Abstract
The development of selective inhibitors for discrete anti-apoptotic BCL-2 family proteins implicated in pathologic cell survival remains a formidable but pressing challenge. Precisely tailored compounds would serve as molecular probes and targeted therapies to study and treat human diseases driven by specific anti-apoptotic blockades. In particular, MCL-1 has emerged as a major resistance factor in human cancer. By screening a library of Stabilized Alpha-Helix of BCL-2 domains (SAHBs), we determined that the MCL-1 BH3 helix is itself a potent and exclusive MCL-1 inhibitor. X-ray crystallography and mutagenesis studies defined key binding and specificity determinants, including the capacity to harness the hydrocarbon staple to optimize affinity while preserving selectivity. MCL-1 SAHB directly targets MCL-1, neutralizes its inhibitory interaction with pro-apoptotic BAK, and sensitizes cancer cells to caspase-dependent apoptosis. By leveraging nature’s solution to ligand selectivity, we generated an MCL-1-specific agent that defines the structural and functional features of targeted MCL-1 inhibition.
Beginning with the discovery of BCL-2 at the t14;18 chromosomal breakpoint of follicular lymphoma1, the anti-apoptotic members of the BCL-2 family have emerged as key pathogenic proteins in human diseases characterized by unchecked cellular survival, such as cancer and autoimmunity2. A series of anti-apoptotic proteins including BCL-2, BCL-XL, BCL-w, MCL-1, and BFL1/A1 promote cellular survival by trapping the critical apoptosis-inducing BCL-2 homology domain 3 (BH3) α-helix of pro-apoptotic BCL-2 family members3. Cancer cells exploit this physiologic survival mechanism through anti-apoptotic protein overexpression, establishing an apoptotic blockade that secures their immortality. To overcome this potentially fatal resistance mechanism, a pharmacologic quest is underway to develop targeted therapies that bind and block BCL-2 family survival proteins.
Anti-apoptotic proteins contain a hydrophobic binding pocket on their surface that engages BH3 α-helices3,4. Because nature’s solution to anti-apoptotic targeting involves selective interactions between BH3 death domains and anti-apoptotic pockets5,6, molecular mimicry of the BH3 α-helix has formed the basis for developing small molecule inhibitors of anti-apoptotic proteins7–9. Promising compounds undergoing clinical evaluation, such as ABT-26310, obatoclax8, and AT-10111, each target three or more anti-apoptotic proteins. The development of precise inhibitors that target individual anti-apoptotic proteins remains a significant challenge due to the subtle differences among BH3-binding pockets. Reminiscent of the long-term goals in kinase therapeutics, anti-apoptotic inhibitors with tailored specificity would provide finely-tuned therapies to treat distinct diseases while potentially avoiding unwanted side-effects. In addition, such compounds would serve as invaluable research tools to dissect the differential biological functions of anti-apoptotic proteins.
The specificity of anti-apoptotic proteins for BH3 domains is conferred by the topography of the canonical binding groove and the distinctive amino acid composition of the interacting BH3 helix. Whereas some BH3 domains, such as that of pro-apoptotic BIM, can tightly engage the BH3-binding groove of all anti-apoptotic proteins, others are more selective such as the BAD BH3 that binds BCL-2, BCL-XL, and BCL-w and the NOXA BH3 that targets MCL-1 and BFL-1/A15. The differential binding capacity of BH3 domains and their mimetics is clinically relevant, as exemplified by the close relationship between inhibitor binding spectrum and biological activity. For example, ABT-737, the prototype small molecule BH3 mimetic modeled after the BH3 domain of BAD, was designed to specifically target BCL-2 and BCL-XL, and induces apoptosis in select cancers that are driven by these proteins9. However, ABT-737 fails to show efficacy against cancer cells that overexpress MCL-1, as this anti-apoptotic lies outside the molecule’s range of binding activity12,13. In an effort to overcome the challenge of designing precision small molecules to selectively target interaction surfaces that are comparatively large and more complex, we investigated whether nature’s BH3 domains could provide a pharmacologic solution to anti-apoptotic specificity.
We chose MCL-1 as the template for this study because of its emerging role as a critical survival factor in a broad range of human cancers14. MCL-1 overexpression has been linked to the pathogenesis of a variety of refractory cancers, including multiple myeloma15, acute myeloid leukemia12, melanoma16, and poor prognosis breast cancer17. MCL-1 exerts its pro-survival activity at the mitochondrial outermembrane where it neutralizes pro-apoptotic proteins such as NOXA, PUMA, BIM, and BAK. The critical role of MCL-1 in apoptotic resistance has been highlighted by the sensitizing effects of small interfering RNAs that downregulate MCL-1 protein levels18–20. Given the clear therapeutic rationale for targeting MCL-1, we sought to develop a selective MCL-1 inhibitor to elucidate the binding and specificity determinants, and interrogate its functional capacity to sensitize cancer cell apoptosis.
RESULTS
The MCL-1 BH3 helix is a selective inhibitor of MCL-1
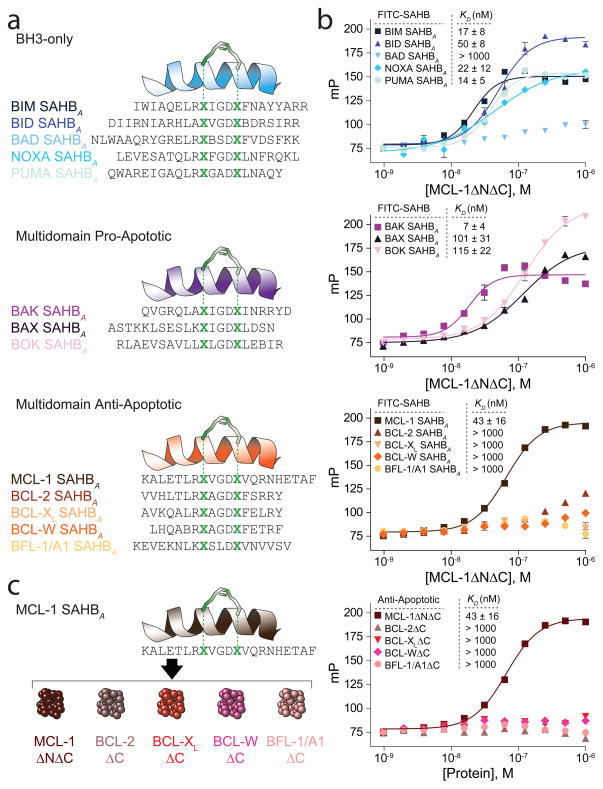
We previously applied hydrocarbon stapling to transform unfolded BID, BAD, and BIM BH3 peptides into protease-resistant and cell-permeable α-helices that engage and modulate their intracellular targets for therapeutic benefit in preclinical models21,22 and for mechanistic analyses23,24. Here, we generated a library of Stabilized Alpha-Helix of BCL-2 domains (SAHBs) modeled after the BH3 domains of human BCL-2 family proteins in order to identify potent and selective inhibitors of MCL-1. We incorporated a pair of non-natural amino acids containing olefin tethers25 at the indicated (i, i+4) positions of the non-interacting face of the BH3 helices (staple position “A”), followed by ruthenium-catalyzed olefin metathesis26, to yield a panel of hydrocarbon-stapled BH3 peptides (Fig. 1a, Supplementary Table 1). Fluorescence polarization assays (FPA) were performed to measure the binding affinity of fluorescently labeled SAHBs for recombinant human MCL-1ΔNΔC (amino acids 172–320), a deletion construct that contains the BH3-binding pocket and affords enhanced expression, purity, and stability. SAHBs corresponding to the BH3 domains of (1) BH3-only proteins NOXA, PUMA, BID, and BIM, (2) multi-domain pro-apoptotic BAK, and (3) anti-apoptotic MCL-1 exhibited high affinity binding for MCL-1 (KD ≤50 nM) (Fig. 1b). To identify MCL-1-selective SAHBs, we first screened for recombinant BCL-XLΔC binding, which eliminated PUMA, BID, BIM, and BAK SAHBAs (Supplementary Table 2a), and then for recombinant BFL1/A1ΔC binding, which eliminated NOXA SAHBA (Supplementary Table 2b). Indeed, binding analysis of MCL-1 SAHBA using an expanded panel of anti-apoptotic proteins, including MCL-1ΔNΔC, BCL-2ΔC, BCL-XLΔC, BCL-wΔC and BFL-1/A1ΔC, confirmed that MCL-1 SAHBA displayed potent and selective binding affinity for MCL-1 alone (KD, 43 nM) (Fig. 1c).
Figure 1.
Identification of an MCL-1-selective BH3 domain. (a) A panel of Stabilized Alpha-Helix of BCL-2 domains (SAHBs) was designed based on the BH3 domains of pro- and anti-apoptotic BCL-2 family members. A pair of crosslinking non-natural amino acids (X) were substituted at the indicated i, i+4 position of the non-interacting helical surface and “stapled” by ruthenium-catalyzed olefin metathesis. To optimize the activity of the Grubbs’ ruthenium catalyst, sulfur-containing methionines were replaced with norleucines, which are designated by the letter B. (b) Dissociation constants for the binding of fluorescently labeled SAHBs to MCL-1ΔNΔC were determined by fluorescence polarization assay (FPA) and nonlinear regression analysis. (c) Among the SAHBs that bound MCL-1ΔNΔC with high affinity, only MCL-1 SAHBA displayed a potent and exclusive interaction with MCL-1ΔNΔC, as evidenced by FPA performed with FITC-MCL-1 SAHBA against a broad panel of anti-apoptotic targets. Data are mean and s.d. for experiments performed in at least triplicate.
Binding and specificity determinants of the MCL-1 BH3 domain
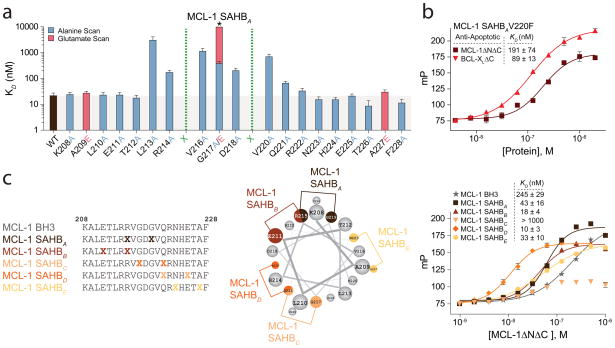
To define the binding and specificity determinants for the interaction between the MCL-1 BH3 helix and MCL-1ΔNΔC, we performed alanine scanning, site-directed mutagenesis, and staple scanning. Amino acid residues within MCL-1 SAHBA were sequentially replaced with alanine and the corresponding fluorescently labeled SAHBs were tested for MCL-1ΔNΔC binding by FPA. The alanine scan was supplemented with glutamate mutagenesis of alanine and glycine residues. Whereas mutagenesis of N- and C- terminal residues had little to no impact on MCL-1ΔNΔC binding affinity, alanine mutagenesis of L213, R214, V216, G217, D218 and V220 decreased the binding affinity of MCL-1 SAHBA for MCL-1ΔNΔC by 10- to 100-fold, revealing the key MCL-1 BH3 residues for MCL-1ΔNΔC engagement (Fig. 2a). Comparative analysis of BH3 domain sequences indicated that the combination of core hydrophobic residues L213, V216, and V220 is unique to MCL-1 BH3 (Fig. 1a) and alanine mutagenesis of any one of these hydrophobic residues is especially detrimental to MCL-1ΔNΔC binding. Interestingly, BAD BH3, which exhibits a restricted binding profile to BCL-2, BCL-XL, and BCL-w, and BIM BH3, which broadly engages anti-apoptotic proteins, possess a phenylalanine at the position corresponding to V220 in MCL-1 BH3 (Supplementary Fig. 1). Scanning mutagenesis of the BIM BH3 sequence previously documented that replacement of this phenylalanine with alanine, glutamate, or lysine abrogated BCL-XL binding but had minimal impact on MCL-1 binding27,28. We find that a single V220F point mutation in MCL-1 SAHBA abolished selectivity for MCL-1ΔNΔC, conferring binding activity to both MCL-1ΔNΔC (KD, 191 nM) and BCL-XLΔC (KD, 89 nM) (Fig. 2b). Whereas certain binding determinants such as the conserved amino acids L213, R214, G217, and D218 are shared among many BH3 domains, other discrete residues in the appropriate context, such as V220 in MCL-1 BH3, can dictate selectivity.
Figure 2.
Binding and specificity determinants of the MCL-1 BH3 helix. (a) A panel of sequential alanine mutants (alanine scan) of FITC-MCL-1 SAHBA was generated for FPA binding analysis, revealing key residues within the core BH3 sequence required for high affinity MCL-1ΔNΔC binding. Glutamate mutagenesis was also performed to evaluate the contribution of native alanine and glycine residues to MCL-1ΔNΔC binding. *, KD >10 μM. (b) A single point mutation of V220F eliminated the MCL-1 specificity of MCL-1 SAHBA, conferring binding affinity to both MCL-1ΔNΔC and BCL-XLΔC, as demonstrated by FPA. (c) Sampling a variety of staple positions along the α-helical surface revealed disruption of MCL-1ΔNΔC binding only by the G217,Q221 staple (MCL-1 SAHBC), which is located at the hydrophobic binding interface. MCL-1 SAHBD exhibited the strongest binding activity (KD, 10 nM), with 4-fold improvement over the parental MCL-1 SAHBA. Data are mean and s.d. for experiments performed in at least triplicate.
We next performed a “staple scan” that effectively replaced pairs of amino acid residues within the BH3 sequence with crosslinked norleucine-like side chains to (1) address which surface along the MCL-1 BH3 helix is essential to MCL-1ΔNΔC engagement and (2) sample alternate staple positions to identify constructs with optimal α-helicity and binding activity for biological studies. In agreement with the alanine scan, mutagenesis of residues E211, R215, G219, Q221, N223, E225, and A227, and insertion of staples at i, i+4 pairings of these sites, did not disrupt the MCL-1ΔNΔC interaction (Fig. 2c). However, placement of the crosslink at positions G217 to Q221 abrogated binding activity, consistent with disruption of the critical hydrophobic interface between MCL-1 SAHBC and MCL-1ΔNΔC by the hydrocarbon staple. Among the MCL-1 SAHBs generated, MCL-1 SAHBD exhibited high α-helical content (~90%) and the strongest binding activity (KD, 10 nM), achieving 4-fold enhancement in MCL-1ΔNΔC affinity compared to the parental MCL-1 SAHBA while retaining MCL-1ΔNΔC selectivity (Fig. 2c, Supplementary Fig. 2, 3).
Structural analysis of the MCL-1 SAHBD/MCL-1 interaction
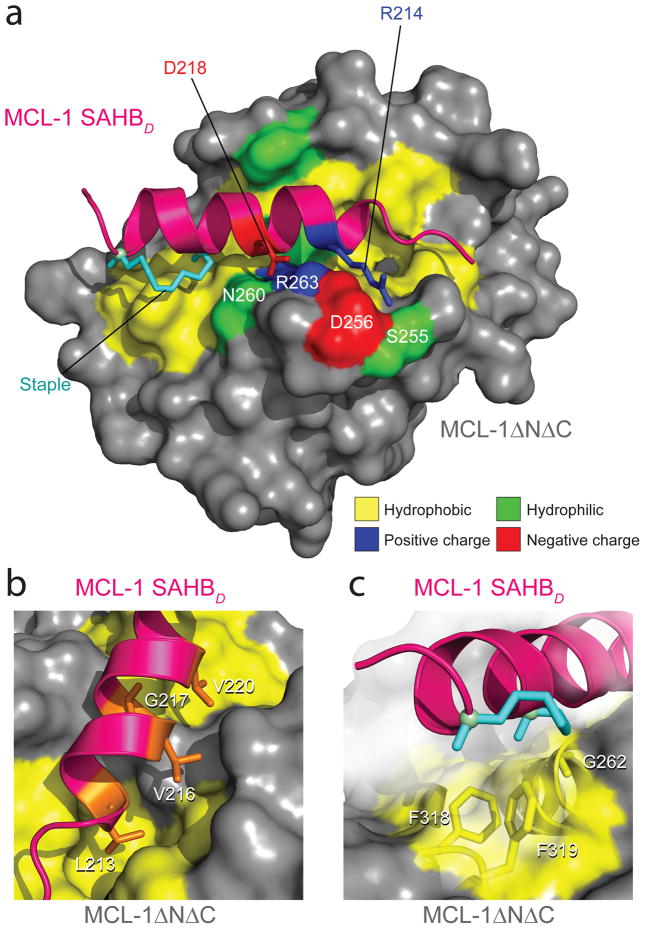
To structurally define the interactions between a selective MCL-1 ligand and its target, we determined the crystal structure of our strongest interactor, MCL-1 SAHBD, in complex with MCL-1ΔNΔC at 2.32-Å resolution (Fig. 3, Supplementary Table 3, PDB 3MK8). Analysis of the three-dimensional structure revealed that MCL-1 SAHBD is an α-helix that engages MCL-1ΔNΔC at the canonical BH3-binding groove comprised of helices α2 (BH3) and portions of α3, α4, α5 (BH1), and α8 (BH2) (Fig. 3a). Hydrophobic residues L213, V216, G217, and V220 of MCL-1 SAHBD make direct contact with the hydrophobic groove at the surface of MCL-1ΔNΔC (Fig. 3b), consistent with the negative ramifications of alanine mutagenesis of these amino acids in MCL-1 SAHBA (Fig. 2a). The hydrophobic interactions are reinforced by a salt bridge between MCL-1 SAHBD D218 and MCL-1ΔNΔC R263; these residues also participate in a hydrogen bond cluster that includes MCL-1ΔNΔC D256 and N260 (Fig. 3a). Adjacent to this cluster, MCL-1 SAHBD R214 lies in close proximity to MCL-1ΔNΔC S255 and D256, which forms the edge of an intricate polar network that complements the binding surface of MCL-1 SAHBD N-terminal to the staple site.
Figure 3.
Crystal structure of the MCL-1 SAHBD/MCL-1ΔNΔC complex. (a) MCL-1 SAHBD engages MCL-1ΔNΔC at the canonical BH3 binding groove of anti-apoptotic proteins, as determined by x-ray crystallography at 2.32-Å resolution (PDB 3MK8). Hydrophobic interactions at the binding interface are reinforced by a complementary polar interaction network that involves MCL-1 SAHBD residues R214 and D218 and MCL-1ΔNΔC residues S255, D256, N260, and R263. The side chains of hydrophobic, positively charged, negatively charged and hydrophilic residues are colored yellow, blue, red and green, respectively. (b) The core BH3 residues L213, V216, G217 and V220 of MCL-1 SAHBD make direct contact with a hydrophobic cleft at the surface of MCL- 1ΔNΔC. (c) The hydrocarbon staple, bearing an olefin in the cis conformation, contributes additional hydrophobic contacts at the perimeter of the core interaction site.
The differential binding activities of MCL-1 SAHBs A-E are consistent with the structure of the MCL-1 SAHBD/MCL-1ΔNΔC complex. MCL-1 SAHBC is the only construct that exhibits poor binding activity and, based on the structure, it bears the only staple location (G217,Q221) that would sterically clash with the binding surface. Interestingly, the hydrocarbon staple of MCL-1 SAHBD, whose alkene functionality is in the cis conformation, makes discrete hydrophobic contacts with the perimeter of the MCL-1ΔNΔC binding site. A methyl group of the α,α-dimethyl functionality occupies a groove consisting of MCL-1ΔNΔC G262, F318, and F319, and additional contacts are also evident for the aliphatic side chain (Fig. 3c). Thus, the superior binding affinity of MCL-1 SAHBD may derive both from its enhanced α-helicity (Supplementary Fig. 2) and the recruitment of additional hydrophobic contacts by the staple itself. Indeed, these structural data highlight the potential to harness the staple functionality to optimize the potency of SAHB ligands while retaining their natural biological specificities.
MCL-1 SAHBD targets MCL-1 and sensitizes apoptosis
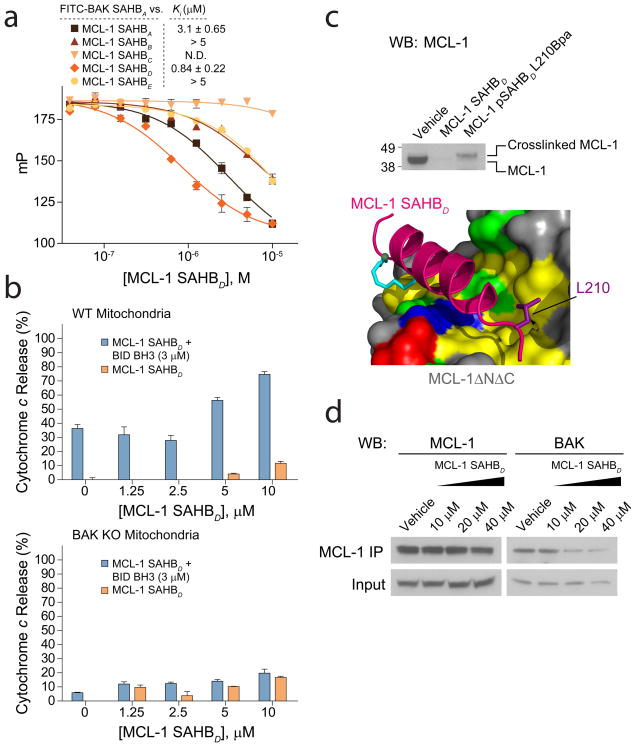
We next conducted a series of functional studies to determine if MCL-1 SAHBD could effectively target MCL-1 and sensitize mitochondrial apoptosis in vitro and in cells. We first performed a competitive FPA to measure the capacity of MCL-1 SAHBs to dissociate a BAK BH3 helix from MCL-1ΔNΔC, simulating the displacement activity required for in situ function. Consistent with the direct binding data (Fig. 2c), MCL-1 SAHBD was most effective at antagonizing the interaction between FITC-BAK SAHBA and MCL-1ΔNΔC (Fig. 4a). We next conducted mitochondrial assays to determine if the ability of MCL-1 SAHBD to disrupt the FITC-BAK SAHBA/MCL-1ΔNΔC complex translated into SAHB-mediated sensitization of BAK-induced cytochrome c release. Wild-type mouse liver mitochondria that contain BAK were exposed to BID BH3, a direct activator of BAK29, in the presence and absence of a serial dilution of MCL-1 SAHBD. Whereas MCL-1 SAHBD had no effect on the mitochondria in the absence of BID BH3, addition of MCL-1 SAHBD to BID BH3-exposed mitochondria triggered dose-responsive enhancement of BAK-mediated cytochrome c release (Fig. 4b). To confirm that cytochrome c release specifically derived from BAK activation, the identical experiment was performed with Bak−/− mitochondria, and no release was observed (Fig. 4b).
Figure 4.
MCL-1 SAHBD dissociates the inhibitory MCL-1/BAK complex in vitro and in situ, and sensitizes BAK-dependent mitochondrial cytochrome c release. (a) MCL-1 SAHBs effectively prevent sequestration of the BAK BH3 helix by MCL-1ΔNΔC, as demonstrated by competition FPA. N.D., no detected displacement. (b) MCL-1 SAHBD dose-responsively sensitized BID BH3-induced and BAK-dependent mitochondrial apoptosis, as measured by cytochrome c release assay performed on wild type and Bak−/− mitochondria. (c) An OPM2 multiple myeloma cellular lysate was incubated with the indicated biotinylated MCL-1 SAHBD constructs in the presence of ultraviolet light, followed by streptavidin-based purification, stringent washing to remove non-covalent binders, elution, and MCL-1 western analysis. The photoreactive MCL-1 pSAHBD, generated by replacing L210 with a benzophenone-bearing non-natural amino acid (Bpa), directly crosslinked to native MCL-1 within the cellular lysate, whereas no covalent crosslinking was observed for MCL-1 SAHBD, which lacked the photoreactive benzophenone moiety. (d) The native interaction between BAK and MCL-1 was dose-responsively disrupted by treatment of OPM2 cells with MCL-1 SAHBD, as assessed by MCL-1 immunoprecipitation and BAK western analysis. Binding and cytochrome c release data are mean and s.d. for experiments performed in at least triplicate. Vehicle, deionized water.
To extend these findings to a cellular context, we first confirmed that MCL-1 SAHBD could target cellular MCL-1 and dissociate native MCL-1/BAK complexes. We synthesized a photoreactive SAHB (pSAHB) that replaced L210 with a non-natural amino acid bearing a benzophenone moiety (4-benzoylphenylalanine, Bpa), which covalently crosslinks to protein targets upon exposure to ultraviolet (UV) light30. We incubated N-terminal biotinylated adducts of MCL-1 pSAHBD or MCL-1 SAHBD with cellular extract from OPM2 multiple myeloma cells in the presence of UV irradiation, followed by streptavidin-based affinity purification, stringent washes to remove non-covalent binders, elution, electrophoresis, and MCL-1 western analysis. Indeed, the photoreactive analog of MCL-1 SAHBD effectively trapped native MCL-1 contained within the cellular lysate, whereas the negative control SAHB that lacked the benzophenone moiety showed no covalent capture-based isolation of MCL-1 (Fig. 4c, Supplementary Fig. 4a). Cultured OPM2 cells were then treated with vehicle or increasing concentrations of MCL-1 SAHBD, followed by immunoprecipitation of MCL-1. BAK western analysis revealed co-immunoprecipitation of MCL-1/BAK from vehicle-treated cells but dose-responsive dissociation of the MCL-1/BAK interaction by MCL-1 SAHBD (Fig. 4d, Supplementary Fig. 4b). Taken together, these mechanistic data demonstrate that MCL-1 SAHBD can directly target native MCL-1 in a complex protein mixture, disrupt the inhibitory MCL-1/BAK interaction in vitro and in cells, and sensitize BAK-mediated mitochondrial cytochrome c release.
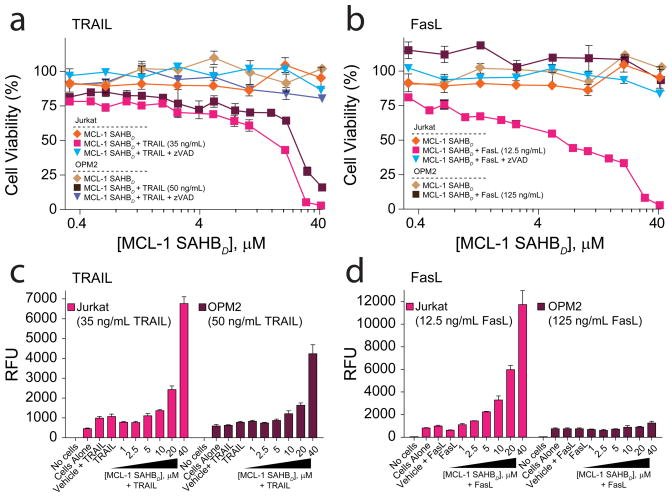
Importantly, selective liberation of pro-apoptotic proteins from MCL-1 may not activate cellular apoptosis if alternative anti-apoptotics are present at sufficient levels to bind and neutralize them. From a functional standpoint, a selective MCL-1 inhibitor would instead be expected to promote apoptosis in cells that employ MCL-1 as a key component of the survival response to a particular stress stimulus. Thus, to examine the functional consequences of selective pharmacologic blockade of MCL-1 in cells, we tested the capacity of MCL-1 SAHBD to sensitize cancer cells to death receptor agonists, whose activity is blunted by MCL-1 and enhanced by MCL-1 knockdown19,20,31,32. Jurkat T-cell leukemia and OPM2 cells were first exposed to serial dilutions of MCL-1 SAHBD and the extrinsic pathway activators TRAIL and Fas ligand (FasL) as single agents to obtain baseline viability measurements (Fig. 5a,b, Supplementary Fig. 5). MCL-1 SAHBD had no effect on cell viability even at 40μM dosing. Jurkat cells exhibited dose-responsive cytotoxicity in response to both TRAIL and FasL, whereas OPM2 cells were sensitive to TRAIL but not FasL (Supplementary Fig. 5). To determine if direct and selective MCL-1 blockade could sensitize the cells to TRAIL- and FasL-induced apoptosis, a serial dilution of MCL-1 SAHBD was combined with low-dose death receptor ligands. MCL-1 SAHBD dose-responsively sensitized Jurkat cells to both TRAIL and FasL (Fig. 5a), and selectively sensitized OPM2 cells to TRAIL (Fig. 5b). MCL-1 SAHBD had no effect on OPM2 cells exposed to FasL, consistent with the lack of response of OPM2 cells to FasL treatment (Supplementary Fig. 5). To confirm that MCL-1 SAHBD-induced sensitization was caspase-dependent, cell viability testing was also conducted in the presence of the pan-caspase inhibitor, z-VAD, which completely abrogated the negative effects on cell viability (Fig. 5a,b). Consistent with these data, MCL-1 SAHBD triggered dose-responsive caspase 3/7 activation when used in combination with low dose TRAIL and FasL in Jurkat cells (Fig. 5c) and with TRAIL, but not FasL, in OPM2 cells (Fig. 5d). Importantly, BFL-1/A1 SAHBA, which displayed no binding activity toward anti-apoptotic proteins, did not sensitize Jurkat cells to TRAIL or FasL (Supplementary Fig. 6a). As a positive control, we sought to generate an additional MCL-1-selective SAHB with a different BH3 sequence. A limited staple scan revealed that relocalizing the staple from R31,K35 in NOXA SAHBA to the A26,R30 position in NOXA SAHBB narrowed the natural MCL-1 and BFL-1/A1 binding selectivity to MCL-1 only, and NOXA SAHBB indeed functioned like MCL-1 SAHBD in this sensitization study (Supplementary Fig. 6b). Whereas MCL-1 SAHBD and NOXA SAHBB showed similar levels of cellular uptake, the negative control BFL-1/A1 SAHBA exhibited even higher intracellular levels, confirming that the inactivity of BFL-1/A1 SAHBA did not derive from a lack of cell penetrance (Supplementary Fig. 6c). Taken together, our mechanistic and functional data demonstrate that MCL-1 SAHBD is a selective, cell-permeable MCL-1 antagonist, which sensitizes cancer cells to apoptotic stimuli that are suppressed by MCL-1.
Figure 5.
Selective MCL-1 targeting by MCL-1 SAHBD sensitizes death receptor signaling and induces caspase-dependent cancer cell apoptosis. (a) Jurkat T-cell leukemia and (b) OPM2 cells were exposed to MCL-1 SAHBD singly and in combination with low dose death receptor agonists TRAIL and Fas ligand (FasL) in the presence or absence of the pan-caspase inhibitor, z-VAD. Cell viability measured by MTT assay at 24 hours revealed dose-responsive and caspase-dependent apoptosis sensitization of Jurkat (TRAIL and FasL) and OPM2 (TRAIL) cells by MCL-1 SAHBD. The capacity of MCL-1 SAHBD to sensitize (c) Jurkat and (d) OPM2 cells to death receptor stimuli correlated with dose-responsive activation of caspase 3/7, as measured by luminescence of DEVD-cleaved substrate. Data are mean and s.d. for experiments performed in at least triplicate. Vehicle, deionized water.
DISCUSSION
BCL-2 proteins, like many protein families, are comprised of numerous members that share a high percentage of sequence identity and functional homology. It is the differences among these homologous proteins, however, that give rise to their unique interactions and spectra of activity. When implicated in pathologic protein interactions, it may be desirable to neutralize all anti-apoptotic family members or a discrete subset, with the drug profile of choice dictated by the nature and severity of the disease. In the case of targeting anti-apoptotic BCL-2 family proteins that cause uncontrolled cell survival, an ideal pharmacologic toolbox would contain agents that target individual, subsets, and all members. Achieving this goal requires careful structural dissection of both the unique and common elements of BH3 interactions with anti-apoptotic targets.
Anti-apoptotic MCL-1 is a high priority target for developmental cancer therapeutics due to its emergence as a formidable and pervasive oncogenic protein14 and chemoresistance factor33,34. Guided by the natural binding selectivities of BH3 helices for discrete anti-apoptotic proteins, we have identified a potent and exclusive inhibitor of MCL-1 based on structural reinforcement of its own α-helical BH3 domain. Indeed, our findings reveal the structural basis for a previous observation that MCL-1 short (MCL-1S), a splice variant of MCL-1 that lacks BH1, BH2, and C-terminal transmembrane domains but retains the BH3, only interacted with MCL-1 in a yeast two hybrid analysis of multidomain pro- and anti-apoptotic BCL-2 family proteins35. The potent and selective interaction between the MCL-1 BH3 helix and MCL-1 is mediated by highly conserved BH3 elements, such as L213, R214, G217, and D218, and a uniquely branched hydrophobic interface comprised of L213, Val216, and V220. A single V220F mutation eliminates the MCL-1 binding exclusivity of MCL-1 SAHBA, underscoring the importance of individual amino acid contacts in dictating the binding spectrum of BH3 α-helices. Compared to the structures of other BH3 peptides in complex with MCL-1 (e.g. BID [2KBW], BIM [2PQK, 2NL9], NOXA [2ROD, 2NLA], PUMA [2ROC]), MCL-1 SAHBD has a shorter structured core, which is confined to approximately three α-helical turns. The critical contacts that lie within this α-helical unit drive the potency and selectivity of the interaction, as demonstrated by site directed mutagenesis of MCL-1 SAHBA. Interestingly, the “D” staple replaces the polar Q221 residue of MCL-1 BH3 and makes hydrophobic contact with G262 Cα (α5) and F318 (α8) of MCL-1, mirroring the binding of a similarly oriented Y35 residue in murine NOXA-A BH3 with G243 (α5) and F299 (α8) of murine MCL-1 (2ROD). Thus, by engaging in additional hydrophobic contacts at the perimeter of the core binding interface, the hydrocarbon staple may directly contribute to the enhanced affinity of MCL-1 SAHBD compared to its differentially stapled analogs without compromising specificity.
From a functional standpoint, MCL-1 SAHBD effectively targets native MCL-1, disrupts its capacity to suppress the death pathway through protein interaction, and sensitizes caspase-dependent cancer cell apoptosis in the context of death receptor stimulation. Whereas TRAIL ligand and antibody-based TRAIL receptor agonists are currently being evaluated in clinical trials, they can be rendered ineffective by MCL-1 expression36,37, underscoring the clinical relevance of our findings. By identifying the critical binding and specificity determinants for selective MCL-1 inhibition by a natural BH3 domain, our data provide a blueprint for the development of novel therapeutics to reactivate apoptosis in diseases driven by pathologic MCL-1-mediated cell survival and chemoresistance.
METHODS
Peptide synthesis
SAHBs were synthesized, purified, and characterized by circular dichroism as described in detail38. All peptides were purified by liquid chromatography-mass spectroscopy to >95% purity and quantitated by amino acid analysis. For CD analysis, MCL-1 SAHBs were dissolved in a 5 mM potassium phosphate solution (pH 7.5) to achieve a target concentration of 20 μM. For all other experiments, MCL-1 SAHBs were reconstituted in deionized water. The compositions of human SAHBs used in this study are listed in Supplementary Table 1.
Anti-apoptotic protein production
Recombinant and tagless MCL-1ΔNΔC, BCL-2ΔC, BCL-XLΔC, BCL-wΔC, and BFL1/A1ΔC were expressed and purified as previously reported39 and as described in the Supplementary Methods.
Fluorescence polarization binding assays
Binding assays were performed as previously described39. FITC-SAHB (50 nM) was added to serial dilutions of recombinant protein in binding buffer (50 mM Tris, 100 mM NaCl, pH 8.0). For competition assays, serial dilutions of acetylated MCL-1 SAHBs were mixed with FITC-BAK SAHB (25 nM), followed by addition of MCL-1ΔNΔC (100 nM) diluted in binding buffer. Multiwell plates were incubated in the dark at room temperature until equilibrium was reached and fluorescence polarization (mP units) measured by microplate reader (SpectraMax, Molecular Devices). For direct binding experiments, dissociation constants (KD) were calculated by nonlinear regression analysis of dose-response curves using Prism software (Graphpad), as described39. For competition experiments, Ki values were determined by nonlinear regression analysis of dose-response curves using a one-site competition model.
X-ray crystallography
MCL-1ΔNΔC protein (6.3 mg/mL) in 50 mM NaCl, 20 mM Tris, 2 mM DTT, pH 7.4 was incubated with an equimolar ratio of MCL-1 SAHBD reconstituted in water. The mixture (1 μL) was added to an equal volume of the reservoir solution (0.1 M Bis Tris, 28% PEG MME 2000, pH 6.5), and crystals were grown by vapor diffusion using the sitting drop method. Crystals were flash frozen in liquid nitrogen and x-ray diffraction data for the P212121 crystal were collected at the Argonne National Laboratory using the advanced photon beamline 24-ID-C, and scaled to 2.32-Å. HLK200040 was used for data processing and phases were obtained by molecular replacement of chain A of PDB 3KJ0 (MCL-1 chain) using PHASER41. Iterative rounds of refinement using TLS and model building were performed using PHENIX42 and COOT43, respectively (PDB 3MK8; Rwork/Rfree, 23.1/27.5). Topology and parameter files were created for non-natural amino acid residues using published bond lengths and angles44. MolProbity45 was used to validate the structure (94.74% Ramachandran favored, 0.66% Ramachandran outliers, 0 bad rotamers).
Cytochrome c release assays
Mouse liver mitochondria (0.5 mg/mL) were isolated and cytochrome c release assays performed according to established methods39 and as described in the Supplementary Methods.
MCL-1 SAHB photocrosslinking
MCL-1 SAHB photocrosslinking was performed using MCL-1 pSAHBD (10 μM), OPM2 cellular lysates, and 365 nm ultraviolet light as described in detail in the Supplementary Methods.
MCL-1 immunoprecipitation assay
OPM2 cells (1 × 107) were incubated with vehicle or MCL-1 SAHBD at the indicated concentrations in Opti-MEM medium (Invitrogen) at 37°C for 4 hours. Cells were washed once with cold PBS and lysed on ice with 500 μL of cold NP-40 lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM DTT, 0.5% NP40, complete protease inhibitor pellet). Cellular debris was pelleted at 14,000×g for 10 minutes at 4°C and the supernatant was collected and exposed to pre-equilibrated protein A/G sepharose beads. The pre-cleared supernatant was incubated with anti-MCL-1 antibody (S-19, Santa Cruz Biotechnology) overnight at 4°C, followed by the addition of protein A/G sepharose beads for 1 hour. The beads were then pelleted, washed with NP-40 lysis buffer for 10 minutes at 4°C three times, and the protein sample eluted from the beads by heating at 90°C for 10 minutes in SDS loading buffer. The immunoprecipitates were subjected to electrophoresis and western analysis using the BAK(NT) antibody (CalBiochem).
Cell viability assay
OPM2 multiple myeloma and Jurkat T-cell leukemia cells were maintained in RPMI 1640 medium (Invitrogen) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine, 50 mM HEPES and 50 μM β-mercaptoethanol. For viability testing, OPM2 and Jurkat cells (4 × 104) were treated with the indicated agents in Opti-MEM media at 37°C in a final volume of 100 μL. Cell viability was measured at 24 hours by MTT assay (Sigma). For synergy studies with TRAIL or FasL, cells were treated simultaneously with MCL-1 SAHBD and the death receptor ligands in the presence or absence of the pan-caspase inhibitor z-VAD (50 μM), which was administered to cells 30 minutes prior to treatment with the pro-apoptotic agents.
Capsase 3/7 activation assay
OPM2 and Jurkat cells (2 × 104 cells) were treated with the indicated agents in Opti-MEM media at 37°C in a final volume of 50μL. Caspase 3/7 activation was measured at 4 hours using the ApoONE Caspase 3/7 kit (Promega). For synergy studies with TRAIL or FasL, cells were treated simultaneously with MCL-1 SAHBD and the death receptor ligands.
Supplementary Material
Acknowledgments
We thank E. Smith for editorial and graphics support, R.A. Grant for input on the crystallography experiments, x-ray data collection and structural analysis, and C.H. Yun and M.J. Eck for assistance in generating the hydrocarbon staple parameter file used in the structural refinement. This work was supported by NIH grant 5P01CA92625 and a Burroughs Wellcome Career Award in the Biomedical Sciences to L.D.W., a Ruth L. Kirschstein National Research Service Award 1F31CA144566 to M.L.S., and NIH award 5RO1GM084181 to A.E.K. X-ray diffraction data were acquired at the Advanced Photon Source on the Northeastern Collaborative Access Team beamlines, which are supported by award RR-15301 from the National Center for Research Resources at the NIH. Use of the Advanced Photon Source is supported by the U.S. Department of Energy, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
Footnotes
AUTHOR CONTRIBUTIONS
M.L.S. and L.D.W. designed, synthesized, and characterized SAHB compounds for biochemical, structural, and cellular analyses. M.L.S. performed the x-ray crystallography experiments with E.F. in the laboratory of A.E.K., who supervised the structural analyses. M.L.S. conducted all biochemical and cellular analyses, with guidance from L.D.W. L.D.W. and M.L.S. wrote the manuscript, which was reviewed and edited by E.F. and A.E.K.
Loren Walensky is a scientific advisor board member and consultant for Aileron Therapeutics.
References
- 1.Tsujimoto Y, Cossman J, Jaffe E, Croce CM. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985;228:1440–3. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- 2.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–19. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 3.Sattler M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 4.Muchmore SW, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–41. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen L, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 6.Zhai D, Jin C, Huang Z, Satterthwait AC, Reed JC. Differential regulation of Bax and Bak by anti-apoptotic Bcl-2 family proteins Bcl-B and Mcl-1. J Biol Chem. 2008;283:9580–6. doi: 10.1074/jbc.M708426200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitada S, et al. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen M, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–7. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 10.Tse C, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–8. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 11.Wang G, et al. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 12.Konopleva M, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–88. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 13.van Delft MF, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–99. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, Gojo I, Fenton RG. Myeloid cell factor-1 is a critical survival factor for multiple myeloma. Blood. 2002;99:1885–93. doi: 10.1182/blood.v99.6.1885. [DOI] [PubMed] [Google Scholar]
- 16.Boisvert-Adamo K, Longmate W, Abel EV, Aplin AE. Mcl-1 is required for melanoma cell resistance to anoikis. Mol Cancer Res. 2009;7:549–56. doi: 10.1158/1541-7786.MCR-08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Q, et al. Myeloid Cell Leukemia-1 Inversely Correlates with Glycogen Synthase Kinase-3{beta} Activity and Associates with Poor Prognosis in Human Breast Cancer. Cancer Res. 2007;67:4564–71. doi: 10.1158/0008-5472.CAN-06-1788. [DOI] [PubMed] [Google Scholar]
- 18.Lin X, et al. ‘Seed’ analysis of off-target siRNAs reveals an essential role of Mcl-1 in resistance to the small-molecule Bcl-2/Bcl-XL inhibitor ABT-737. Oncogene. 2007;26:3972–9. doi: 10.1038/sj.onc.1210166. [DOI] [PubMed] [Google Scholar]
- 19.Meng XW, et al. Mcl-1 as a buffer for proapoptotic Bcl-2 family members during TRAIL-induced apoptosis: a mechanistic basis for sorafenib (Bay 43–9006)-induced TRAIL sensitization. J Biol Chem. 2007;282:29831–46. doi: 10.1074/jbc.M706110200. [DOI] [PubMed] [Google Scholar]
- 20.Taniai M, et al. Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res. 2004;64:3517–24. doi: 10.1158/0008-5472.CAN-03-2770. [DOI] [PubMed] [Google Scholar]
- 21.Danial NN, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–53. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walensky LD, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–70. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavathiotis E, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–81. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walensky LD, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Schafmeister C, Po J, Verdine G. An all-hydrocarbon cross-linking system for enhancing the helicity and metabolic stability of peptides. J Am Chem Soc. 2000;122:5891–5892. [Google Scholar]
- 26.Blackwell HE, et al. Ring-Closing Metathesis of Olefinic Peptides: Design, Synthesis, and Structural Characterization of Macrocyclic Helical Peptides. J Org Chem. 2001;66:5291–5302. doi: 10.1021/jo015533k. [DOI] [PubMed] [Google Scholar]
- 27.Boersma MD, Sadowsky JD, Tomita YA, Gellman SH. Hydrophile scanning as a complement to alanine scanning for exploring and manipulating protein-protein recognition: application to the Bim BH3 domain. Protein Sci. 2008;17:1232–40. doi: 10.1110/ps.032896.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day CL, et al. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–71. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- 29.Letai A, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 30.Saghatelian A, Jessani N, Joseph A, Humphrey M, Cravatt BF. Activity-based probes for the proteomic profiling of metalloproteases. Proc Natl Acad Sci U S A. 2004;101:10000–5. doi: 10.1073/pnas.0402784101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clohessy JG, Zhuang J, de Boer J, Gil-Gomez G, Brady HJ. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem. 2006;281:5750–9. doi: 10.1074/jbc.M505688200. [DOI] [PubMed] [Google Scholar]
- 32.Han J, Goldstein LA, Gastman BR, Rabinowich H. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J Biol Chem. 2006;281:10153–63. doi: 10.1074/jbc.M510349200. [DOI] [PubMed] [Google Scholar]
- 33.Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–36. doi: 10.1007/s00018-008-8637-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warr MR, Shore GC. Unique biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–47. doi: 10.2174/156652408783769580. [DOI] [PubMed] [Google Scholar]
- 35.Bae J, Leo CP, Hsu SY, Hsueh AJ. MCL-1S, a splicing variant of the antiapoptotic BCL-2 family member MCL-1, encodes a proapoptotic protein possessing only the BH3 domain. J Biol Chem. 2000;275:25255–61. doi: 10.1074/jbc.M909826199. [DOI] [PubMed] [Google Scholar]
- 36.Kim SH, Ricci MS, El-Deiry WS. Mcl-1: a gateway to TRAIL sensitization. Cancer Res. 2008;68:2062–4. doi: 10.1158/0008-5472.CAN-07-6278. [DOI] [PubMed] [Google Scholar]
- 37.Ricci MS, et al. Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell. 2007;12:66–80. doi: 10.1016/j.ccr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 38.Bird GH, Bernal F, Pitter K, Walensky LD. Chapter 22 Synthesis and biophysical characterization of Stabilized Alpha-helices of BCL-2 Domains. Methods Enzymol. 2008;446:369–86. doi: 10.1016/S0076-6879(08)01622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitter K, Bernal F, LaBelle JL, Walensky LD. Chapter 23 Dissection of the BCL-2 family signaling network with Stabilized Alpha-Helices of BCL-2 Domains. Methods Enzymol. 2008;446:387–408. doi: 10.1016/S0076-6879(08)01623-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 41.Storoni LC, McCoy AJ, Read RJ. Likelihood-enhanced fast rotation functions. Acta Crystallogr D Biol Crystallogr. 2004;60:432–8. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 42.Adams PD, et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58:1948–54. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 43.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 44.Lynch VM, Tanaka T, Fishpaugh JR, Martin SF, Davis BE. Structure of (+/−)-(1R*,4S*,6S*)-1-benzyloxy-4,8,11,11-tetramethyl-6-phenylthio-bicyclo[5.3.1]undec-7-en-3-one. Acta Crystallogr C. 1990;46 ( Pt 7):1351–3. doi: 10.1107/s0108270190002104. [DOI] [PubMed] [Google Scholar]
- 45.Davis IW, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–83. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.