Abstract
The immune system includes a subpopulation of CD8+ T cells equipped to inhibit the expansion of follicular T helper (TFH) cells, resulting in suppression of autoantibody production and associated lupus-like disease. These CD8+ T regulatory (Treg) cells recognize Qa-1/peptide complexes on target TFH cells and depend on the IL-15 cytokine for development and function. Here we show that these CD8+ Treg cells express a triad of surface receptors—CD44, CD122, and the class I MHC receptor Ly49—and account for <5% of CD8+ T cells. Moreover, the development of systemic lupus erythematosus-like disease in B6-Yaa mutant mice is associated with a pronounced defect in CD8+ Treg cell activity, suggesting that this regulatory subset may represent an effective therapeutic approach to systemic lupus erythematosus-like autoimmune disease.
Keywords: Rag2-deficient mice, cell transfer, HLA-E, KIR, memory CD8 cells
Achieving a balance between induction of protective immunity against pathogens and maintenance of self-tolerance is a central feature of the adaptive immune system. Although negative selection in the thymus removes the majority of clones that express T-cell receptors (TCRs) with high affinity for self-peptide MHC products, a significant fraction of autoreactive T cells is spared, and these can differentiate into effector cells in the context of inflammatory stimuli (1–3). Pathological responses to self antigens are generally held in check by aberrant T-cell activation leading to cellular elimination or inactivation (4, 5). However, these cell-intrinsic mechanisms may not be sufficient to prevent the development of autoimmune disorders (6, 7).
Self tolerance also depends on inhibitory interactions between effector T cells and regulatory or suppressive cells (8). A subpopulation of CD8+ T cells regulates the activity of CD4+ follicular helper T (TFH) cells through recognition of Qa-1 expressed at the surface of TFH cells (9). Previous analysis of the surface phenotype of these Qa-1-restricted CD8+ T regulatory (Treg) cells (hereafter CD8+ Treg) indicated that they express high levels of CD44 and CD122 and lower levels of CXCR5 and ICOSL. Expression of CD122 at the surface of these cells is in accord with the cells’ dependence on IL-15 for development and function (9). Here we show that CD8+ Treg cells express, in addition to CD44 and CD122, the class I MHC receptor Ly49, which confers enhanced responsiveness to IL-15 (10–13). These findings indicate that CD44+CD122+Ly49+ CD8 T cells, which represent 3–5% of CD8 T cells, account for virtually all of the Qa-1–restricted suppressive activity invested in CD8 cells and suggest that CD8+ Treg cells display several features of natural killer (NK) cells of the innate immune system.
We also investigated the contribution of CD8+ Tregs to a well-defined murine model of systemic lupus erythematosus (SLE). Qa-1 knock-in mice (D227K) that express an amino acid exchange mutation that impairs CD8+ Treg activity develop a lupus-like autoimmune disorder characterized by dysregulated TFH cells, tissue-specific autoantibodies, and severe glomerulonephritis (9). This constellation of pathological changes is reminiscent of the lupus-like autoimmune syndrome displayed by BXSB-Yaa mice as well as the C57BL/6 (B6)-Yaa substrain (14, 15). The finding that β2 microglobulin deficiency and IL-15 receptor deficiency exacerbate BXSB-Yaa disease suggests that development of this disorder might reflect defective CD8+ Treg cell activity (14). We found that B6-Yaa mice have increased numbers of TFH and germinal center (GC) B cells at an early age, and that CD8+ Tregs from these mice are unable to suppress WT CD4 T cells in adoptive hosts. We examined the possibility that defects in the development of CD8+ Tregs contribute to disease pathogenesis in this model of lupus.
Results
CD8+ Treg Cells Depend on IL-15 for Acquisition of Suppressive Activity.
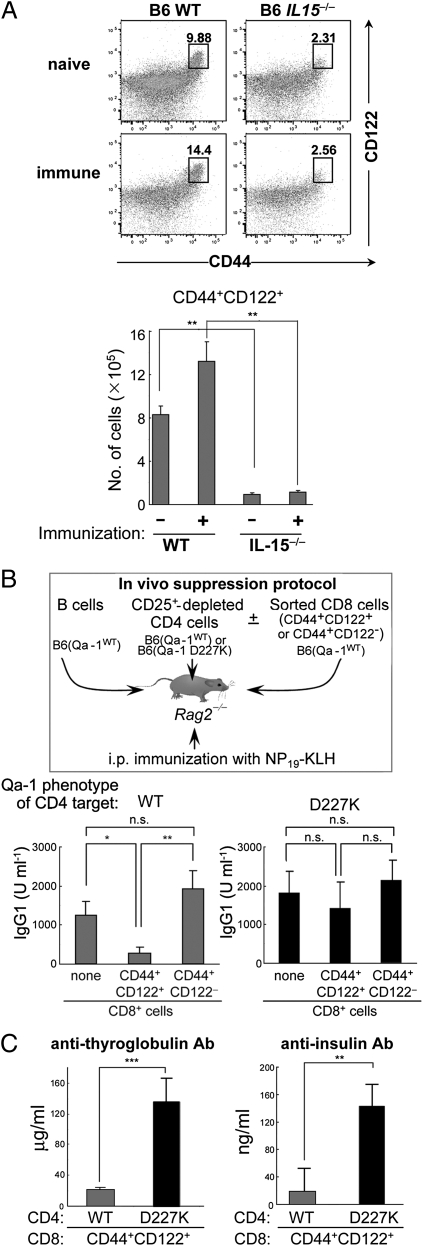
The CD44+ CD8+ subpopulation of T cells is divisible into two subsets according to expression of CD122, a component of the IL-15 receptor (Fig. 1A). Analysis of the CD44+CD122+ fraction of CD8+ T cells from C57BL/6 (B6; WT) and B6(IL-15−/−) mice revealed fivefold more CD44+CD122+ CD8+ T cells in the WT mice. Immunization of B6(WT) mice, but not B6(IL-15−/−) mice, with KLH/CFA resulted in a ∼50% increase in CD44+CD122+ CD8+ T cells (Fig. 1). Moreover, although ∼2–3% of the CD44+ CD8+ cells expressed CD122 in the IL-15−/− mice, the expression level was significantly reduced (Fig. 1A).
Fig. 1.
Qa-1–dependent suppression of Ab response by CD44+CD122+ CD8 T cells. (A) The percentage and number of CD44+CD122+ CD8 T cells from naïve and KLH/CFA-immunized (day 8) WT or IL-15−/− mice. FACS profiles after gating on CD3+CD8+ cells are shown. (B) Ab response after transfer of B, CD4, and CD8 cells into Rag2−/− mice. Here 2 × 106 WT naïve B cells were transferred along with 1 × 106 CD25+ depleted CD4 cells from B6.Qa-1(WT) or B6.Qa-1(D227K) mice into Rag2−/− hosts. CD44+CD122+ or CD44+CD122− CD8+ T cells isolated from KLH/CFA immunized WT B6 mice were also transferred into these Rag2−/− hosts before recipients were immunized i.p. with 100 μg of NP19-KLH in CFA. At day 10, mice were challenged i.p. with 50 μg of NP19-KLH in IFA, and NP-specific Ab responses were measured by ELISA 7 d later. (C) Defective inhibition by CD8 Treg cells leads to autoantibody generation. Anti-thyroglobulin and anti-insulin Abs generated in Rag2−/− recipients were measured by ELISA at day 50. Error bars denote mean ± SEM.
These data suggest that defective regulatory activity of CD44+ CD8+ T cells from IL-15−/− donors might reflect insufficient numbers of CD122hiCD44+ CD8 T cells. Consequently, we evaluated the Qa-1–dependent suppressive activity of these cells. For this, CD44+CD122+ CD8+ cells (>98% purity) and CD44+CD122− CD8+ cells (>96% purity) were sorted from B6(WT) mice before transfer of equal numbers into B6.Rag2−/− hosts along with (CD25−) CD4 cells from Qa-1 (WT) or Qa-1 (D227K) donors and B cells. After challenge with NP19-KLH, CD44+CD122+ CD8 cells, but not CD44+CD122− CD8 cells, displayed Qa-1–restricted suppression of NP-specific Ab responses (Fig. 1B).
We then asked whether CD44+CD122+ CD8+ Treg cells were also responsible for the inhibition of autoantibody responses. We found that the Qa-1 point mutation D227K that disrupts the inhibitory interaction between CD8+ Tregs and Qa-1+ target TH cells is accompanied by a burst of autoantibody to thyroglobulin and insulin (Fig. 1C).
Expression of Inhibitory Ly49 Receptors by CD8+ Treg Cells.
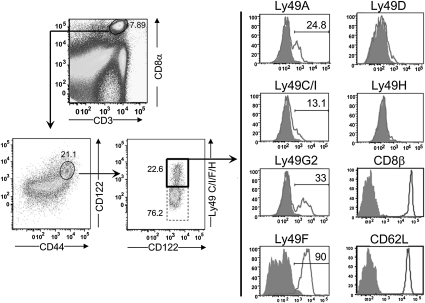
To accurately define the subpopulation of CD8 T cells programmed to express Qa-1–restricted suppression in the CD44+CD122+ CD8 T-cell population, we analyzed a panel of markers expressed by CD8 effector and memory cells. Although CD44+CD122+ CD8 T cells expressed Ly6C, CD200, NKG2D, and VLA-4 (data not shown), these markers did not allow enrichment of CD8 Tregs. However, CD44+CD122+ CD8 T cells can be further dissected into two subgroups according to the expression of Ly49, a family of genes that encode both inhibitory and activating receptors associated with enhanced responsiveness to IL-15 in vitro (11). Ly49+ CD8 cells also depend on CD4+ T cells for age-dependent expansion in vivo (10, 12).
After immunization of WT B6 mice with KLH/CFA, ∼25% of CD8+ (CD44+CD122+) T cells were found to also express Ly49 (detected by anti-Ly49C/I/F/H clone 4B11). This CD44+CD122+Ly49+ subset expressed CD8αβ+ but not CD8αα, as well as high levels of CD62L (Fig. 2). Analysis of the Ly49 gene family expressed by CD44+ CD8 cells revealed that this subset expressed the following inhibitory receptors: Ly49A (24.8%), Ly49C/I (13.1%), Ly49F (90%), and Ly49G2 (33%). These cells did not express activating Ly49 receptors (Ly49D and H) at detectable levels, however (Fig. 2).
Fig. 2.
Ly49 expression by CD8+ Treg cells after immunization. WT B6 mice were immunized i.p. with 100 μg of KLH in CFA, and 10 d later the surface expression of CD44, CD122, Ly49 subtypes, CD8β, and CD62L was analyzed. Staining of Ly49+ cells was done using the following Abs: Ly49C/I/F/H (clone 4B11), Ly49C/I (clone 5E6), Ly49A (clone A1), and Ly49G2 (clone eBio4D11). The numbers represent the percentage of cells expressing each surface protein.
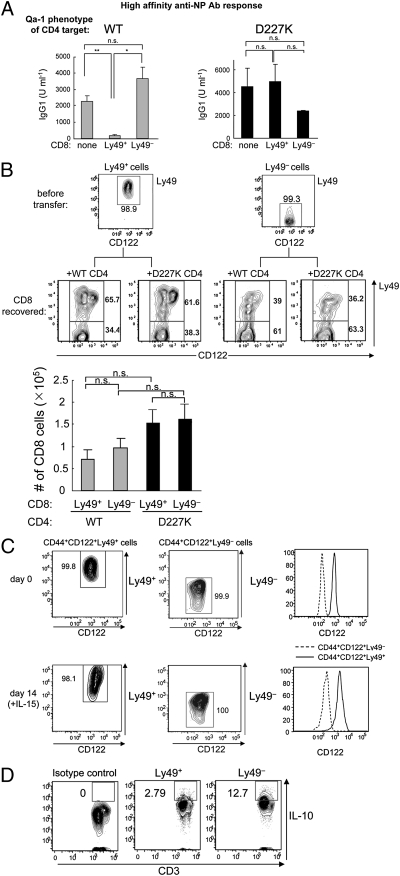
In vivo suppression assays revealed that inhibitory Ly49 receptor expression marked CD44+CD122+ CD8 cells that mediated Qa-1–restricted regulation, because sorted CD44+CD122+Ly49+ CD8 T cells, but not CD44+CD122+Ly49− CD8 T cells, efficiently suppressed Qa-1 WT CD4 T cells, but not Qa-1 mutant (D227K) CD4 T cells (Fig. 3A). Analysis of the fate of Ly49+ CD8 T cells in Rag2−/− hosts at 3 wk after transfer showed that 2/3 of the cell population maintained surface expression of Ly49 and was not affected by the Qa-1 genotype (WT or Qa-1 D227K) of cotransferred CD4 T cells (Fig. 3B). Thus, the suppressive activity exerted by Ly49+ T cells did not reflect a survival advantage in Rag2−/− hosts (Fig. 3B).
Fig. 3.
CD44+CD122+Ly49+ CD8 T cells account for Qa-1–restricted suppressive activity. (A) Ab response after transfer of B, CD4, and CD8 cells into Rag2−/− hosts. Here 2 × 106 WT naïve B cells were transferred along with 0.5 × 106 CD25+-depleted CD4 cells from B6.Qa-1(WT) or B6.Qa-1(D227K) mice into Rag2−/− hosts. CD44+CD122+Ly49+ CD8+ T cells were isolated from KLH/CFA-immunized WT B6 mice, and 0.15 × 106 cells were transferred into Rag2−/− hosts. Immediately after cell transfer, the Rag2−/− recipients were immunized i.p. with 100 μg of NP19-KLH in CFA. At day 10, mice were challenged i.p. with 50 μg of NP19-KLH in IFA, and high-affinity NP-specific Ab responses were measured by ELISA 7 d after the challenge. Data shown represent mean ± SEM. (B) Phenotype of CD8 T cells in Rag2−/− hosts transferred with either CD44+CD122+Ly49+ or CD44+CD122+Ly49− CD8+ T cells along with WT B cells and CD4 T cells (WT or D227K) were analyzed for surface Ly49 expression 3 wk after cell transfer. (Upper) Numbers shown in the gate represent the percentage of cells. (Lower) Total CD8 T cell numbers recovered from Rag2−/− hosts reconstituted with B, CD4, and CD8 cells from different origins are shown. (C) Phenotypic stability of Ly49+ and Ly49− CD8 T cells in vitro. CD44+CD122+Ly49+ and CD44+CD122+Ly49− CD8 T cells were sorted by FACS (purity >99%). Cells were incubated with 100 ng/mL of IL-15 for 14 d, after which the phenotype was reanalyzed. The percentages of Ly49+ and Ly49− cells and levels of CD122 expression in each cell subset are shown. (D) Expression of IL-10 by Ly49+ and Ly49− CD8 cells. Ly49+ and Ly49− CD8 cells were stimulated with phorbol myristate acetate/ionomycin for 8 h, and the production of IL-10 was measured by intracellular cytokine staining. The percentages of IL-10+ Ly49+ and Ly49− cells are shown. Immunofluorescence of Ly49− cells labeled with isotype control Ab is shown as a negative control.
We also analyzed the phenotypic stability of Ly49 expression in vitro. Ly49+ and Ly49− CD8 cells incubated in vitro for 2 wk in the presence of IL-15 (100 ng/mL) maintained their surface phenotype (Fig. 3C). Moreover, the level of CD122 expression remained greater in Ly49+ cells than in Ly49− cells, whereas the proportions of Ly49C/I+, A+, G2+ and F+ cells remained unchanged, provided that cultures were split every 3 d and IL-15 concentrations were maintained at 100 ng/mL. Under these conditions, CD8+ Treg cells expanded at a remarkably rapid rate in vitro without an obvious change of phenotype. For example, we have used these culture conditions to obtain ∼5 × 106 cells at 2 wk after initiating growth with 1 × 105 peripheral CD8+CD44+Ly49+ cells. Further experiments are needed to determine the ability of these cells to mediate Qa-1–restricted regulatory activity. Consistent with our previous studies demonstrating that anti–IL-10 had no effect on the suppressive activity of CD8+ Tregs (9), here we detected only minimal levels of IL-10 production (∼3%) by Ly49+ CD8 cells even after phorbol myristate acetate/ionomycin stimulation (Fig. 3D); Ly49− CD8 T cells produced higher levels of IL-10 (∼13%).
Although the contribution of inhibitory receptors to the suppressive activity of CD8 Tregs requires further study, these findings suggest the possibility that the development of CD8+ Tregs may depend in part on the engagement of receptors normally expressed by NK cells that inhibit activation after engagement by class I MHC products.
Defective CD8 Treg Cell Function in B6-Yaa Mice May Contribute to the Development of Lupus-Like Disease.
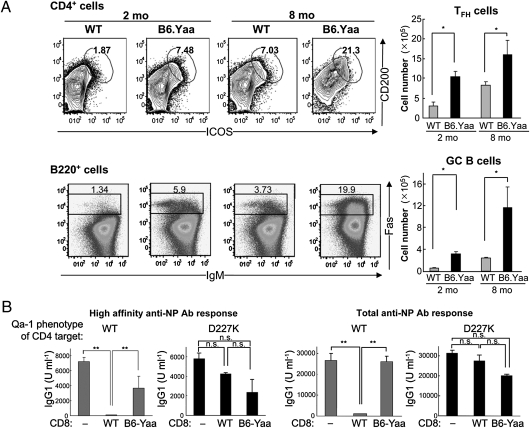
Expression of a point mutation in Qa-1 (Qa-1 D227K) that disrupts the suppressive interaction between CD8+ Tregs and target TFH cells results in the expansion of TFH and GC B cells and development of a lupus-like autoimmune disorder (9). Consequently, we asked whether defective CD8+ Treg activity also may contribute to the pathogenesis of autoimmune disease in Yaa mice, based on findings that the lupus-like autoimmune disease of BXSB-Yaa mice is characterized by increased numbers of TFH and GC B cells (15) and that a deficiency in β2 microglobulin accelerates the onset and intensity of this disease (14). The serious autoimmune disorder characteristic of BXSB-Yaa males is also found when Yaa is transferred to other lupus-prone genetic backgrounds (16–18). When Yaa is transferred onto the B6 background (B6-Yaa) a similar lupus-like autoimmune syndrome also develops, but in a more protracted manner.
Our analysis of B6-Yaa mice revealed that both 2-mo-old and 8-mo-old mice had an approximately threefold increase in both TFH and GC B cells in the spleen, reminiscent of the B6.Qa-1(D227K) phenotype (Fig. 4A). B6-Yaa autoimmune mice demonstrated defective CD8+ Treg activity (Fig. 4B). Thus, although the B6-Yaa mice had at least as many CD44+CD122+Ly49+ CD8+ T cells as did the B6 WT mice at 7 d after KLH/CFA immunization (Fig. S1), these cells lacked suppressive activity, in contrast to CD8+ Treg cells from WT donors, which transferred robust suppression (Fig. 4B). These findings suggest the possibility that TFH cell expansion and associated autoimmunity in B6-Yaa mice reflect defective CD8+ Treg cell function.
Fig. 4.
Expansion of TFH and GC B cells in B6-Yaa mice. (A) Spleen cells from age-matched (2 mo and 8 mo) WT B6 and B6-Yaa mice were stained with CD4, ICOS, and CD200 antibodies for TFH cells and with B220, IgM, and Fas antibodies for GC B cells. (Right) The absolute numbers of TFH and GC B cells are shown. (B) Impaired suppression of Ab response by CD8+ T cells from B6-Yaa mice. WT naïve B cells were transferred along with CD25+-depleted CD4 T cells from Qa-1 (WT) or Qa-1 (D227K) mice into Rag2−/− hosts. FACS-sorted CD44+CD122+ CD8 T cells isolated from KLH/CFA-immunized WT or B6-Yaa mice were transferred into Rag2−/− hosts. Immediately after cell transfer, the Rag2−/− recipients were immunized i.p. with 100 μg of NP19-KLH in CFA and then reimmunized i.p. with 50 μg of NP19-KLH in IFA 20 d later. Secondary Ab responses for high-affinity and total anti-NP IgG1 are shown.
Discussion
Recent analyses of Qa-1 mutant mice have shown that genetic disruption of the inhibitory interaction between CD8+ Tregs and target Qa-1+ TFH cells results in the development of an SLE-like autoimmune disease that is accelerated by viral infection (9). The inhibitory effects of CD8+ Treg cells entails perforin-dependent elimination of target TFH cells, a mechanism that has been shown to be efficiently enhanced by IL-21, the canonical cytokine secreted by activated TFH target cells (19). The data presented herein more precisely define the surface phenotype of CD8+ Tregs based on their expression of inhibitory Ly49 receptors and dependence on the IL-15 cytokine. Taken together, the data show that Qa-1–restricted suppressive activity is invested in a small subpopulation of CD8+ T cells that express CD44, CD122, and inhibitory Ly49 receptors at the surface and is defective in B6-Yaa autoimmune-prone mice.
Further analysis of the surface phenotype of CD8+ Tregs revealed expression of a panel of inhibitory Ly49 receptors—Ly49A, Ly49G2, Ly49C/I, and Ly49F (Fig. 2)—but not Ly49-activating receptors. Although engagement of Ly49 can inhibit CD8 T-cell activation (10), ligation of inhibitory Ly49 molecules also may decrease activation-induced cell death and thereby protect memory CD8 cells from premature elimination (20–24). These considerations suggest that the expression of inhibitory Ly49 receptors by CD8+ Tregs might limit the intensity of early activation but prolong the lifespan in the memory pool. Interestingly, the ligands that bind to the Ly49F receptor, the major Ly49 family member expressed by CD8+ Tregs, remain unidentified. The absence of binding activity of Ly49F to conventional MHC class I molecules has led to speculation that this Ly49 family member might interact with MHC class Ib molecules (25). TCRαβ+CD8αα intestinal intraepithelial lymphocytes also express Ly49 family members, as well as TGF-β and LAG-3, consistent with a potential regulatory function (25). Shared expression of Ly49 inhibitory receptors by TCRαβ+CD8αα and TCRαβ+CD8αβ Treg cells might reflect overlapping developmental or functional properties.
Human CD8 T cells include a small subset of cells that express inhibitory killer cell immunoglobulin-like receptors (KIRs) that are analogous to the murine Ly49 receptor. Both proteins display intracytoplasmic immunoreceptor tyrosine-based inhibitory motifs that deliver inhibitory signals on binding to self MHC class I proteins (26). Human KIR+CD8+αβTCR+ T cells express a memory phenotype, account for virtually all perforin-positive human CD8 cells, and represent ∼4–5% of the CD8+ T-cell pool (27, 29). Moreover, human CD8+ T-cell clones isolated from KIR+CD8αβ+TCR+ T cells recognize HLA-E (the human homolog of Qa-1b) (30–32), consistent with our findings that Ly49+ CD8 T cells in mice exert Qa-1–dependent immune suppression. Further analysis is needed to determine whether this subset of human CD8 T cells mediates HLA-E–dependent suppression of activated human CD4+ T memory helper cells.
We have previously noted that CD44+CD122+ cells also express CXCR5 and ICOSL, as detected by bead-dependent enrichment (9) as well as FACS-dependent cell sorting (Fig. S2). This surface phenotype is of interest because of its potential contribution to the suppressive activity of CD8+ Tregs. CXCR5 expression may allow navigation of CD8+ Tregs into the B/CD4 T-cell follicle, whereas expression of ICOSL by activated emigrant CD8+ Tregs may enhance interaction of CD8+ Treg with ICOS+ TFH cells within the follicular microenvironment. However, antibodies specific for CD44, CD122, and Ly49 are preferable to commercially available anti-CXCR5 and anti-ICOSL antibodies for the rapid and efficient isolation of CD8+ Tregs from heterogeneous cell populations. Thus, sorting of CD8+ T cells based on expression of the CD44/ CD122/ Ly49 protein triad, along with perforin expression, represents the optimal method for isolating CD8+ Treg cells. The expression of Ly49 on CD44+CD122+ cells from nonmutant C57BL/6 mice reliably predicts the expression of Qa-1–dependent suppressive activity.
Definition of this surface phenotype allows a general experimental approach for studies of CD8+ Tregs. The FACS-sorted CD44+CD122+Ly49+ fraction of CD8 cells can be analyzed for suppressive activity in adoptive Rag2−/− hosts or in irradiated B6 (WT) hosts. The most stringent test for Qa-1–restricted suppressive activity by these cells involves a comparison of their ability to regulate CD4 target cells expressing WT Qa-1 or Qa-1(D227K), a mutation that disrupts Qa-1 recognition. Although CD4 cells from Qa-1–deficient donors also may be used as negative controls, in view of the extreme sensitivity of Qa-1−/− CD4 cells to NK lysis (33), adoptive transfer must be carried out in perforin-deficient Rag2−/− hosts.
Finally, we analyzed B6-Yaa mice, which harbor a translocation of ∼20 genes from the X chromosome to the Y chromosome (18, 34); duplication of Tlr7 is largely responsible for the Yaa-associated autoimmune phenotype in males. Although appreciable end-organ pathology does not develop in B6-Yaa mice until age 8–12 mo, we found expansion of TFH and GC B cells and defective CD8+ T regulatory activity by age 2 mo (Fig. 4A). Chronic activation of CD8+ memory cells by activated TLR7hi dendritic cells possibly might support excessive development of cytokine-secreting CD8+ memory cells that displace or override CD8+ Treg cells. Interestingly, the number of CD44+CD122+Ly49+ CD8+ T cells was similar in Qa-1(D227K) mice and WT B6 mice (Fig. S1), indicating that an analysis of surface phenotype must be combined with the identification of intracellular gene products, such as perforin, for complete characterization of CD8+ Treg activity in mutant autoimmune mouse strains.
In summary, we have shown that the IL-15 dependence of Qa-1–restricted CD8+ Treg cells is associated with expression of the triad of CD44+CD122+Ly49+ receptors at the cell surface. Genetic interruption of the interaction between CD8 Tregs and target CD4 T cells results in autoantibody responses by conventional B6 mice, whereas the autoimmune disorder that develops in a murine model of SLE—BXSB-Yaa—reflects, in part, defective CD8+ Treg activity. These findings suggest that manipulation of CD8+ Treg activity might represent an effective therapeutic approach to autoimmune disease.
Methods
Mice.
C57BL/6J (B6), B6-Yaa (Jackson Laboratory), B6.Rag2−/−, B6.IL15−/− (Taconic), and B6.Qa-1(D227K) mice (backcrossed for 11 generations) were housed in pathogen-free conditions. All experiments were performed in compliance with federal laws and institutional guidelines, as approved by Dana-Faber Cancer Institute's Animal Care and Use Committee.
Reagents and Flow Cytometry.
Single-cell suspensions were prepared and maintained in the dark at 4 °C for immunofluorescence analysis, washed in ice-cold FACS buffer (2% FCS and 0.1% NaN3 in PBS), incubated with each antibody for 30 min, and then washed in FACS buffer before analysis. Anti-CD4, anti-CD3, anti-CD8α, anti-B220, anti-CD44, anti-CD62L, anti-Fas, anti-ICOS, anti-IgM, anti-CD200, anti–IL-10 (BD Biosciences), anti-CD8β, anti-Ly49C/I/F/H, anti-Ly49A, Ly-49G2, Ly49C/I, Ly49D, Ly49F, and Ly49H (eBioscience) were used, followed by analysis of cells using a FACSCanto analyzer (BD Biosciences) and FlowJo software (TriStar).
Cell Purification and Adoptive Transfer.
Naïve B cells were isolated from spleens of Qa-1 WT mice using a BD Biosciences B Lymphocyte Enrichment Set. Naïve CD4+CD25− cells were purified from spleens of Qa-1 WT and mutant mice using a BD Biosciences CD4 Cell Enrichment Set and biotinylated anti-CD25 Ab. B cell and CD4 cell purity was >95%. To generate immune CD8 cells, WT B6 mice were immunized i.p. with 100 μg of KLH in CFA, and splenic CD8 cells were obtained 7–10 d later (using a CD8 Cell Enrichment Set; BD Bioscience) and sorted for CD44+CD122+Ly49+ CD8 T cells. These cells were transferred i.v. into Rag2−/− recipients. Immediately after cell transfer, Rag2−/− mice were immunized i.p. with 100 μg of NP19-KLH in CFA, followed 10 d later by reimmunization i.p. with 50 μg of NP19-KLH in IFA.
In Vitro Culture of CD8+ Treg Cells.
CD44+CD122+Ly49+ and CD44+CD122+Ly49− CD8+ T cells were sorted from WT B6 mice immunized with 100 μg KLH/CFA. Cell culture medium (RPMI, 10% FCS, 50 units/ml penicillin, 50 μg/ml streptomycin, 10 mM Hepes buffer, 55 μM 2-mercaptoethanol, 1 mM sodium pyruvate, 2 mM l-glutamine) was supplemented with 100 ng/mL of IL-15. Cells were incubated in 12-well plates and divided when cell density reached 1 × 106/mL.
ELISA.
For detection of NP-specific antibodies, ELISA plates were coated with 0.5 μg/mL of NP4-BSA or 1 μg/mL NP23-BSA (Biosearch Technologies). Serum harvested at 14 d after immunization with NP19-KLH in CFA and reimmunization with NP19-KLH in IFA was used as a standard. Here, a 1:4,000 dilution of this immune serum was defined as 100 U/mL. NP-specific IgG1 Abs were detected by incubating plates with biotinylated anti-mouse IgG1 Ab, followed by streptavidin-peroxidase. For the detection of autoantibodies, porcine thyroglobulin (Sigma-Aldrich) and porcine insulin (Sigma-Aldrich) were used to detect relevant Abs.
Statistics.
Statistical analyses were performed using the Wilcoxon–Mann–Whitney rank-sum test for comparison of two conditions and the Kruskal–Wallis test for comparison of more than two conditions. A P value < 0.05 was considered statistically significant (*P < 0.05; **P < 0.01; ***P < 0.001), as shown in Figs. 1, 3, and 4 and Fig. S2.
Supplementary Material
Acknowledgments
We thank A. Angel for manuscript and figure preparation. This work was supported in part by National Institutes of Health Research Grant AI 037562 and a gift from the Schecter Research Foundation (to H.C.), National Research Service Award Fellowship DFCI/NCI T32 CA070083 (to H.-J.K.), and Alliance for Lupus Research Target Identification in Lupus (to D.C.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018974108/-/DCSupplemental.
References
- 1.Bouneaud C, Kourilsky P, Bousso P. Impact of negative selection on the T cell repertoire reactive to a self-peptide: A large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 2.Goldrath AW, Bevan MJ. Selecting and maintaining a diverse T-cell repertoire. Nature. 1999;402:255–262. doi: 10.1038/46218. [DOI] [PubMed] [Google Scholar]
- 3.Slifka MK, et al. Preferential escape of subdominant CD8+ T cells during negative selection results in an altered antiviral T cell hierarchy. J Immunol. 2003;170:1231–1239. doi: 10.4049/jimmunol.170.3.1231. [DOI] [PubMed] [Google Scholar]
- 4.Martin DA, et al. Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc Natl Acad Sci USA. 1999;96:4552–4557. doi: 10.1073/pnas.96.8.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. doi: 10.1016/1074-7613(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 6.Anderton SM, Radu CG, Lowrey PA, Ward ES, Wraith DC. Negative selection during the peripheral immune response to antigen. J Exp Med. 2001;193:1–11. doi: 10.1084/jem.193.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panoutsakopoulou V, et al. Analysis of the relationship between viral infection and autoimmune disease. Immunity. 2001;15:137–147. doi: 10.1016/s1074-7613(01)00172-8. [DOI] [PubMed] [Google Scholar]
- 8.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Kim HJ, Verbinnen B, Tang X, Lu L, Cantor H. Inhibition of follicular T-helper cells by CD8(+) regulatory T cells is essential for self tolerance. Nature. 2010;467:328–332. doi: 10.1038/nature09370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coles MC, McMahon CW, Takizawa H, Raulet DH. Memory CD8 T lymphocytes express inhibitory MHC-specific Ly49 receptors. Eur J Immunol. 2000;30:236–244. doi: 10.1002/1521-4141(200001)30:1<236::AID-IMMU236>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 11.Judge AD, Zhang X, Fujii H, Surh CD, Sprent J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J Exp Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anfossi N, et al. Expansion and function of CD8+ T cells expressing Ly49 inhibitory receptors specific for MHC class I molecules. J Immunol. 2004;173:3773–3782. doi: 10.4049/jimmunol.173.6.3773. [DOI] [PubMed] [Google Scholar]
- 13.Rifa’i M, Kawamoto Y, Nakashima I, Suzuki H. Essential role of CD8+CD122+ regulatory T cells in the maintenance of T cell homeostasis. J Exp Med. 2004;200:1123–1134. doi: 10.1084/jem.20040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bubier JA, et al. Treatment of BXSB-Yaa mice with IL-21R-Fc fusion protein minimally attenuates systemic lupus erythematosus. Ann NY Acad Sci. 2007;1110:590–601. doi: 10.1196/annals.1423.063. [DOI] [PubMed] [Google Scholar]
- 15.Bubier JA, et al. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci USA. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izui S, Higaki M, Morrow D, Merino R. The Y chromosome from autoimmune BXSB/MpJ mice induces a lupus-like syndrome in (NZW × C57BL/6)F1 male mice, but not in C57BL/6 male mice. Eur J Immunol. 1988;18:911–915. doi: 10.1002/eji.1830180612. [DOI] [PubMed] [Google Scholar]
- 17.Morel L, et al. Genetic reconstitution of systemic lupus erythematosus immunopathology with polycongenic murine strains. Proc Natl Acad Sci USA. 2000;97:6670–6675. doi: 10.1073/pnas.97.12.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 19.Zeng R, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–148. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ugolini S, et al. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat Immunol. 2001;2:430–435. doi: 10.1038/87740. [DOI] [PubMed] [Google Scholar]
- 21.Young NT, Uhrberg M, Phillips JH, Lanier LL, Parham P. Differential expression of leukocyte receptor complex–encoded Ig-like receptors correlates with the transition from effector to memory CTL. J Immunol. 2001;166:3933–3941. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- 22.Roger J, Chalifour A, Lemieux S, Duplay P. Cutting edge: Ly49A inhibits TCR/CD3-induced apoptosis and IL-2 secretion. J Immunol. 2001;167:6–10. doi: 10.4049/jimmunol.167.1.6. [DOI] [PubMed] [Google Scholar]
- 23.Chwae YJ, et al. Molecular mechanism of the activation-induced cell death inhibition mediated by a p70 inhibitory killer cell Ig-like receptor in Jurkat T cells. J Immunol. 2002;169:3726–3735. doi: 10.4049/jimmunol.169.7.3726. [DOI] [PubMed] [Google Scholar]
- 24.Gati A, et al. CD158 receptor controls cytotoxic T-lymphocyte susceptibility to tumor-mediated activation-induced cell death by interfering with Fas signaling. Cancer Res. 2003;63:7475–7482. [PubMed] [Google Scholar]
- 25.Denning TL, et al. Mouse TCRαβ+CD8αα intraepithelial lymphocytes express genes that down-regulate their antigen reactivity and suppress immune responses. J Immunol. 2007;178:4230–4239. doi: 10.4049/jimmunol.178.7.4230. [DOI] [PubMed] [Google Scholar]
- 26.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: Witness of the past, actors of the future. Nat Rev Immunol. 2004;4:190–198. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 27.Mingari MC, et al. Human CD8+ T lymphocyte subsets that express HLA class I–specific inhibitory receptors represent oligoclonally or monoclonally expanded cell populations. Proc Natl Acad Sci USA. 1996;93:12433–12438. doi: 10.1073/pnas.93.22.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Speiser DE, et al. In vivo expression of natural killer cell inhibitory receptors by human melanoma-specific cytolytic T lymphocytes. J Exp Med. 1999;190:775–782. doi: 10.1084/jem.190.6.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anfossi N, Pascal V, Vivier E, Ugolini S. Biology of T memory type 1 cells. Immunol Rev. 2001;181:269–278. doi: 10.1034/j.1600-065x.2001.1810123.x. [DOI] [PubMed] [Google Scholar]
- 30.Pietra G, et al. The analysis of the natural killer–like activity of human cytolytic T lymphocytes revealed HLA-E as a novel target for TCRα/β-mediated recognition. Eur J Immunol. 2001;31:3687–3693. doi: 10.1002/1521-4141(200112)31:12<3687::aid-immu3687>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 31.Moretta L, Romagnani C, Pietra G, Moretta A, Mingari MC. NK-CTLs, a novel HLA-E–restricted T-cell subset. Trends Immunol. 2003;24:136–143. doi: 10.1016/s1471-4906(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 32.Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 33.Lu L, Kim HJ, Werneck MB, Cantor H. Regulation of CD8+ regulatory T cells: Interruption of the NKG2A–Qa-1 interaction allows robust suppressive activity and resolution of autoimmune disease. Proc Natl Acad Sci USA. 2008;105:19420–19425. doi: 10.1073/pnas.0810383105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Subramanian S, et al. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci USA. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.