Abstract
DNA replication in archaea and eukaryotes is executed by family B DNA polymerases, which exhibit full activity when complexed with the DNA clamp, proliferating cell nuclear antigen (PCNA). This replication enzyme consists of the polymerase and exonuclease moieties responsible for DNA synthesis and editing (proofreading), respectively. Because of the editing activity, this enzyme ensures the high fidelity of DNA replication. However, it remains unclear how the PCNA-complexed enzyme temporally switches between the polymerizing and editing modes. Here, we present the three-dimensional structure of the Pyrococcus furiosus DNA polymerase B-PCNA-DNA ternary complex, which is the core component of the replisome, determined by single particle electron microscopy of negatively stained samples. This structural view, representing the complex in the editing mode, revealed the whole domain configuration of the trimeric PCNA ring and the DNA polymerase, including protein–protein and protein–DNA contacts. Notably, besides the authentic DNA polymerase-PCNA interaction through a PCNA-interacting protein (PIP) box, a novel contact was found between DNA polymerase and the PCNA subunit adjacent to that with the PIP contact. This contact appears to be responsible for the configuration of the complex specific for the editing mode. The DNA was located almost at the center of PCNA and exhibited a substantial and particular tilt angle against the PCNA ring plane. The obtained molecular architecture of the complex, including the new contact found in this work, provides clearer insights into the switching mechanism between the two distinct modes, thus highlighting the functional significance of PCNA in the replication process.
Keywords: fidelity control, protein–DNA complex, replication fork, single particle analysis, structural bioinformatics
In eukaryotes and archaea, genome replication is conducted efficiently and accurately by family B DNA polymerases (PolB), including Pol α, δ, and ϵ in eukaryotes and PolB in archaea. Several crystal structures of PolB have been determined, and they share essentially the same architecture, consisting of five domains: finger, palm, thumb (the three polymerase domains), N-term, and 3′ → 5′ exonuclease domains (1–10). The exonuclease domain, which is responsible for the 3′ → 5′ editing of incorrect synthesis by the polymerizing activity, ensures the high fidelity of DNA replication (11, 12). In addition, fully processive DNA synthesis in vivo by the replicative polymerase requires proliferating cell nuclear antigen (PCNA), which has a trimeric ring structure that encircles the DNA and acts as a DNA clamp (13). PCNA also interacts with various protein factors, in addition to DNA polymerases, to control DNA replication, DNA repair, and cell cycle progression and thus functions as a major conductor for the recruitment and release of these crucial players (14, 15). These proteins generally interact with PCNA through a consensus sequence, called the PCNA interacting protein (PIP) box (16). Although the entire structure of the DNA polymerase-PCNA-DNA complex has not been reported yet, ternary complex models were proposed for both the polymerizing and editing modes of a bacteriophage system, based on the two crystal structures of the DNA polymerase-DNA complexes in each mode (1, 7), and for that of the sliding clamp complexed with the clamp-binding fragment of the DNA polymerase, by using proteins from bacteriophage RB69 (1). Recently, the crystal structure of an archaeal full-length DNA polymerase complexed with a monomeric PCNA allowed us to construct a reasonable model of the ternary complex with DNA, thus providing clearer insights into the switching mechanism between the polymerizing and editing modes (10). Analyses of these structures suggested that the complex must undergo a considerable conformational change during the switch between the polymerizing and editing modes. Our studies, using single particle electron microscopy (EM), revealed that structural views of complexes at medium resolution can provide valuable information about the molecular mechanisms of the clamp loading and the DNA ligation reaction on PCNA (17, 18). Here, we report direct visualization of the 3D structure of the Pyrococcus furiosus (Pfu) PolB-PCNA-DNA complex examined by single particle electron microscopy. This structure reveals an unexpected configuration of the complex, fixed by the second PolB-PCNA contact identified in this work, which provides clearer insights into the switching mechanism between the polymerizing and editing modes of the complex.
Results and Discussion
Electron Microscopy and Overall Structure of the Complex.
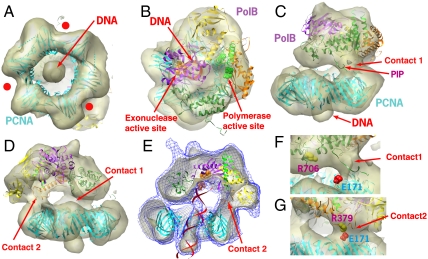
EM images of the PfuPolB-PCNA-DNA complex (total molecular mass: 210 kDa) revealed that the complex particles were well-dispersed, with almost the same size (Fig. 1A; see also Fig. S1A). About 19,000 complex images were extracted and utilized for a single particle analysis. The final EM map, obtained at 19-Å resolution, exhibited the two-layered structure of the complex, which consists of lower and upper layers corresponding to PCNA and PolB, respectively (Fig. 2; see also Fig. 1B and Fig. S1B). The dsDNA was clearly visualized as a 2-nm thick rod, running through the center of the clamp channel (Fig. 2A). The dsDNA was slightly tilted (13°) relative to the threefold axis of the PCNA ring (Fig. 2E). Notably, the dsDNA rod was located at almost the exact center of the clamp channel and lacked obvious contacts with the inner wall of the channel. This view contrasts with the highly tilted (22°) dsDNA in the crystal structure of the bacterial β-clamp-DNA complex, which contacted both sides of the clamp channel (19). In the 3D structure of the DNA ligase-PCNA-DNA complex obtained by EM analysis (18), the dsDNA was tilted by 16° against the PCNA axis and had only one contact with the DNA. Meanwhile, in the clamp loading complex composed of primed DNA, PCNA, and replication factor C (RFC), the dsDNA was almost parallel to the PCNA axis, and no significant interactions were observed between DNA and PCNA (17). These results suggest that the configurations of PCNA are variable relative to the clamped dsDNA, depending upon the protein factors within the complexes. Consistently, the most recently reported crystal structure of the PCNA-DNA complex (20) exhibited the highest tilt of DNA (40°). Presumably, this versatile property of the DNA clamp would allow protein factors to occupy the appropriate positions for their functions.
Fig. 1.
Electron microscopy of the PfuPolB-PCNA-DNA complex. (A) Electron micrograph of the negatively stained PfuPolB-PCNA-DNA complex. (Scale bar: 50 nm). (B) The 2D class averages (Upper), with the corresponding reprojections of the final 3D structure of the PfuPolB-PCNA-DNA complex (Lower). The side length of the individual images is 17.4 nm.
Fig. 2.
Three-dimensional structure of the PfuPolB-PCNA-DNA complex. (A) Bottom view. (B) Top view. (C) Front view. (D) Side view. The red dots in A indicate the concave edges of the hexagonal donut-shaped PCNA trimer. The flat edge corresponds to the IDCL of the PCNA, which serves as the universal binding platform for various PCNA-binding proteins. The DIE motif in the exonuclease active site and the DTDG motif in the polymerase active site in B are depicted by orange and green sphere models, respectively. The DNA is shown by a red ribbon model. The five domains of PolB are shown in different colors (N terminal: yellow, exonuclease: purple, palm: orange, fingers: pale green, and thumb: green). The side view (D) clearly indicates the bidentate interactions (contacts 1 and 2) between Pol B and PCNA. (E) Another side view of the sliced complex. The net-surface presentation corresponds to the complex surface of the lower threshold level. Note that the DNA rod reaches the DNA polymerase at this level and that the DNA duplex is substantially tilted relative to the PCNA ring (13°), without any apparent contacts between PCNA and DNA. The 141DIETLYHE residues in the Exo I motif are depicted by the orange sphere model. (F) Molecular contact through the PIP box between PolB and PCNA. Glu171 of the PCNA, corresponding to the switching hook, is marked. (G) The second PolB-PCNA contact, found in the present work. E171 of the PCNA subunit adjacent to the PIP-motif bound subunit participates in this second contact site.
Fitting of the PolB and PCNA Crystal Structures.
The crystal structure of PfuPCNA (21) fit nicely into the hexagonal ring of the lower layer (Fig. 2A and Fig. S2). This ring exhibited 3-fold symmetry, rather than genuine 6-fold symmetry, as characterized by the alternating flat and concave edges, corresponding to the interdomain connecting loop (IDCL) and the intersubunit interface, respectively. The IDCL of PCNA is known to serve as the universal platform for PCNA-binding proteins (16), and hence the observation of 3-fold symmetry in the map was important to uniquely place the trimeric PfuPCNA crystal structure in the hexagonal ring, and also to validate the reliability of the docking of the PolB model into the upper layer. In the EM map, the upper PolB region entirely covers the upper surface of PCNA (Fig. 2). The docked atomic model of PfuPolB shows that the origin (i.e., the N terminus) of the C-terminal loop with the PIP-box motif lies close to the C-terminal and IDCL regions of the PCNA, and hence the PIP box could interact with PCNA, when considering the flexibility of this loop. Intriguingly, besides the PolB-PCNA contact at the authentic PIP motif (contact 1), another contact was found between the N terminus of the palm domain of PolB and the adjacent PCNA subunit (contact 2; Fig. 2 D and E). In comparison with the model predicted from the bacteriophage replication system (7), the PolB molecule in our complex structure adopts a rather horizontal configuration and lies on top of the PCNA ring. The stability of this configuration, which has never been observed before, could be attributable to the second contact.
The density of the dsDNA rod encircled by PCNA becomes faint, as it approaches the inner wall of the PolB. This may reflect the general tendency of the poor visualization of DNA molecules complexed with proteins by negative staining. However, the DNA-PolB contact was partially visualized by lowering the threshold level of the map (Fig. 2E, net-surface presentation). The contact between the rod density and the PolB lies close to the exonuclease active site (Fig. 2 B and E), thus indicating that the complex structure is in the editing mode. It should be noted that the exonuclease had to be inactivated, by replacing the two catalytic aspartate residues in the exonuclease active site by alanine, in order to crystallize the RB69 DNA polymerase complex with DNA in the polymerizing mode (7). By contrast, the crystals of the complex in the editing mode were prepared using the wild-type enzyme (1). Therefore, the wild-type PfuPolB in our ternary complex should correspond to the editing mode.
Visualization of the priDNA Termini by the Streptavidin Labeling.
The bacteriophage RB69 DNA polymerase-DNA complexes were obtained using primed DNAs with a short ssDNA region (1, 7), and hence the entrance of the ssDNA in the complex has not been identified yet. Our initial attempt also failed to visualize the ssDNA region in the EM map, in spite of the sufficient length of the primed DNA. To identify the termini and the orientation of the DNA in the complex, we biotinylated the DNA termini and labeled them with streptavidin (SA) (Fig. 3A). The SA molecule at the dsDNA terminus was successfully visualized as a bulky density below PCNA, thus confirming that the primer DNA was assembled within the complex in the correct orientation, i.e., the nascent dsDNA was on the PCNA side and the template ssDNA was on the polymerase side (Fig. 3B and Fig. S3A). Meanwhile, the complex with the SA label on the ssDNA terminus revealed that the 5′ terminus of the template DNA protrudes from the side gap between PolB and PCNA (Fig. 3 C and D and Fig. S3B). This view is in good agreement with the ssDNA entrance proposed from the crystal structure of the RB69 complex (1).
Fig. 3.
SA-labeled complex. (A) Schematic views of the complexes SA-labeled in the double-stranded (5′ end of primer) terminus (Left), and single-stranded (5′ end of template) terminus (Right) of the DNA, respectively. (B) EM map of the complex with the SA label at the double-stranded terminus of the primed DNA (magenta surface). Yellow surface: unlabeled complex. (C) EM map of the complex (cyan surface) with the SA-labeled single-stranded terminus. The yellow map depicts the unlabeled complex. The bulk density derived from the SA labels provides information about the primed DNA path. (D) Top view of the single-stranded terminus-labeled complex. The DIE motif in the exonuclease active site is depicted by the orange sphere model. The domains of PolB are indicated with the same color code as those in Fig. 2.
The Conserved Glutamate at the Second Contact.
In the PolB-PCNA-DNA ternary complex, the relative orientations between each component are fixed through the DNA–protein interactions, the authentic PIP-motif dependent interaction, and the second contact between PolB and PCNA (Fig. 2). The docked PCNA crystal structure shows that the outermost turn with E171 is located at the second contact (Fig. 2G). In the previously reported PfuPolB-PCNA crystal structure, this turn was referred to as a “switching hook,” and E171 was proposed to play a key role in switching between the editing and polymerizing modes (10). This switching hook was considered to function in the vicinity of the PIP-box contact site on the same PCNA subunit. By contrast, the second contact in the present ternary complex appears to be formed between the switching hook on the adjacent PCNA subunit and the arginine cluster (379RRLR; R379 is the closest residue) on the short helix connected to the PolB palm domain. The sequence alignment around the second contact site shows that glutamate (or aspartate) at the switching hook is conserved among the clamp proteins from various species (Fig. S4). These findings support the proposal that the second contact, together with the PCNA E171-PolB R706 switch in the first contact, participates in switching between the editing and the polymerizing modes. Intriguingly, this glutamate (or aspartate) is missing in the human Rad1 subunit, which constitutes the Rad9-Hus1-Rad1 clamp involved in translesion synthesis lacking the editing activity.
The Switching Mechanism.
We previously proposed a model where E171, which is close to the authentic PIP-box contact, is detached from R706 of PfuPolB upon the transition from the polymerizing mode to the editing mode (10). This switching model is essentially consistent with our current model of the editing mode of PolB. In fact, the E171 residue on the same subunit is quite far from R706 of PolB (Fig. 2F). Conversely, the corresponding acidic residue on the adjacent subunit contacts R379 of PolB (Fig. 2G). Notably, although the arginine cluster was not strictly conserved as a sequence motif, basic residues were frequently found in the corresponding regions of other archaeal PolBs (Fig. S5).
To verify the proposed model, in which the arginine cluster acts on the switching mechanisms between polymerizing and editing modes, we prepared the three PolB mutants with the substitutions of R379E, R379/382E, and R379/380/382E and compared their PCNA-dependent exonuclease activities with that of the wild-type PolB. As shown in Fig. 4, the PCNA-dependent exonuclease activity weakened with the increase of the substitutions in the arginine cluster, whereas the difference was not observed in the absence of PCNA (see also Fig. S6). This result is consistent with the notion that the strength of the second contact between PolB and PCNA, in fact, affects the stability of the exonuclease mode. Intriguingly, single particle EM of the complex, composed of the R379E mutant, exhibited a structural heterogeneity, suggesting that the inhibition of the second contact may fail to retain the stable architecture (Fig. S6).
Fig. 4.
PCNA-dependent exonuclease activity affected by the mutations at the arginine cluster (379RRLR) in the palm domain of PfuPolB. (A) The exonuclease activities of the single (R379E), double (R379/382E), and triple (R379/380/382E) PolB mutants were assayed, using linearized pGEM-T plasmid substrate, and were compared with that of the wild-type. The size marker DNA (New England Biolabs) was loaded on the left. (B) The residual substrate DNA bands, indicated by a thick arrow in A, were quantified to calculate cleavage efficiencies for each reaction, and the results were shown as % cleavage.
The swinging motion (40°) of the dsDNA in the RB69 polymerase between the two modes (7) suggests that an alteration of the PCNA orientation should be accompanied, thereby affecting the second contact (Fig. 5). In order to simulate the configuration change of the ternary complex, especially that involving the second contact, we constructed detailed atomic models of the ternary complex in the editing and polymerizing mode (Fig. 5 and Fig. S7; see also Table S1), by superposing and assembling the known crystal structures (detailed description in SI Material and Methods). In the model of the polymerizing mode, the PolB is apparently rising up, in comparison with that in our EM map (Figs. 2 and 5), thus disrupting the second contact observed in the editing complex. Indeed, we could not model the ternary complex in the polymerizing mode without a clash between the DNA and PCNA or the disruption of this contact. Taken together, we presume that each E171 residue on the two adjacent PCNA subunits would play key roles in switching between the two modes, in which the interactions with the PolB counterparts are reversed (Fig. 5).
Fig. 5.
Switching mechanism of the PolB-PCNA-DNA complex between the polymerizing and editing modes. (A) The complex in the editing mode, shown in a ribbon representation. PolB and PCNA are colored blue and green, respectively. The template and primer strands are depicted by orange and red ribbons. The two E171 residues in the PCNA switching hook are shown by red spheres. R706 in the thumb domain and R379 in the palm domain of PfuPolB are represented by yellow spheres. The DIE motif in the exonuclease active site and the DTDG motif in the polymerase active site are represented by orange and green spheres, respectively. Note that while the switching hook near the PIP-box contact is detached from R706, the one on the adjacent PCNA is in contact with R379 of the PolB, stabilizing the closed configuration of the complex. (B) The complex in the polymerizing mode. The interactions between the PCNA hooks and the PolB counterparts are reversed, relative to those in the editing mode.
Multiple Interactions Between PCNA and Replicative Factors.
The primary functional role of PCNA is considered to be the tethering of multiple protein factors on DNA. Consistently, the crystal structure of the human Fen1-PCNA complex, harboring three Fen1 proteins bound on a PCNA ring (22), led to the “tool-belt model” (23). In fact, Fen1, DNA ligase, and DNA polymerase can bind simultaneously to the Sulfolobus solfataricus PCNA heterotrimer (24). Together with the previously reported ternary complex of DNA ligase-PCNA-DNA (18), in which the second contact between the PCNA and the protein factor was observed as well, the present structure suggests another important functional role of PCNA. Notably, these two complexes, with DNA and tethered protein factors bound simultaneously, should represent the structural views that are closest to the functional states.
PCNA may frequently provide a second contact site, which resides on a different subunit from that tethering the protein factor through the PIP box. The PIP-box motifs are generally located at either the N or C terminus or in the flexible loops of PCNA-binding proteins (25) and thus are considered to function mainly for tethering the factors to PCNA. Meanwhile, the second contact seems to fix each factor in its optimal orientation, thereby modulating the regulatory activities. These extra contacts may function to block the access of other factors to PCNA, so that the order of the incoming factors can be correctly maintained during the DNA transaction. In other words, the extra contact might function as an intrinsic checkpoint of the protein factors during the sequential catalytic reactions required for high-fidelity genome replication. Thus, we presume that similar extra interactions, besides the major PIP-box contact, would broadly exist between PCNA and the various proteins involved in DNA transaction processes.
Materials and Methods
Purification of the PfuPolB and PfuPCNA Proteins.
PfuPolB and PfuPCNA were purified basically as described previously (21, 26, 27), using Escherichia coli containing the plasmids for overproducing these proteins. The heat-treated supernatant of the cell extracts of these E. coli were treated with polyethyleneimine to precipitate nucleic acids, and the proteins in the supernatants were precipitated by adding ammonium sulfate (80% saturation) and were subjected to column chromatographies with various principles, including anion exchange, cation exchange, and affinities by hydrophobicity and a specific ligand. (See SI Materials and Methods for detailed description.) Amino acid substitutions were introduced into the pol gene on the expression plasmid at R379, R380, and R382 by PCR-mediated mutagenesis using QuikChange site-directed mutagenesis kit (Stratagene). The sequences of the primers used in the mutagenesis are available upon request.
Exonuclease Assay.
The in vitro exonuclease activities of the wild-type and mutant PfuPolBs at 70 °C for 15 min, in the presence and absence of PfuPCNA, were measured as basically described previously (10). The pGEM-T plasmid (Promega) was used as DNA substrate after linearization by NcoI digestion. The reaction products were separated by 0.8% agarose gel electrophoresis, followed by staining with SYBR Green I (Invitrogen). The gel image was visualized by Typhoon Trio+ (GE Healthcare). The residual substrate DNA bands were quantified to calculate cleavage efficiencies for each reaction.
Electron microscopy and single particle image analysis.
The purified PfuPolB (10 μM) and PfuPCNA (30 μM) were mixed with synthetic DNA (10 μM of pri25/49 or pri30/40, the sequences are described in SI Materials and Methods), and incubated in 34 μL of reconstruction buffer, containing 50 mM Tris-HCl (pH 8.0) and 5 mM MgCl2, at 37 °C for 15 min. The mixture was subsequently loaded onto a gel filtration column (Superdex 200 PC 3.2-30, GE Healthcare) equilibrated with the same buffer (Fig. S1A).
A 3-μL aliquot of sample solution was applied to a copper grid supporting a continuous thin-carbon film, left for 1 min, and then stained with three drops of 2% uranyl acetate. Images of molecules were recorded by a BioScan CCD camera (Gatan) with a pixel size of 3.1 Å/pixel, using a JEM1010 electron microscope (JEOL) operated at an accelerating voltage of 100 kV. The magnification of the images was calibrated using tobacco mosaic virus as a reference sample. A minimum dose system (MDS) was used to reduce the electron radiation damage of the sample.
A total of 18,897 images of the PfuPolB-PfuPCNA-DNA complex were selected, using the BOXER program in EMAN (28). Alignment, classification, and averaging of the particle images were performed using the image analysis tools in IMAGIC (29). The initial 3D model was obtained using the common-line method. Subsequent iterative alignment and 3D reconstruction were performed using the REFINE routine in EMAN. The resolution of the final map was estimated by means of the Fourier shell correlation (FSC) method, using the 0.5 FSC criteria. The visualization of the EM map and the fitting of the crystal structures into the map were performed with the Chimera software (30). The crystal structures of PfuPolB and PfuPCNA were docked into the map to obtain the initial atomic models, using the “Fit Model in Map” tool in Chimera (see SI Materials and Methods for detailed description).
Single Particle Analysis of Labeled Complexes.
The pri25/40 (SI Materials and Methods) with the 5′ biotinylated primer strand was used to label the ds terminus of the complex. The ternary complex of PfuPolB-PfuPCNA-biotinylated DNA was reconstructed under the same conditions as that for the nonlabeled complex. Before gel filtration, a twofold excess of SA was added to the complex solution, and the mixture was further incubated for 15 min.
The pri19/40 (SI Materials and Methods) with the 5′ biotinylated template strand was used to label the ss terminus of the complex, and the SA-labeled complex was purified by the same method. EM images of the SA-labeled complex were recorded with a pixel size of 5.1 Å/pixel. The total numbers of boxed images used for single particle analyses of the ds-terminus-labeled and ss-terminus-labeled complexes were 3,645 and 4,833, respectively.
Construction of the Atomic Model of the DNA Polymerase B-PCNA-DNA Complex.
The atomic models of the PfuPolB-PfuPCNA-DNA complex were constructed from the known structures in the Protein Data Bank (PDB) (31) listed in Table S1. Modeling was executed as shown in Fig. S7, using the in-house program SEARCHCMP. The homologous protein structures of PfuPolB and PfuPCNA were retrieved from the PDB. The structures were assembled through various combinations and orders of superposition among the known crystal structures, and a cluster of probable models was generated. The models were visually inspected, and it was found that the models, fitted into the experimental EM density, were generated. (See SI Materials and Methods for detailed step-by-step description.) The SANDER module of the AMBER 9 program suite (32) was used to refine the initial models of the editing and the polymerizing modes by energy minimization.
Supplementary Material
Acknowledgments.
K. Mayanagi, S.K., Y.I., M.S., and T.S. were supported by BIRD-JST. H.N. was partly supported by a grant from the Genome Network Project of the Ministry of Education, Culture, Sports, Science, and Technology of Japan and also by a research grant endorsed by the New Energy and Industrial Technology Development Organization. Y.I. was supported by a grant from the Human Frontier Science Program and a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. K. Morikawa was supported by the Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (CREST-JST).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: Data have been deposited in the Electron Microscopy Data Bank, www.EMDataBank.org (accession no. EMD-5220).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010933108/-/DCSupplemental.
References
- 1.Shamoo Y, Steitz TA. Building a replisome from interacting pieces: Sliding clamp complexed to a peptide from DNA polymerase and a polymerase editing complex. Cell. 1999;99:155–166. doi: 10.1016/s0092-8674(00)81647-5. [DOI] [PubMed] [Google Scholar]
- 2.Hopfner KP, et al. Crystal structure of a thermostable type B DNA polymerase from Thermococcus gorgonarius. Proc Natl Acad Sci USA. 1999;96:3600–3605. doi: 10.1073/pnas.96.7.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao Y, et al. Crystal structure of an archaebacterial DNA polymerase. Structure. 1999;7:1189–1199. doi: 10.1016/s0969-2126(00)80053-2. [DOI] [PubMed] [Google Scholar]
- 4.Hashimoto H, et al. Crystal structure of DNA polymerase from hyperthermophilic archaeon Pyrococcus kodakaraensis KOD1. J Mol Biol. 2001;306:469–477. doi: 10.1006/jmbi.2000.4403. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez AC, Park HW, Mao C, Beese LS. Crystal structure of a pol alpha family DNA polymerase from the hyperthermophilic archaeon Thermococcus sp.9 degrees N-7. J Mol Biol. 2000;299:447–462. doi: 10.1006/jmbi.2000.3728. [DOI] [PubMed] [Google Scholar]
- 6.Kim SW, Kim DU, Kim JK, Kang LW, Cho HS. Crystal structure of Pfu, the high fidelity DNA polymerase from Pyrococcus furiosus. Int J Biol Macromol. 2008;42:356–361. doi: 10.1016/j.ijbiomac.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Franklin MC, Wang J, Steitz TA. Structure of the replicating complex of a pol alpha family DNA polymerase. Cell. 2001;105:657–667. doi: 10.1016/s0092-8674(01)00367-1. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, et al. Crystal structure of a pol alpha family replication DNA polymerase from bacteriophage RB69. Cell. 1997;89:1087–1099. doi: 10.1016/s0092-8674(00)80296-2. [DOI] [PubMed] [Google Scholar]
- 9.Firbank SJ, Wardle J, Heslop P, Lewis RJ, Connolly BA. Uracil recognition in archaeal DNA polymerases captured by X-ray crystallography. J Mol Biol. 2008;381:529–539. doi: 10.1016/j.jmb.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Nishida H, et al. Structural determinant for switching between the polymerase and exonuclease modes in the PCNA-replicative DNA polymerase complex. Proc Natl Acad Sci USA. 2009;106:20693–20698. doi: 10.1073/pnas.0907780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunkel TA. DNA replication fidelity. J Biol Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 12.Patel PH, Loeb LA. Getting a grip on how DNA polymerases function. Nat Struct Biol. 2001;8:656–659. doi: 10.1038/90344. [DOI] [PubMed] [Google Scholar]
- 13.Waga S, Stillman B. The DNA replication fork in eukaryotic cells. Annu Rev Biochem. 1998;67:721–751. doi: 10.1146/annurev.biochem.67.1.721. [DOI] [PubMed] [Google Scholar]
- 14.Maga G, Hubscher U. Proliferating cell nuclear antigen (PCNA): A dancer with many partners. J Cell Sci. 2003;116:3051–3060. doi: 10.1242/jcs.00653. [DOI] [PubMed] [Google Scholar]
- 15.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Warbrick E. PCNA binding through a conserved motif. Bioessays. 1998;20:195–199. doi: 10.1002/(SICI)1521-1878(199803)20:3<195::AID-BIES2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 17.Miyata T, et al. Open clamp structure in the clamp-loading complex visualized by electron microscopic image analysis. Proc Natl Acad Sci USA. 2005;102:13795–13800. doi: 10.1073/pnas.0506447102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayanagi K, et al. Mechanism of replication machinery assembly as revealed by the DNA ligase-PCNA-DNA complex architecture. Proc Natl Acad Sci USA. 2009;106:4647–4652. doi: 10.1073/pnas.0811196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgescu RE, et al. Structure of a sliding clamp on DNA. Cell. 2008;132:43–54. doi: 10.1016/j.cell.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McNally R, Bowman GD, Goedken ER, O'Donnell M, Kuriyan J. Analysis of the role of PCNA-DNA contacts during clamp loading. BMC Struct Biol. 2010;10:3. doi: 10.1186/1472-6807-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsumiya S, Ishino Y, Morikawa K. Crystal structure of an archaeal DNA sliding clamp: Proliferating cell nuclear antigen from Pyrococcus furiosus. Protein Sci. 2001;10:17–23. doi: 10.1110/ps.36401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakurai S, et al. Structural basis for recruitment of human flap endonuclease 1 to PCNA. EMBO J. 2005;24:683–693. doi: 10.1038/sj.emboj.7600519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barry ER, Bell SD. DNA replication in the archaea. Microbiol Mol Biol Rev. 2006;70:876–887. doi: 10.1128/MMBR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dionne I, Nookala RK, Jackson SP, Doherty AJ, Bell SD. A heterotrimeric PCNA in the hyperthermophilic archaeon Sulfolobus solfataricus. Mol Cell. 2003;11:275–282. doi: 10.1016/s1097-2765(02)00824-9. [DOI] [PubMed] [Google Scholar]
- 25.Warbrick E. The puzzle of PCNA’s many partners. Bioessays. 2000;22:997–1006. doi: 10.1002/1521-1878(200011)22:11<997::AID-BIES6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 26.Komori K, Ishino Y. Functional interdependence of DNA polymerizing and 3′ → 5′ exonucleolytic activities in Pyrococcus furiosus DNA polymerase I. Protein Eng. 2000;13:41–47. doi: 10.1093/protein/13.1.41. [DOI] [PubMed] [Google Scholar]
- 27.Cann IK, et al. Functional interactions of a homolog of proliferating cell nuclear antigen with DNA polymerases in Archaea. J Bacteriol. 1999;181:6591–6599. doi: 10.1128/jb.181.21.6591-6599.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ludtke SJ, Baldwin PR, Chiu W. EMAN: Semiautomated software for high-resolution single-particle reconstructions. J Struct Biol. 1999;128:82–97. doi: 10.1006/jsbi.1999.4174. [DOI] [PubMed] [Google Scholar]
- 29.van Heel M, Harauz G, Orlova EV, Schmidt R, Schatz M. A new generation of the IMAGIC image processing system. J Struct Biol. 1996;116:17–24. doi: 10.1006/jsbi.1996.0004. [DOI] [PubMed] [Google Scholar]
- 30.Pettersen EF, et al. UCSF Chimera—A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 31.Berman H, Henrick K, Nakamura H. Announcing the worldwide Protein Data Bank. Nat Struct Biol. 2003;10:980. doi: 10.1038/nsb1203-980. [DOI] [PubMed] [Google Scholar]
- 32.Duan Y, et al. A point-charge force field for molecular mechanics simulations of proteins based on condensed-phase quantum mechanical calculations. J Comput Chem. 2003;24:1999–2012. doi: 10.1002/jcc.10349. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.