Abstract
Resistance to tamoxifen in breast cancer patients is a serious therapeutic problem and major efforts are underway to understand underlying mechanisms. Resistance can be either intrinsic or acquired. We derived a series of subcloned MCF7 cell lines that were either highly sensitive or naturally resistant to tamoxifen and studied the factors that lead to drug resistance. Gene-expression studies revealed a signature of 67 genes that differentially respond to tamoxifen in sensitive vs. resistant subclones, which also predicts disease-free survival in tamoxifen-treated patients. High-throughput cell-based screens, in which >500 human kinases were independently ectopically expressed, identified 31 kinases that conferred drug resistance on sensitive cells. One of these, HSPB8, was also in the expression signature and, by itself, predicted poor clinical outcome in one cohort of patients. Further studies revealed that HSPB8 protected MCF7 cells from tamoxifen and blocked autophagy. Moreover, silencing HSBP8 induced autophagy and caused cell death. Tamoxifen itself induced autophagy in sensitive cells but not in resistant ones, and tamoxifen-resistant cells were sensitive to the induction of autophagy by other drugs. These results may point to an important role for autophagy in the sensitivity to tamoxifen.
Keywords: functional screen, estrogen receptor
The two thirds of women with estrogen receptor- (ER) or progesterone receptor-positive breast cancers are excellent candidates for antihormone therapy. Selective ER modulators (SERMs), like tamoxifen, block ER activation and have impacted both therapy and survival. However, the success of tamoxifen therapy is limited by intrinsic and acquired drug resistance. Several pathways have been implicated in antiestrogen resistance, including: the PI3K/AKT/mTOR (mammalian target of rapamycin) pathway, which is implicated in cell survival; the EGFR family; and the RAS/RAF/MEK1/2/ERK1/2 family, which regulate cell proliferation (1, 2). Loss of ER expression or function may also be an important mechanism of de novo resistance to tamoxifen, either through relatively rare ER mutations or changes in coactivators and corepressors (3).
Several groups have used gene-expression analysis to identify genes regulated through ER (4) that are affected by SERMs in breast cancer cells (5, 6). Others have used tumor samples to develop gene signatures that can predict clinical responses to tamoxifen (7–10). Genetic strategies have also been used to identify genes that drive tamoxifen resistance. Receptor tyrosine kinases and MAPK signaling were detected using expression of pooled cDNA libraries in ZR-75-1, an approach often biased toward the most abundantly expressed genes and which requires recovery of hits by PCR (11). The analysis of antiestrogen-sensitive and -resistant MCF7 cells by SNP and comparative genomic hybridization pointed to changes in protein abundance rather than somatic genomic changes (12). An RNA interference screen of kinases identified CDK10, CRK7, and MAP2K7, whose knockdown cause tamoxifen resistance in MCF7 cells (13).
Kinases play an essential role in cellular physiology and several have been shown to cause tamoxifen resistance. It is likely that other kinases contribute to hormone independence.
Results
Ectopic Kinase Expression Screen for Tamoxifen Resistance.
Tamoxifen-sensitive and -resistant MCF7 subclones.
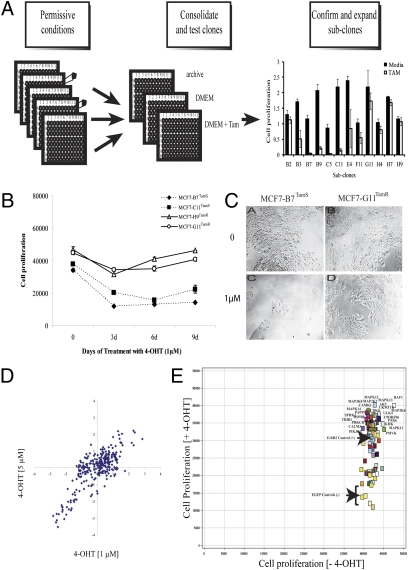
We selected the MCF7 line for a cell-based screen because it requires estrogen for proliferation and is growth-inhibited by antihormone therapy. We noted, however, that MCF7 displayed a heterogeneous response to hormonal manipulation, revealing partial but not complete cell killing after tamoxifen. Thus, we used limiting dilution to separate seven well-behaved, subcloned cell lines of which three were sensitive and four were resistant to tamoxifen based on the ratio of growth in tamoxifen/control (Fig. 1A). We used resazurin uptake (Fig. 1B) and microscopic observation (Fig. 1C) to further characterize two each of these that were either sensitive (>90% cell death), MCF7-B7TamS and MCF7-C11TamS, or tamoxifen-resistant [proliferate in presence of 4-OHT (4-hydroxy-tamoxifen)], MCF7-G11TamR and MCF7-H9TamR. The inhibitory concentration for 50% of the sensitive cells (IC50) was 5 μM for tamoxifen (TAM) or 1 μM for 4-OHT after 6 d, whereas the resistant cells continued to grow at these conditions.
Fig. 1.
MCF7 subclones and kinome screen. (A) MCF7 cells were diluted and plated into 96-well plates. Condensed plates were split into three different plates: archive, control, and tamoxifen. Cell proliferation was measured and 12 subclones were selected. (B) Cell proliferation assay showed that resistant subclones continued to proliferate in presence of 4-OHT (1 μM) and tamoxifen-sensitive cells were inhibited. (C) Photomicrographs of MCF7-B7TamS and MCF7-G11TamR showed growth inhibition of sensitive but not resistant cells. (D) Scatter plot of the z-scores showing the correlation of results for kinase cDNA expression on cell growth of MCF7-C11TamS after 4-OHT at 1 and 5 μM and puromycin (1 μg/mL). (E) Scatter plot of cell proliferation based on resazurin of 80 candidate kinases in the presence and absence of 4-OHT (1 μM) and puromycin.
We confirmed that the subclones were all derived from the parental line by demonstrating 100% concordance with the parent and other MCF7 cells for a panel of 24 SNPs and less than 5% concordance across other non-MCF7 cells (Table S1).
High-throughput functional cell-based screens.
We screened our collection of >500 full-length and fully-sequenced cDNAs of human kinases (14) to find those that confer tamoxifen resistance in the sensitive subclones. We introduced the ectopic kinases using retroviral transduction because it was more efficient and showed less variability than transfection (Fig. S1A). Assay conditions were adjusted using ERBB2 as a positive control because its ectopic overexpression conferred resistance to MCF7 (1) (Fig. S1B).
We adapted the MCF7-C11TamS for transduction in high-throughput screen (15). Using a partial set of 250 human kinases in a pilot screen, we established reproducible conditions on different days at different drug concentrations with a calculated correlation coefficient of 0.80 (Fig. 1D). We then executed three independent screens with the complete pJP1520-Kinase set (505). From these and the pilot screen, we identified 80 kinases that conferred resistance to 4-OHT (z score > 1.5). The viral supernatants for these 80 chosen candidate kinases were prepared and tested on MCF7-C11TamS and MCF7-B7TamS six times independently to narrow down the best candidate kinases. Growth in the presence of puromycin and in the absence of 4-OHT indicated good transduction efficiency (Fig. 1E). In the presence of 4-OHT, the majority of these kinase genes enhanced cell proliferation by two- to threefold compared with the control-EGFP.
From the above criteria, 60 kinases among 80 were found to be positive in at least one experiment, and 31 were positive in at least four of six independent experiments (Table S2). A pathway analysis of the candidates that scored at least twice (47 in total) indicated 20 genes that enable tamoxifen escape (Table S3). EGFR, ERBB2, and IGF1R are therapeutic targets for breast cancer and inhibitors are already clinically available (16). We also identified kinases, such as HSPB8, C9ORF96, and TRIB1 that have relatively unknown functions and had not been associated with tamoxifen resistance.
Microarray Expression Analyses of Tamoxifen-Sensitive and -Resistant MCF7.
We further compared the differences between the sensitive and resistant subclones by performing gene-expression profile analysis after estrogen and tamoxifen challenge.
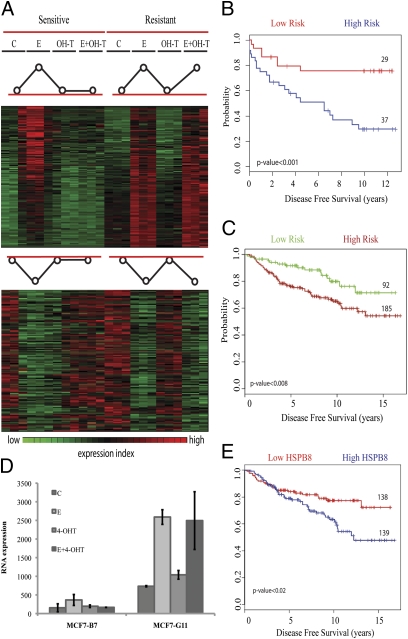
As expected in sensitive cells, tamoxifen blocked the ability of estrogen to induce (or repress) genes. We focused specifically on identifying the genes that failed to show an effect of tamoxifen in resistant cells. These 227 estrogen-responsive genes responded to tamoxifen in sensitive cells but not resistant ones (Fig. 2A). The genes included CCND1, IGF1R, MYC, and RERG, which are known to contribute to the development of resistance, as well as ERBB2, BCAR3, PIK3C2B, PIK3R3, and some negative regulators of cell cycle progression, such as CDKN1A (p21) and CDKN2B (p15) (1) (Table S4). We found that p53, TGFβ, p21 regulation, ErbB, cell cycle, and Jak-STAT signaling were the pathways that demonstrated statistically significant association with tamoxifen deregulation in resistant cells (Table S5).
Fig. 2.
Microarray expression profiles of tamoxifen-sensitive and -resistant subclones. (A) Gene cluster analysis for E-regulated genes showed 115 induced and 112 repressed genes, which no longer respond to tamoxifen in resistant cells. Stimulated genes are shown in red, inhibited genes in green, with black intermediate between the two. (B) From the genes in A, a set of 67 genes were selected to test prognostic significance. Kaplan-Meier survival analysis from Miller's dataset (18) showed 37 patients with early recurrence compared with 29 showing better disease-free survival; and (C) 92 patients showed better disease-free survival compared with 185 at high risk in Loi's dataset (19). (D) Expression of HSPB8 increased in both cell lines after E addition, but is suppressed by tamoxifen in MCF7-B7TamS and not in MCF7-G11TamR. (E) A Kaplan-Meier curve for disease-free survival, sorted based only on HSPB8 expression, showed better outcome when expression is low. From this set, 67 of the 92 low-risk patients showed low expression of HSPB8 (73% overlap). Similarly, 114 of the 139 patients with high HSPB8 expression were in the high-risk group (82% overlap).
Clinical outcome prediction.
We wondered if these 227 genes might also be predictive for outcomes in breast cancer patients who received tamoxifen. Because tumor tissue cannot be challenged with drugs, we focused on the subset of genes with an expression difference between the sensitive and resistant cells at baseline (no treatment). Using the PAM algorithm (17), we found 72 probes for 67 unique genes (Table S4). The entire microarray dataset is available through the Gene Expression Omnibus (accession no. GSE 26459).
Using clinical studies with long-term outcomes in tamoxifen-treated women that examined gene expression in tumors (18), the expression profile for each patient's tumor was sorted into “resistance-like” or “sensitive-like” groups. These genes, selected entirely for their differential tamoxifen responses in our sensitive and resistant subclones, predicted better disease-free survival of patients that matched the “sensitive” signature (Fig. 2B). Presumably, these patients were more likely to respond to tamoxifen and, hence, had a lower probability of relapse compared with patients whose patterns were closer to the resistant cells. In contrast, the “resistance pattern” patients had a one in two chance of relapse after 5 y of tamoxifen treatment. We further confirmed this result using a different dataset (19) (Fig. 2C).
Univariate analysis showed that the tamoxifen resistance signature was associated with a strong hazard ratio (2.12), comparable to tumor size (Table S6). Moreover, multivariate analysis demonstrated that as a prognostic predictor of relapse, the resistance signature was independent of the clinical variables available in this study, including histological grade.
Comparison of the Screen and Microarray Analyses for Pathways Involved in Tamoxifen Resistance.
A number of genes were common to both the cell based screen and the gene-expression analysis, including ERBB2, HSPB8, IGF1R, and SGK3. We focused our interest on HSPB8 because: it has never been previously studied in endocrine resistance; it repeated in five of six screens; it gave one of the strongest responses, and it was also present in the 67-gene signature that predicted relapse for women taking tamoxifen.
At baseline, HSPB8 was expressed at higher levels in the resistant compared with the sensitive cells and it was not inhibited by tamoxifen in the resistant cells (Fig. 2D). Moreover, when the 277 patients from Loi et al. (19) were sorted based on HSBP8 expression alone, a high expression level of HSPB8 predicted an earlier relapse on tamoxifen (Fig. 2E). A similar trend was observed in the Miller dataset (18), but was not significant.
HSPB8 as an Important Protein in the Development of Tamoxifen Resistance.
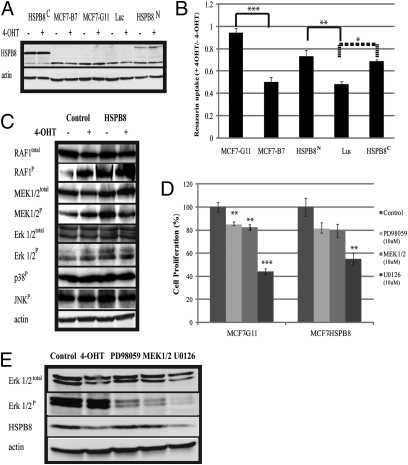
We established stable cell lines expressing 3xFlag-tagged versions of the protein in MCF7-B7TamS, with either carboxyl or amino-terminal modification, with the former showing greater abundance (Fig. 3A). Compared with the luciferase control, ectopic expression of both HSPB8 constructs provided a proliferation advantage with 4-OHT treatment (Fig. 3B). Considering its lower abundance, the amino-tagged construct was more active than the carboxyl-tagged, perhaps because of the tag position. Neither was as active as the naturally resistant clones, pointing to other pathways that contribute to tamoxifen resistance, consistent with our gene-expression studies.
Fig. 3.
Stable ectopic expression of HSPB8 blocks autophagy after tamoxifen. (A) Stable cell lines in MCF7-B7TamS expressing HSPB8 flag-tagged at either HSPB8C or HSPB8N were compared by immunoblot with MCF7-B7TamS and MCF7-G11TamR after 4-OHT (1 μM) for 72 h. Luciferase (Luc) was used as a negative control. (B) Cell proliferation assay of above cells was performed in 96-well plates. Data represent resazurin uptake in presence of 4-OHT divided by resazurin uptake in absence of drug. (*P < 0.07, **P < 0.05, ***P < 0.005 calculated based on Welch Two Samples t test.) (C) Western blot analysis of the phosphorylation status of MAP kinases. RAFP and ERK1/2P were elevated in HSPB8 and increased further in presence of 4-OHT. (D) Stable HSPB8 and MCF7-G11TamR cells were treated for 72 h with three MEK inhibitors: PD98059, MEK1/2, and U0126 and control (**P < 0.01, ***P < 0.00001). (E) ERK1/2P and HSPB8 were measured by western-blot after MCF7-G11TamR was treated with MEK inhibitors.
To determine if HSPB8 were affecting known tamoxifen resistance pathways, we evaluated MAP kinase signaling. Although p38P and JNKP did not change with added tamoxifen or HSPB8 expression, RAFP and ERKP were induced by both, with a particularly strong combined effect for RAFP. The effect on MEK was mixed (Fig. 3C). To probe the contribution of the MEK pathway to cell survival in tamoxifen, we used three different MEK inhibitors: PD98059, MEK1/2, and U0126 (20). At effective concentrations, neither PD98059 nor MEK1/2 had a marked effect on cell proliferation for either the naturally resistant cells or the HSPB8 cells (Fig. 3D). However, U0126 significantly reduced cell proliferation of both cell lines, which may point to activities unrelated to MEK that contribute to this effect. Interestingly, the addition of U0126 reduced HSPB8 protein levels in the MCF7-G11TamR cells, but neither PD98059 nor MEK1/2 had this effect (Fig. 3E).
HSPB8 Expression Is Essential for Cell Proliferation in MCF7 Cells.
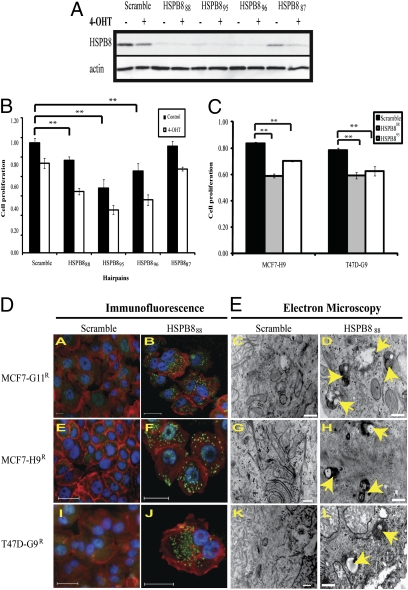
As ectopic HSPB8 expression supported proliferation in tamoxifen-treated cells, we tested whether HSPB8 was required for proliferation in resistant cells. We observed that three out of four targeted shRNAs reduced both HSPB8 protein (Fig. 4A) and cell proliferation (Fig. 4B) in MCF7-G11TamR. The same effect was observed in MCF7-H9TamR and T47D-G9TamR cells, although less pronounced in the latter (Fig. 4C). After silencing HSPB8, cells rounded up and lost cell-to-cell contact.
Fig. 4.
Reducing HSPB8 in MCF7-G11TamR leads to autophagy. (A) Protein expression of HSPB8 was reduced with three of four different targeted hairpins in MCF7-G11TamR compared with vector-expressing scrambled sequences. (B) Silencing HSPB8 reduced cell proliferation of MCF7-G11TamR. The effect was more pronounced in the presence of 4-OHT (1 μM). Resazurin data were normalized against scramble in the presence of puromycin and in absence of 4-OHT after 48 h postdrug treatment (**P < 0.001). (C) Hairpins HSPB888 and HSPB895 reduced cell proliferation compared with scramble in two additional tamoxifen-resistant subclones, MCF7-H9TamR and T47D-G9TamR, in the presence of 4-OHT (**P < 0.001). (D) Immunofluorescence images after silencing HSPB888 in all resistant subclones showed the characteristic redistribution of anti-MAP LC3 into punctuate green structures (B, F, and J, respectively), indicating active autophagy, but scrambled hairpin showed only diffuse staining (A, E, and I). Blue: nucleus by Hoechst; red: actin by Phalloidin; green: autophagy marker anti-MAP LC3. (Scale bars 50 μM.) (E) EM consistently identified a large number of autophagosomes containing organelles undergoing degenerative changes (arrows) in all of the cells where HSPB8 was silenced (D, H, and L). (Scale bars, 500 nm.)
These data suggest that HSPB8 is essential to survival. Relatively little is known about HSPB8 signaling, which has been reported to participate in both autophagy (21) and apoptosis (22). Silencing HSPB8 did not result in significant apoptosis, suggesting that this was not the predominant response causing cell death (Fig. S2A). However, using the MAP LC3 antibody, we observed a punctuate pattern characteristic of ongoing autophagy with all of the hairpins (Fig. 4D). We confirmed autophagosome formation when we examined these cells by transmission electron microscopy (EM), which revealed the characteristic double membrane structures (Fig. 4E).
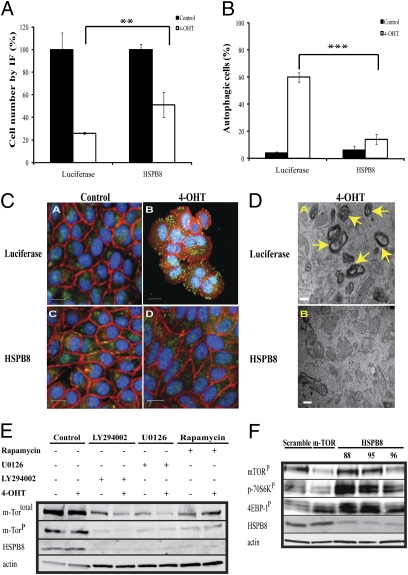
In the converse experiment, we tested whether ectopically expressed HSPB8 blocked autophagy. Consistent with our previous results, HSPB8 conferred a twofold proliferation advantage to tamoxifen-treated cells (Fig. 5A). In addition, HSPB8 led to more than a threefold reduction in the number of cells displaying autophagy (Fig. 5B), as indicated by MAP LC3 (Fig. 5C) and autophagosomes in EM (Fig. 5D). Thus, silencing HSPB8 increased autophagy and cell death, whereas ectopic expression reduced autophagy and improved cell survival in the presence of tamoxifen.
Fig. 5.
Effect of autophagy in sensitive and resistant cells. (A) Fluorescence images of quadruplicate experiments were taken after LC3 staining in MCF7-B7TamS cells stably expressing either HSPB8 or luciferase (control). Total number of cells were counted in the presence and absence of 4-OHT (1 μM) after 72 h and expressed as a percentage of no treatment controls (**P < 0.01). (B) HSPB8 reduced the percentage of autophagic cells (mean ± SD) (***P < 0.003). (C) Representative images from B showing reduction of autophagy. Blue: nucleus by Hoechst; red: actin by Phalloidin; green: autophagy marker anti-MAP LC3. (Scale bars, 50 μM.) (D) EM images showed the presence of clusters of autophagosomes after control cells were treated with 4-OHT. Autophagosomes were markedly reduced when the cells were ectopically expressing HSPB8. (Scale bar, 500 nm.) (E) Western blot analysis showed lower expression levels of mTOR and HSPB8 in MCF7-G11TamR 72 h posttreatment with autophagy-inducing drugs: rapamycin (0.1 μM), LY294002 (5 μM), U0126 (10 μM), but not with 4-OHT (1 μM). (F) Short hairpin RNAs directed at HSPB8, mTOR, or scramble were transduced into MCF7-G11TamR cells. Protein expression levels of HSPB8, mTORP, p70S6kP, and 4EBP-1P were measured 72 h postinfection. β-Actin served as a loading control.
HSPB8 and Autophagy.
The role of autophagy in the effect of HSPB8 suggested an interesting alternative explanation for why U0126 had a more pronounced effect on the proliferation of resistant cells (Fig. 3D). Unlike the other MEK inhibitors, U0126 affects the mTOR pathway by inhibiting p70S6K, leading to autophagy (23). This finding pointed to a potentially important role for autophagy, as well as apoptosis, in cell death triggered by tamoxifen. Indeed, we observed that tamoxifen induced both processes in sensitive but not in resistant cells (Fig. S2B). Consistent with this finding, two other drugs that induce autophagy, LY294002 and rapamycin (24), also inhibited the growth of the resistant cells (Fig. S3) and reduced HSPB8 levels (Fig. 5E).
Many drugs that induce autophagy act through the mTOR pathway. Interestingly, we observed that silencing HSPB8 did not affect the protein abundance of mTOR, nor did silencing mTOR affect HSPB8 (Fig. 5F). Decreasing mTOR affected known downstream targets, including decreasing p70S6kP and the complex effect of increasing the amount of 4EBP-1P, but shifting it to a faster migrating band (25), confirming that the hairpins inhibited the mTOR pathway compared with the control. Interestingly, silencing HSPB8 activated both p70S6kP and 4EBP-1P, suggesting that HSPB8 may ordinarily inhibit these phosphorylations and pointing to related but different roles for HSPB8 and mTOR in autophagy.
Discussion
SERMs such as tamoxifen are part of the standard treatment regimen for many ER-positive breast cancer patients and have been demonstrated to reduce deaths. Unfortunately, many patients become resistant by mechanisms that have been only partly characterized (1, 2).
We used the estrogen-dependent and tamoxifen-responsive cell line MCF7 to explore tamoxifen resistance in a high-throughput screen that identified 31 kinases of 500 (6%) that conferred strong tamoxifen resistance when ectopically expressed in at least four of six experiments (Table S2). To generate a clean drug response for a high-throughput screen, we reduced the previously reported heterogeneity in the MCF7 cells (6, 26) by limiting-dilution subcloning. A relatively large fraction of the cells (∼60%) grew out in permissive conditions, of which about half were already resistant to tamoxifen, suggesting that MCF7 cells do not require tamoxifen conditioning because resistant cells are already present in the heterogeneous population (27, 28). This finding emphasizes the importance of caution when interpreting hormone-response experiments done on commonly available MCF7 cells.
The availability of sister subclones enabled us to identify estrogen-responsive genes that were blunted by tamoxifen in a sensitive cell line, but not in a matched resistant one. Many of these genes (>90%) have not previously been linked to tamoxifen resistance, although about one-third of them could be traced in the literature to SERMS. Some of these genes are not linked into any known pathways or gene ontology classifications (Table S5). This demonstration is unique in showing that the effect of tamoxifen on these genes is altered in resistant cells. Moreover, this response in cultured cells was also relevant to clinical outcome in patients receiving tamoxifen therapy. A 67-gene subset was used to predict tumor recurrence in two independent cohorts. The tamoxifen response signature was an independent predictor of outcome compared with other clinical variables, and the hazard ratio was among the strongest observed for the study. Signatures like this one may help personalize therapy by planning regimens of adjuvant therapy best suited to each particular patient.
We found a 15% overlap of the genes when we compared our tamoxifen-resistance signature with those of Lippman et al. (7) and Chanrion et al. (8). Looking more broadly at our entire set of 227 genes with an altered tamoxifen response, we identified 21 genes that overlapped across the tamoxifen resistance studies (Table S4).
A considerable body of evidence has emerged regarding the ER, growth factor, and kinase-signaling pathways and their role in tamoxifen resistance (1). Thus, it was reassuring that many of the 31 kinases identified here were in the MAPK\ERK1/2 and receptor tyrosine kinases pathways (Table S3). Other proteins might contribute by affecting the cell cycle (ATR, AURKA, CDC2, CDK9, and STK6) or metabolism (AK5, AK2, NME3, NME4, NME7, KHK, RBKS, PMVK).
HSPB8 is a 22-kDa member of the small heat-shock protein superfamily, which contains a well-conserved α-crystallin domain at the C terminal (29). HSPB8 has not been described in connection with tamoxifen resistance, but it is overexpressed in breast cancer, particularly in ER-positive cancers (6, 30).
The ectopic expression of HSPB8 enabled cell proliferation in the presence of tamoxifen in sensitive cells, although not to the level of naturally resistant MCF7 cells. In addition to HSPB8, our gene-expression studies pointed to numerous genes that were also aberrantly regulated by tamoxifen in these resistant subclones, many of which are known to play key roles in the oncogenic phenotype, including cyclin D1, myc, myb, p21, ERBB2, and p15, which might contribute to resistance.
Interestingly, silencing HSPB8 in tamoxifen-resistant cells led to cell death via autophagy, not apoptosis (Fig. 4D and Fig. S2A). Tamoxifen induces both processes in sensitive cells (31, 32) but neither in any of the resistant subclones (Fig. S2B). The ectopic expression of HSPB8 in sensitive cells prevented the induction of autophagy by tamoxifen (Fig. 5) and presumably conferred a proliferation advantage on the treated cells (Fig. 3B). A constant low level of autophagy has been suggested for cancer cells; therefore, it is possible that prevention of autophagy in cells may lead to a general proliferation advantage that could even extend to untreated cells in culture.
Autophagy is a catabolic process involving self-digestion of cellular organelles as a means of cell survival; however, if taken too far, autophagy can lead to cell death (33, 34). The regulation of autophagy signaling is tightly linked to oncogenic signaling, with both positive and negative roles in cancer. Like HSPB8, several common oncogenes also inhibit autophagy, including: class I PI3K, AKT, mTOR, and BCL-2, arguing that oncogenes might prevent autophagy from limiting the ability of cancer cells to proliferate. Consistent with this notion, several tumor-suppressor genes, such as Beclin 1, DAPk, p53, PTEN, and TSC1/TSC2 stimulate autophagy (35). However, the well-described oncogenes Ras and MYC both stimulate autophagy, suggesting that this interplay is likely to be complex and not completely understood (34, 36).
There is controversy about whether one should turn autophagy on or off to treat cancer. At least in the context here, when inhibition of autophagy promotes cancer-cell survival in response to drugs, then induction of autophagic cell death could be predicted to have therapeutic value (35, 37).
The suppression of either HSPB8 or mTOR induces autophagy, and appears to do so independently, in as much as neither affects the levels of the other. Silencing HSPB8 activated both 4EBP1and p70S6k, suggesting that HSPB8 may ordinarily inhibit these phosphorylation events independently of mTOR (Fig. 5E). The crosstalk between signaling pathways that activate or inactivate autophagy remains under investigation (38). Recent publications suggest that 4EBP-1 may have unrecognized actions that affect mTOR signaling or autophagy (39), and there are conflicting reports on the role of p70S6k to either activate or suppress autophagy, depending on the cellular context (40). The strong effect that silencing HSPB8 has on downstream targets of mTOR that are known to participate in autophagy is certainly intriguing. It will be interesting to determine how these downstream effects are mediated and which ones are important to the establishment of autophagy and cell death. The expression of HSPB8 also affected RAF and ERK1/2 (Fig. 3C), and this might explain some degree of tamoxifen resistance. However, inhibition of the downstream ERK1/2 phosphorylation with drugs (Fig. 3D) did not seem to negate the resistance to tamoxifen and the cells continued to proliferate despite almost complete inhibition of ERK1/2 at the higher drug dose, suggesting that there must be other pathways acting here.
Clearly, the mechanism of action and specific interacting partners of HSPB8 still needs to be determined.
Materials and Methods
MCF7 Subclones.
MCF7 cells were diluted into 10 96-multiwell plates (1/3 cell/well) and grown for approximately 1 mo, including several changes of DMEM 10% FBS without selection. For those wells in which cells grew to confluence (∼192), cells were trypsinized and condensed into two plates. After condensing all subclones, they were grown again to confluence and split into three different plates: archive, control, and tamoxifen. After 6 d posttreatment, cell proliferation was measured by MTS Assay (Promega). We selected 12 subclones for further analysis, and from those, seven that behaved well in culture. Tamoxifen-sensitive cells were regularly maintained in DMEM supplemented with 5% of FBS, and resistant cells were grown in same media supplemented with 4-OHT (1 μM).
High-Throughput Functional Cell-Based Screens.
Kinase plasmids were fully sequence verified and validated (14). DNA preparation, transfections, and virus preparation have been described previously (15). For data analysis, we always subtract the signal obtained from an empty well and considered this as the background reading. Any wells whose cell viability in the presence of puromycin was less than 30% of those in the absence of puromycin were considered to have an inadequate viral titer, called dropouts (Fig. S1D). To identify genes that provided a growth advantage in the presence of tamoxifen, a z-score was computed for each well [(well fluorescence − mean of all well fluorescence for the plate)/SD of the mean of well fluorescence]. The genes for which the z score against entire plate was higher than z total > 1.5 were considered for further analysis.
Complete details of methods are available in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Joan Brugge for providing MCF7 cells, Mauricio Fernandez for all the engineering technical support with the robot instruments, John Doench and Dorre Gruenberg for manuscript suggestions and corrections, Dorre Gruenberg for providing the shRNA construct for mammalian target of rapamycin, and Milen Vitanov for his technical support at Arizona State University. This work was supported by the National Cancer Institute Specialized Programs of Research Excellence in Breast Cancer at Harvard, the Breast Cancer Research Foundation, and the National Cancer Institute Program Project Grant P01 C080111. The Expedition Inspiration for Breast Cancer Research supported L.G.-M.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018157108/-/DCSupplemental.
References
- 1.Musgrove EA, Sutherland RL. Biological determinants of endocrine resistance in breast cancer. Nat Rev Cancer. 2009;9:631–643. doi: 10.1038/nrc2713. [DOI] [PubMed] [Google Scholar]
- 2.Riggins RB, Schrecengost RS, Guerrero MS, Bouton AH. Pathways to tamoxifen resistance. Cancer Lett. 2007;256(1):1–24. doi: 10.1016/j.canlet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004;11:643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 4.Yu J, et al. A transcriptional fingerprint of estrogen in human breast cancer predicts patient survival. Neoplasia. 2008;10(1):79–88. doi: 10.1593/neo.07859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frasor J, et al. Gene expression preferentially regulated by tamoxifen in breast cancer cells and correlations with clinical outcome. Cancer Res. 2006;66:7334–7340. doi: 10.1158/0008-5472.CAN-05-4269. [DOI] [PubMed] [Google Scholar]
- 6.Fan M, et al. Diverse gene expression and DNA methylation profiles correlate with differential adaptation of breast cancer cells to the antiestrogens tamoxifen and fulvestrant. Cancer Res. 2006;66:11954–11966. doi: 10.1158/0008-5472.CAN-06-1666. [DOI] [PubMed] [Google Scholar]
- 7.Lippman ME, Rae JM, Chinnaiyan AM. An expression signature of estrogen-regulated genes predicts disease-free survival in tamoxifen-treated patients better than progesterone receptor status. Trans Am Clin Climatol Assoc. 2008;119:77–92. [PMC free article] [PubMed] [Google Scholar]
- 8.Chanrion M, et al. A gene expression signature that can predict the recurrence of tamoxifen-treated primary breast cancer. Clin Cancer Res. 2008;14:1744–1752. doi: 10.1158/1078-0432.CCR-07-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jansen MP, et al. Molecular classification of tamoxifen-resistant breast carcinomas by gene expression profiling. J Clin Oncol. 2005;23:732–740. doi: 10.1200/JCO.2005.05.145. [DOI] [PubMed] [Google Scholar]
- 10.Paik S, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 11.Meijer D, van Agthoven T, Bosma PT, Nooter K, Dorssers LC. Functional screen for genes responsible for tamoxifen resistance in human breast cancer cells. Mol Cancer Res. 2006;4:379–386. doi: 10.1158/1541-7786.MCR-05-0156. [DOI] [PubMed] [Google Scholar]
- 12.Johnson N, Speirs V, Curtin NJ, Hall AG. A comparative study of genome-wide SNP, CGH microarray and protein expression analysis to explore genotypic and phenotypic mechanisms of acquired antiestrogen resistance in breast cancer. Breast Cancer Res Treat. 2008;111(1):55–63. doi: 10.1007/s10549-007-9758-6. [DOI] [PubMed] [Google Scholar]
- 13.Iorns E, et al. Identification of CDK10 as an important determinant of resistance to endocrine therapy for breast cancer. Cancer Cell. 2008;13(2):91–104. doi: 10.1016/j.ccr.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Park J, et al. Building a human kinase gene repository: Bioinformatics, molecular cloning, and functional validation. Proc Natl Acad Sci USA. 2005;102:8114–8119. doi: 10.1073/pnas.0503141102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pearlberg J, et al. Screens using RNAi and cDNA expression as surrogates for genetics in mammalian tissue culture cells. Cold Spring Harb Symp Quant Biol. 2005;70:449–459. doi: 10.1101/sqb.2005.70.047. [DOI] [PubMed] [Google Scholar]
- 16.Agrawal A, Gutteridge E, Gee JM, Nicholson RI, Robertson JF. Overview of tyrosine kinase inhibitors in clinical breast cancer. Endocr Relat Cancer. 2005;12(Suppl 1):S135–S144. doi: 10.1677/erc.1.01059. [DOI] [PubMed] [Google Scholar]
- 17.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci USA. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller LD, et al. An expression signature for p53 status in human breast cancer predicts mutation status, transcriptional effects, and patient survival. Proc Natl Acad Sci USA. 2005;102:13550–13555. doi: 10.1073/pnas.0506230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loi S, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- 20.Duncia JV, et al. MEK inhibitors: The chemistry and biological activity of U0126, its analogs, and cyclization products. Bioorg Med Chem Lett. 1998;8:2839–2844. doi: 10.1016/s0960-894x(98)00522-8. [DOI] [PubMed] [Google Scholar]
- 21.Carra S, Seguin SJ, Landry J. HspB8 and Bag3: A new chaperone complex targeting misfolded proteins to macroautophagy. Autophagy. 2008;4:237–239. doi: 10.4161/auto.5407. [DOI] [PubMed] [Google Scholar]
- 22.Li B, et al. Overload of the heat-shock protein H11/HspB8 triggers melanoma cell apoptosis through activation of transforming growth factor-beta-activated kinase 1. Oncogene. 2007;26:3521–3531. doi: 10.1038/sj.onc.1210145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fukazawa H, Uehara Y. U0126 reverses Ki-ras-mediated transformation by blocking both mitogen-activated protein kinase and p70 S6 kinase pathways. Cancer Res. 2000;60:2104–2107. [PubMed] [Google Scholar]
- 24.Takeuchi H, et al. Synergistic augmentation of rapamycin-induced autophagy in malignant glioma cells by phosphatidylinositol 3-kinase/protein kinase B inhibitors. Cancer Res. 2005;65:3336–3346. doi: 10.1158/0008-5472.CAN-04-3640. [DOI] [PubMed] [Google Scholar]
- 25.Yu K, et al. mTOR, a novel target in breast cancer: The effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–258. doi: 10.1677/erc.0.0080249. [DOI] [PubMed] [Google Scholar]
- 26.Resnicoff M, et al. Subpopulations of MCF7 cells separated by Percoll gradient centrifugation: A model to analyze the heterogeneity of human breast cancer. Proc Natl Acad Sci USA. 1987;84:7295–7299. doi: 10.1073/pnas.84.20.7295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samaddar JS, et al. A role for macroautophagy in protection against 4-hydroxytamoxifen-induced cell death and the development of antiestrogen resistance. Mol Cancer Ther. 2008;7:2977–2987. doi: 10.1158/1535-7163.MCT-08-0447. [DOI] [PubMed] [Google Scholar]
- 28.Seibert K, et al. Clonal variation of MCF-7 breast cancer cells in vitro and in athymic nude mice. Cancer Res. 1983;43:2223–2239. [PubMed] [Google Scholar]
- 29.Kappe G, et al. The human genome encodes 10 alpha-crystallin-related small heat shock proteins: HspB1-10. Cell Stress Chaperones. 2003;8(1):53–61. doi: 10.1379/1466-1268(2003)8<53:thgecs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Creighton CJ, et al. Activation of mitogen-activated protein kinase in estrogen receptor alpha-positive breast cancer cells in vitro induces an in vivo molecular phenotype of estrogen receptor alpha-negative human breast tumors. Cancer Res. 2006;66:3903–3911. doi: 10.1158/0008-5472.CAN-05-4363. [DOI] [PubMed] [Google Scholar]
- 31.Bardon S, Vignon F, Montcourrier P, Rochefort H. Steroid receptor-mediated cytotoxicity of an antiestrogen and an antiprogestin in breast cancer cells. Cancer Res. 1987;47:1441–1448. [PubMed] [Google Scholar]
- 32.Bursch W, et al. Active cell death induced by the anti-estrogens tamoxifen and ICI 164 384 in human mammary carcinoma cells (MCF-7) in culture: The role of autophagy. Carcinogenesis. 1996;17:1595–1607. doi: 10.1093/carcin/17.8.1595. [DOI] [PubMed] [Google Scholar]
- 33.Codogno P, Meijer AJ. Autophagy and signaling: Their role in cell survival and cell death. Cell Death Differ. 2005;12(Suppl 2):1509–1518. doi: 10.1038/sj.cdd.4401751. [DOI] [PubMed] [Google Scholar]
- 34.Kondo Y, Kanzawa T, Sawaya R, Kondo S. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734. doi: 10.1038/nrc1692. [DOI] [PubMed] [Google Scholar]
- 35.Ng G, Huang J. The significance of autophagy in cancer. Mol Carcinog. 2005;43(4):183–187. doi: 10.1002/mc.20097. [DOI] [PubMed] [Google Scholar]
- 36.Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 37.Rubinsztein DC, Gestwicki JE, Murphy LO, Klionsky DJ. Potential therapeutic applications of autophagy. Natl Rev. 2007;6:304–312. doi: 10.1038/nrd2272. [DOI] [PubMed] [Google Scholar]
- 38.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balakumaran BS, et al. MYC activity mitigates response to rapamycin in prostate cancer through eukaryotic initiation factor 4E-binding protein 1-mediated inhibition of autophagy. Cancer Res. 2009;69:7803–7810. doi: 10.1158/0008-5472.CAN-09-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klionsky DJ, Meijer AJ, Codogno P. Autophagy and p70S6 kinase. Autophagy. 2005;1(1):59–60. doi: 10.4161/auto.1.1.1536. discussion 60–61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.