of optimal subunit vaccines.
Full text
PDF



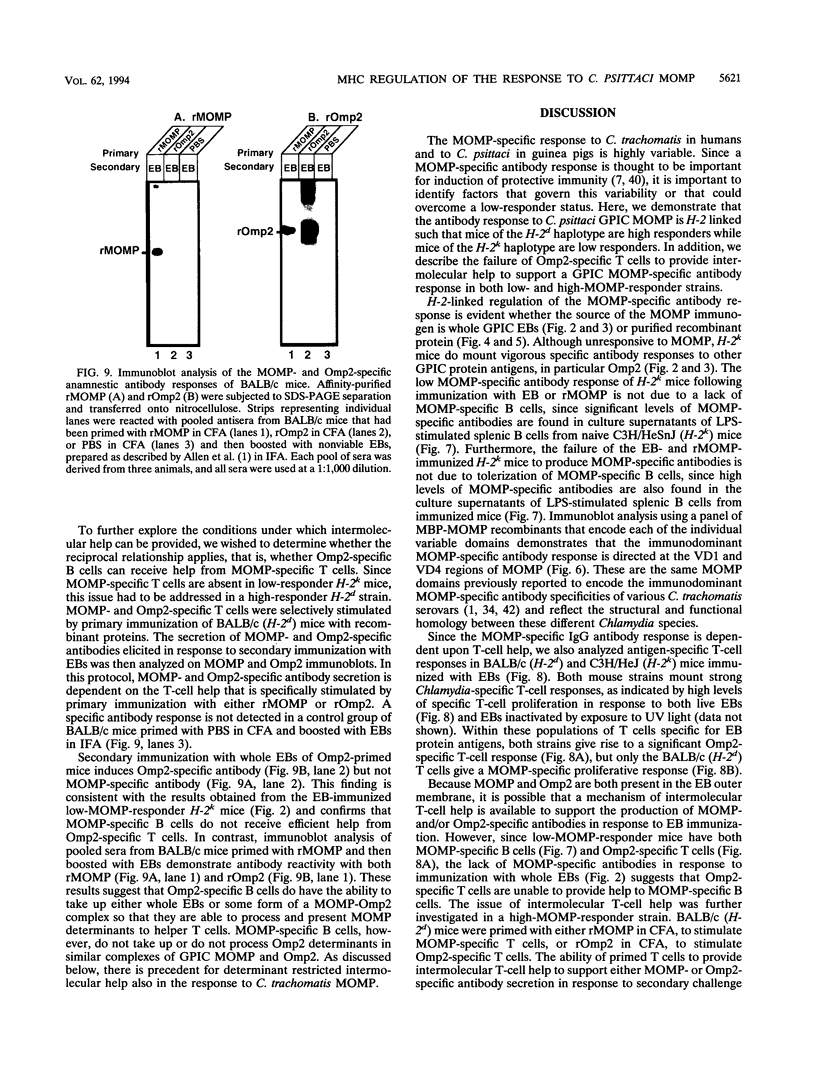


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. E., Locksley R. M., Stephens R. S. A single peptide from the major outer membrane protein of Chlamydia trachomatis elicits T cell help for the production of antibodies to protective determinants. J Immunol. 1991 Jul 15;147(2):674–679. [PubMed] [Google Scholar]
- Allen J. E., Stephens R. S. An intermolecular mechanism of T cell help for the production of antibodies to the bacterial pathogen, Chlamydia trachomatis. Eur J Immunol. 1993 May;23(5):1169–1172. doi: 10.1002/eji.1830230529. [DOI] [PubMed] [Google Scholar]
- Amann E., Brosius J. "ATG vectors' for regulated high-level expression of cloned genes in Escherichia coli. Gene. 1985;40(2-3):183–190. doi: 10.1016/0378-1119(85)90041-1. [DOI] [PubMed] [Google Scholar]
- Baehr W., Zhang Y. X., Joseph T., Su H., Nano F. E., Everett K. D., Caldwell H. D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Newhall W. J., 5th, Jones R. B. Differences in outer membrane proteins of the lymphogranuloma venereum and trachoma biovars of Chlamydia trachomatis. Infect Immun. 1985 Nov;50(2):488–494. doi: 10.1128/iai.50.2.488-494.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batteiger B. E., Rank R. G., Bavoil P. M., Soderberg L. S. Partial protection against genital reinfection by immunization of guinea-pigs with isolated outer-membrane proteins of the chlamydial agent of guinea-pig inclusion conjunctivitis. J Gen Microbiol. 1993 Dec;139(12):2965–2972. doi: 10.1099/00221287-139-12-2965. [DOI] [PubMed] [Google Scholar]
- Bavoil P., Stephens R. S., Falkow S. A soluble 60 kiloDalton antigen of Chlamydia spp. is a homologue of Escherichia coli GroEL. Mol Microbiol. 1990 Mar;4(3):461–469. doi: 10.1111/j.1365-2958.1990.tb00612.x. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Caldwell H. D., Judd R. C. Structural analysis of chlamydial major outer membrane proteins. Infect Immun. 1982 Dec;38(3):960–968. doi: 10.1128/iai.38.3.960-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascher C., Roll D., Bavoil P. M. Expression and translocation of the chlamydial major outer membrane protein in Escherichia coli. Microb Pathog. 1993 Dec;15(6):455–467. doi: 10.1006/mpat.1993.1094. [DOI] [PubMed] [Google Scholar]
- Davidson H. W., Watts C. Epitope-directed processing of specific antigen by B lymphocytes. J Cell Biol. 1989 Jul;109(1):85–92. doi: 10.1083/jcb.109.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duplay P., Bedouelle H., Fowler A., Zabin I., Saurin W., Hofnung M. Sequences of the malE gene and of its product, the maltose-binding protein of Escherichia coli K12. J Biol Chem. 1984 Aug 25;259(16):10606–10613. [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Kellermann O. K., Ferenci T. Maltose-binding protein from Escherichia coli. Methods Enzymol. 1982;90(Pt E):459–463. doi: 10.1016/s0076-6879(82)90171-9. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- MURRAY E. S. GUINEA PIG INCLUSION CONJUNCTIVITIS VIRUS. I. ISOLATION AND IDENTIFICATION AS A MEMBER OF THE PSITTACOSIS-LYMPHOGRANULOMA-TRACHOMA GROUP. J Infect Dis. 1964 Feb;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- Manca F., Kunkl A., Fenoglio D., Fowler A., Sercarz E., Celada F. Constraints in T-B cooperation related to epitope topology on E. coli beta-galactosidase. I. The fine specificity of T cells dictates the fine specificity of antibodies directed to conformation-dependent determinants. Eur J Immunol. 1985 Apr;15(4):345–350. doi: 10.1002/eji.1830150408. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison R. P., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. Ocular hypersensitivity elicited by a genus-specific 57-kD protein. J Exp Med. 1989 Mar 1;169(3):663–675. doi: 10.1084/jem.169.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newhall W. J., Batteiger B., Jones R. B. Analysis of the human serological response to proteins of Chlamydia trachomatis. Infect Immun. 1982 Dec;38(3):1181–1189. doi: 10.1128/iai.38.3.1181-1189.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki S., Berzofsky J. A. Antibody conjugates mimic specific B cell presentation of antigen: relationship between T and B cell specificity. J Immunol. 1987 Jun 15;138(12):4133–4142. [PubMed] [Google Scholar]
- Stephens R. S., Sanchez-Pescador R., Wagar E. A., Inouye C., Urdea M. S. Diversity of Chlamydia trachomatis major outer membrane protein genes. J Bacteriol. 1987 Sep;169(9):3879–3885. doi: 10.1128/jb.169.9.3879-3885.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Caldwell H. D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992 Jan 1;175(1):227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Morrison R. P., Watkins N. G., Caldwell H. D. Identification and characterization of T helper cell epitopes of the major outer membrane protein of Chlamydia trachomatis. J Exp Med. 1990 Jul 1;172(1):203–212. doi: 10.1084/jem.172.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuffrey M., Alexander F., Woods C., Taylor-Robinson D. Genetic susceptibility to chlamydial salpingitis and subsequent infertility in mice. J Reprod Fertil. 1992 May;95(1):31–38. doi: 10.1530/jrf.0.0950031. [DOI] [PubMed] [Google Scholar]
- Washington A. E., Johnson R. E., Sanders L. L., Jr Chlamydia trachomatis infections in the United States. What are they costing us? JAMA. 1987 Apr 17;257(15):2070–2072. [PubMed] [Google Scholar]
- Yuan Y., Zhang Y. X., Watkins N. G., Caldwell H. D. Nucleotide and deduced amino acid sequences for the four variable domains of the major outer membrane proteins of the 15 Chlamydia trachomatis serovars. Infect Immun. 1989 Apr;57(4):1040–1049. doi: 10.1128/iai.57.4.1040-1049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Morrison S. G., Caldwell H. D., Baehr W. Cloning and sequence analysis of the major outer membrane protein genes of two Chlamydia psittaci strains. Infect Immun. 1989 May;57(5):1621–1625. doi: 10.1128/iai.57.5.1621-1625.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. X., Stewart S., Joseph T., Taylor H. R., Caldwell H. D. Protective monoclonal antibodies recognize epitopes located on the major outer membrane protein of Chlamydia trachomatis. J Immunol. 1987 Jan 15;138(2):575–581. [PubMed] [Google Scholar]
- Zhong G. M., Reid R. E., Brunham R. C. Mapping antigenic sites on the major outer membrane protein of Chlamydia trachomatis with synthetic peptides. Infect Immun. 1990 May;58(5):1450–1455. doi: 10.1128/iai.58.5.1450-1455.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong G., Brunham R. C. Antibody responses to the chlamydial heat shock proteins hsp60 and hsp70 are H-2 linked. Infect Immun. 1992 Aug;60(8):3143–3149. doi: 10.1128/iai.60.8.3143-3149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]