Abstract
Activated macrophages express high levels of Nrf2, a transcription factor that positively regulates the gene expression of antioxidant and detoxication enzymes. In this study, we examined how Nrf2 contributes to the anti-inflammatory process. As a model system of acute inflammation, we administered carrageenan to induce pleurisy and found that in Nrf2-deficient mice, tissue invasion by neutrophils persisted during inflammation and the recruitment of macrophages was delayed. Using an antibody against 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2), it was observed that macrophages from pleural lavage accumulate 15d-PGJ2. We show that in mouse peritoneal macrophages 15d-PGJ2 can activate Nrf2 by forming adducts with Keap1, resulting in an Nrf2-dependent induction of heme oxygenase 1 and peroxiredoxin I (PrxI) gene expression. Administration of the cyclooxygenase 2 inhibitor NS-398 to mice with carrageenan-induced pleurisy caused persistence of neutrophil recruitment and, in macrophages, attenuated the 15d-PGJ2 accumulation and PrxI expression. Administration of 15d-PGJ2 into the pleural space of NS-398-treated wild-type mice largely counteracted both the decrease in PrxI and persistence of neutrophil recruitment. In contrast, these changes did not occur in the Nrf2-deficient mice. These results demonstrate that Nrf2 regulates the inflammation process downstream of 15d-PGJ2 by orchestrating the recruitment of inflammatory cells and regulating the gene expression within those cells.
When animals are exposed to environmental electrophiles, including xenobiotics, drugs, toxins, and carcinogens, even at nontoxic doses, the expression of a battery of genes that are essential to cellular defense mechanisms is induced. This process of gene induction is mediated by the antioxidant-responsive element (ARE) (31, 32). An increasing number of studies have identified genes regulated by ARE. These include genes encoding the phase II detoxication enzymes, such as glutathione S-transferase and quinone reductase, as well as antioxidative defense enzymes, such as heme oxygenase 1 (HO-1) and enzymes involved in glutathione synthesis (32). The cooperative activity of these enzymes serves to detoxify electrophiles and oxidative stress products.
Nrf2 is a member of the leucine zipper transcription factor family, and its activity is pivotal for the coordinate induction of phase II detoxifying and antioxidative enzymes whose expression is under the regulatory influence of ARE (15, 16, 18, 41). Nrf2 thus contributes to cytoprotection against environmental electrophiles and oxidative stresses (1, 4, 7, 11, 16, 33). Consistent with its assigned role in protection from environmental stress, Nrf2 is highly expressed in detoxication organs, including the gastrointestinal tract, liver, kidney, and lung. In addition, Nrf2 is abundantly expressed in activated macrophages, thyroid glands, and brown adipose tissue, suggesting additional physiological roles beyond detoxication (5).
The inflammatory response requires a coordinated integration of various signaling pathways, including cyclooxygenases (COX), nitric oxide, and cytokines (28, 40). COX enzymes catalyze the conversion of arachidonic acid to prostaglandin H2 (PGH2), from which other prostaglandins (PGs) are derived by the concomitant action of a variety of PG synthetases. Of the COX enzymes, COX-2 is found mainly in inflammatory cells and tissues. COX-2 is found to be upregulated during acute inflammation (37). By producing PGH2, COX-2 promotes the synthesis of PGE2, an important component of the inflammatory cascade that manifests many of the cardinal signs of inflammation (10). Intriguingly, since COX-2 is also expressed in the late phase of inflammation, it is widely accepted that COX-2 is associated with the resolution, as well as the establishment, of the acute inflammatory response (13). However, in contrast to the case during early stages of inflammation, pleural exudates of rats taken during the resolution stage of inflammation were found to contain high concentrations of PGD2 and 15-deoxy-Δ12,14-PGJ2 (15d-PGJ2) with minimal levels of PGE2 expression.
PGs can be divided into two subtypes: conventional PGs and cyclopentenone PGs (cyPGs) (44). Conventional PGs, such as PGE2 and PGD2, bind to cell surface receptors to exert their actions. However, no cell surface receptors have been identified for cyPGs, such as 15d-PGJ2 and PGA2. Rather, cyPGs are actively transported into cells, where they accumulate in nuclei and act as potent repressors of cell growth and inducers of cell differentiation (12, 29). It has been reported that 15d-PGJ2 exerts its anti-inflammatory activity through activation of peroxisome proliferator-activated receptor γ (PPARγ) (21, 34) or by directly inhibiting nuclear factor kappa B (NF-κB) activation by binding covalently to the IκB kinase (36).
Recently, Nrf2 target genes in macrophages were suspected of playing anti-inflammatory roles. For instance, HO-1 exerts anti-inflammatory functions in various systems, including carrageenan-induced pleurisy, through generation of carbon monoxide (30, 43). Furthermore, human peroxiredoxin I (PrxI or PAG) was recently identified as a negative regulator of macrophage migration inhibitory factor (MIF) (22), a crucial factor in the regulation of inflammation (26) and sepsis (35). These observations led us to explore possible roles for Nrf2 in the acute inflammatory response. To this end, we exploited carrageenan-induced pleurisy in mice as a model system. The results of this study reveal that the Nrf2-ARE system regulates the acute inflammation process by orchestrating the recruitment of inflammatory cells. We also found that COX-2 mediates the intracellular accumulation of 15d-PGJ2 and that 15d-PGJ2, which is shown to activate Nrf2, in turn regulates the expression of PrxI and other anti-oxidative stress enzymes in activated inflammatory macrophages.
MATERIALS AND METHODS
RNA blot hybridization analysis.
Total cellular RNAs were extracted from macrophages by use of RNAzol (Tel-Test, Friendswood, Tex.). The RNA samples (10 μg) were electrophoresed and transferred to Zeta-Probe GT membranes (Bio-Rad). The membranes were probed with 32P-labeled cDNA probes as indicated in the figures. β-Actin cDNA was used as a positive control.
RT-PCR analysis.
Total RNAs (1 μg) were reverse transcribed into cDNA and used for a reverse transcription-PCR (RT-PCR) analysis (Qiagen, Hilden, Germany). GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as a positive control. The PCR products were separated in a 1.5% agarose gel, and positive signals were quantified by densitometry analysis after staining with ethidium bromide.
Immunoblotting.
The nuclei of peritoneal macrophages were solubilized with sodium dodecyl sulfate (SDS) sample buffer without loading dye and 2-mercaptoethanol, and protein concentrations were estimated by the bicinchoninic acid protein assay (Pierce, Rockford, Ill.). Proteins were separated by SDS-polyacrylamide gel electrophoresis in the presence of 2-mercaptoethanol and electrotransferred onto Immobilon membranes (Millipore). To detect immunoreactive proteins, we used horseradish peroxidase-conjugated anti-rabbit immunoglobulin G and ECL blotting reagents (Amersham). An anti-Nrf2 antibody was used as previously described (16). Inflammatory cell pellets from pulmonary lavage were lysed by sonication in buffer containing 50 mM Tris-HCl (pH 7.4), 25 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1% Nonidet P-40, and protease inhibitors. After centrifugation at 8,000 × g for 5 min at 4°C, protein concentrations in the supernatants were determined by the Bio-Rad protein assay. Samples were boiled with gel loading buffer (62.5 mM Tris-HCl, 2% SDS, 25% glycerol, and 0.01% bromophenol blue) at a ratio of 1:1 for 5 min. Total protein equivalents for each sample were separated by SDS-5 to 15% polyacrylamide gel electrophoresis in the presence of 2-mercaptoethanol and were transferred to Sequi-Blot polyvinylidene difluoride membranes (Bio-Rad). Blots were incubated with polyclonal rabbit antibody against murine PrxI (17).
Carrageenan-induced pleurisy.
Wild-type and nrf2−/− mice of the ICR/129SV background weighing 20 to 25 g were used throughout the experiments. A 0.25% lambda carrageenan solution in saline (0.1 ml) was injected into the right pleural cavities of the animals. At 2, 6, 12, 24, 48, and 72 h and 7 days after the injection of carrageenan, the chest was carefully opened and the pleural cavity was washed with 1 ml of saline solution containing heparin. The pleural cavity was washed five times consecutively in the same way, and the pleural lavage fluid was collected into a tube.
Leukocyte counts.
A 30-μl sample of collected pleural lavage fluid was diluted with Turk's solution, and total leukocytes were counted under an optical microscope. Differential leukocyte counts were determined in cytospin smears stained with Wright-Giemsa stain (Diff-Quick; Sysmex, Kobe, Japan).
Albumin concentration.
The first washing was centrifuged at 400 × g for 5 min at 4°C, and the albumin concentration in the supernatant was determined with the albumin reagent from Sigma (St. Louis, Mo.).
Immunohistochemical analysis.
Cells were smeared onto poly-l-lysine-coated slides and allowed to air dry. Endogenous peroxidases were quenched with 0.3% H2O2 in methanol, and sections were washed with 0.1% Triton X-100 in phosphate-buffered saline. The sections were reacted with anti-15d-PGJ2 monoclonal antibody (38), anti-PrxI antibody (16), anti-HO-1 antibody (a generous gift from Shigeru Taketani), anti-F4/80 antibody (Serotec), anti-COX-2 antibody (Santa Cruz), or anti-hematopoietic PG synthetase (PGDS) antibody (Santa Cruz) and incubated for another hour with Histofine Simple Stain MAX-PO (Nichirei, Tokyo, Japan). Diaminobenzidine was used as a chromogen.
NS-398 and indomethacin treatment.
NS-398 (Cayman Chemical, Ann Arbor, Mich.) (10 mg/kg) and indomethacin (Sigma) (10 mg/kg) were administered intraperitoneally 1 h before the injection of carrageenan. The pleural cavity was washed at 2, 12, and 24 h after the injection of carrageenan for the determination of inflammatory cell numbers and albumin concentration. To determine the effects of NS-398 at 48 and 72 h and 7 day, NS-398 was administered every 24 h thereafter. At 48 h, 72 h, and 7 days after the injection of carrageenan, the pleural cavity was washed for the determination of inflammatory cell numbers and albumin concentration.
15d-PGJ2 administration.
At 1 h after the intraperitoneal injection of NS-398, 15d-PGJ2 (100 μg/kg) was injected into the pleural cavity. At 24 h after the injection of carrageenan, the pleural cavity was washed for the determination of inflammatory cell numbers and anti-inflammatory gene expression levels.
Statistical analysis.
Statistical analysis was done by analysis of variance followed by a Bonferroni posttest. Albumin concentration data were analyzed by using Welch's t test. A P value of less than 0.05 was accepted as statistically significant.
RESULTS
Persistence of inflammatory cells in nrf2−/− mice during carrageenan-induced pleurisy.
To explore the influence of Nrf2 during acute inflammation, we examined the effect of nrf2 gene disruption on carrageenan-induced pleurisy in mice. In our preliminary experiments we administered 1% carrageenan to mice, a dose that is commonly used to induce carrageenan pleurisy in rodents, but we found that this particular dose provoked severe and protracted inflammation in mice as judged by neutrophil infiltration into the pleural cavity. We therefore carefully tested the correlation between the carrageenan dose and the duration of inflammation, finding that administration of 0.25% carrageenan reproduces the time course of acute inflammation and recovery (data not shown). Vehicle treatment caused a transient infiltration of neutrophils, which peaked at 12 h, but did not significantly alter the albumin concentration (Table 1) and macrophage recruitment (data not shown) at the 12- and 24-h time points. In contrast, 0.25% carrageenan provoked a significant increase of neutrophil and albumin concentrations in the pleural lavage fluid (Table 1) compared to the vehicle-treated mice.
TABLE 1.
Number of neutrophils and albumin concentration in pleural lavage fluid
| Time (h) and treatment | Neutrophils (104)a
|
Albumin (mg/ml)a
|
||
|---|---|---|---|---|
| Nrf2+/+ mice | Nrf2−/− mice | Nrf2+/+ mice | Nrf2−/− mice | |
| 0 | 1 ± 0 | 1 ± 0 | 0.27 ± 0.07 | 0.30 ± 0.14 |
| 12 | ||||
| Vehicle | 179 ± 53c | 128 ± 48 | 0.38 ± 0.14 | 0.26 ± 0.09 |
| Carrageenan | 492 ± 59b,c | 508 ± 56b,c | 0.68 ± 0.22b,c | 0.87 ± 0.36b,c |
| 24 | ||||
| Vehicle | 27 ± 18 | 21 ± 14 | 0.30 ± 0.18 | 0.31 ± 0.22 |
| Carrageenan | 229 ± 10b,c | 386 ± 56b,c | 1.1 ± 0.30b,c | 1.5 ± 0.26b,c |
Data are means ± SEM. At least three mice were examined for each group.
Significantly different from time-matched vehicle control mice (P < 0.05).
Significantly different from genotype-matched 0-h control mice (P < 0.05).
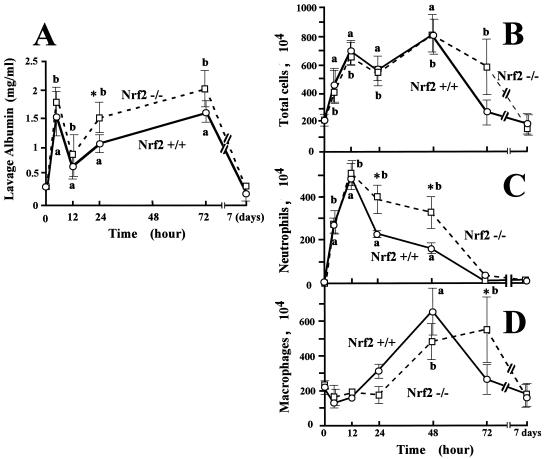
The albumin concentration in the pleural lavage fluid showed two peaks, one at 2 h and the other at 72 h after carrageenan injection, and then returned to normal levels by day 7 of pleurisy in both wild-type and nrf2−/− mice (Fig. 1A). The albumin concentration in pleural lavage fluid was significantly higher in nrf2−/− mice during pleurisy than in wild-type mice at the 24-h time point. The total inflammatory cell number in the pleural lavage fluid peaked at 12 and 48 h of pleurisy in both wild-type and nrf2−/− mice (Fig. 1B), but the infiltration of the cells persisted until 72 h of pleurisy in nrf2−/− mice. We speculated that an increase in neutrophils and macrophages at different time points might be responsible for the two peaks of infiltrated cells. We therefore examined the numbers of neutrophils (Fig. 1C) and macrophages (Fig. 1D) in the pleural lavage fluid. In wild-type mice, the neutrophil number peaked at 12 h and returned to the background level at 72 h of pleurisy, indicating that the first peak of total infiltrated cells mainly reflected an increase in neutrophils. Although in nrf2−/− mice, the magnitude of neutrophil infiltration was not significantly different from that in wild-type mice, the increased number of neutrophils persisted to later time points of pleurisy. The number of neutrophils in the pleural lavage fluid from nrf2−/− mice was significantly higher than that in wild-type mice at 24 and 48 h (Fig. 1C).
FIG. 1.
Persistence of inflammatory cells in Nrf2-deficient mice in carrageenan-induced pleurisy. (A) Concentrations of pleural lavage albumin were measured at the indicated time points of pleurisy, and the means and standard deviations of triplicates are shown. (B to D) The numbers of total inflammatory cells (B), neutrophils (C), and macrophages (D) were counted microscopically at the indicted time points of pleurisy, and the means and standard deviations of triplicates are shown. Solid lines indicate the results for nrf2+/+ mice, whereas dashed lines indicate the results for nrf2−/− mice. The means from four experiments are presented with standard errors of the mean. *, significantly different from time-matched wild-type mice (P < 0.05). a, significantly different from non-carrageenan-treated wild-type mice at the 0-h time point (P < 0.05). b, significantly different from non-carrageenan-treated nrf2−/− mice at the 0-h time point (P < 0.05).
In contrast, macrophages were recruited to the pleural space at a later phase of pleurisy than the peak of neutrophil infiltration (Fig. 1D). The increase of macrophage number peaked at 48 h after the carrageenan administration and returned to the control level at the 72-h time point in wild-type mice, indicating that the second peak of total infiltrated cells mainly reflected an increase in macrophages. In contrast, macrophage recruitment peaked at the 72-h time point in nrf2−/− mice. The number of macrophages at this time point was significantly different from that in wild-type mice (Fig. 1D). Collectively, these results demonstrate that Nrf2 deficiency leads to a persistence of neutrophil occupation and a delay of macrophage recruitment in carrageenan-induced pleurisy of mice.
15d-PGJ2 accumulates in pleural inflammatory macrophages.
Since 15d-PGJ2 has been implicated as a key regulator of carrageenan pleurisy (8, 13, 44), we examined whether 15d-PGJ2 accumulates in pleural inflammatory cells by using a specific monoclonal antibody against 15d-PGJ2. The antibody has successfully detected the accumulation of 15d-PGJ2 in both RAW264.7 cells activated by lipopolysaccharide and foamy macrophages of human atherosclerotic lesions (38). Furthermore, this antibody has shown to react almost exclusively with 15d-PGJ2 (38). Immunohistochemical analysis with this antibody revealed that accumulation of 15d-PGJ2 specifically occurred in pleural macrophages that were positive for F4/80 antigens but did not occur in neutrophils (data not shown).
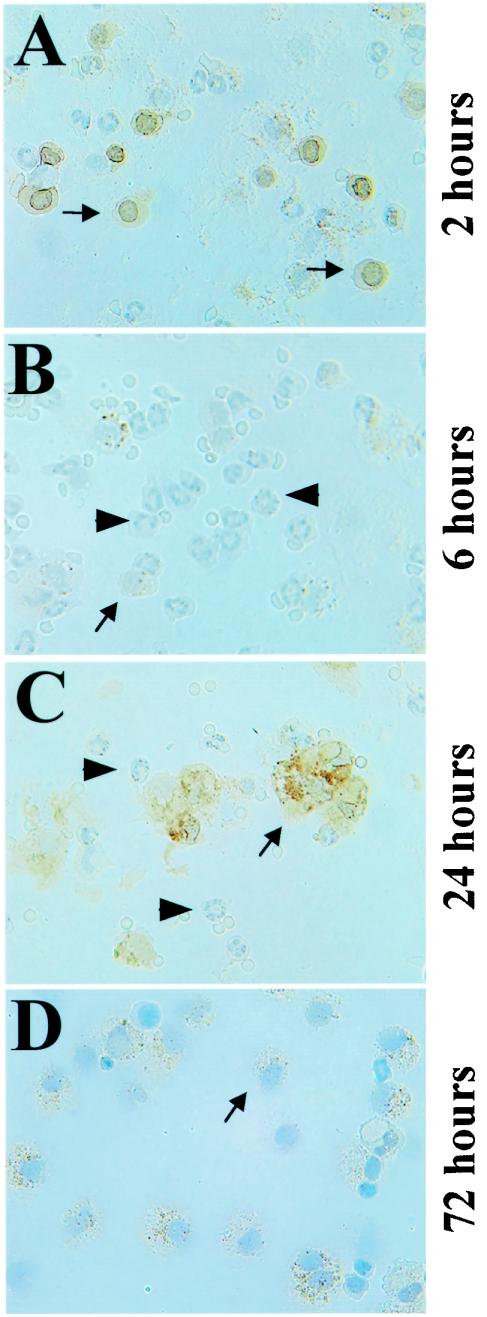
As reported previously for rat carrageenan pleurisy, the accumulation of 15d-PGJ2 showed two peaks (13). The accumulation of 15d-PGJ2 was transiently observed at 2 h of pleurisy (Fig. 2A), but the accumulation then decreased at 6 h of pleurisy (Fig. 2B). At 2 h of pleurisy, the inducible accumulation of 15d-PGJ2 in macrophages appeared to occur strongly around the nuclear membranes of resident pleural macrophages, which are small and round. The level increased again at 12 h after the carrageenan injection and remained high until 48 h of pleurisy (Fig. 2C and data not shown), followed by another increase at 72 h of pleurisy (Fig. 2D). At the 24-h time point, the accumulation of 15d-PGJ2 was observed mainly in the cytoplasm of macrophages, which were large and had a foamy appearance (Fig. 2C). These results thus demonstrate that 15d-PGJ2 specifically accumulates in pleural inflammatory macrophages and suggest that 15d-PGJ2 may act through modifying macrophage function.
FIG. 2.
Accumulation of 15d-PGJ2 (brown) in pleural macrophages during carrageenan-induced pleurisy. Pleural inflammatory cells were examined immunohistochemically at the indicated times of pleurisy with anti-15d-PGJ2 antibody. Arrows indicate macrophages, while arrowheads indicate neutrophils.
The Nrf2-Keap1 pathway mediates the induction of a range of genes by 15d-PGJ2 in mouse peritoneal macrophages.
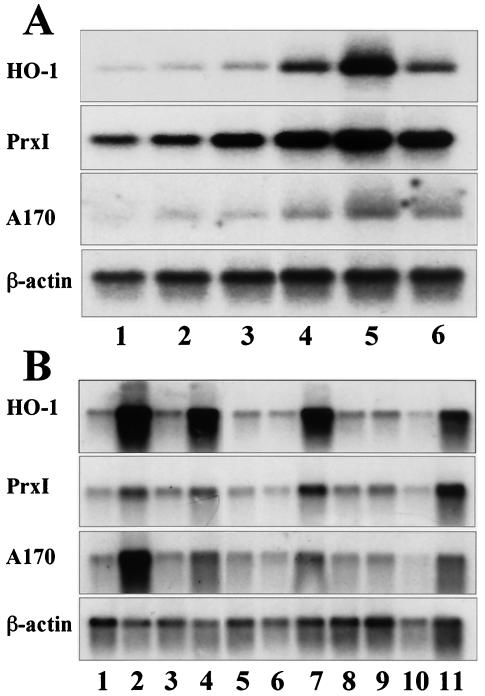
cyPGs, including 15d-PGJ2, have a reactive α,β-unsaturated carbonyl group in the cyclopentane ring. This ring structure renders this molecule capable of forming Michael adducts with nucleophilic cellular molecules and covalent modification of specific proteins. We speculate that this feature of cyPGs may activate Nrf2 (39). In order to obtain solid evidence for activation of the Nrf2 pathway by 15d-PGJ2, we first examined the effect of exogenous 15d-PGJ2 on the induction of three Nrf2 target genes in primary cultures of mouse peritoneal macrophages, which were used previously for the study of Nrf2 (16). The results clearly indicated that 15d-PGJ2 activates, in a dose-dependent manner, the expression of the HO-1, PrxI, and A170 genes (Fig. 3A), all of which were shown to be inducible by electrophiles in peritoneal macrophages (16).
FIG. 3.
cyPGs activate a set of antioxidant and anti-inflammatory genes in mouse peritoneal macrophages. (A) Peritoneal macrophages were treated with dimethyl sulfoxide alone (lane 1); with 15d-PGJ2 at 0.5 μM (lane 2), 1 μM (lane 3), 5 μM (lane 4), or 10 μM (lane 5) for 5 h; or with 100 μM diethylmaleate for 5 h (lane 6), and total RNAs were analyzed by RNA blot analysis with HO-1, PrxI, and A170 cDNAs as probes. A β-actin cDNA probe was used for a loading control. (B) Peritoneal macrophages were treated with dimethyl sulfoxide alone (lane 1), PGA1 (50 μM) (lane 2), PGB2 (50 μM) (lane 3), PGD2 (50 μM) (lane 4), PGE2 (50 μM) (lane 5), PGF1a (50 μM) (lane 6), 15d-PGJ2 (10 μM) (lane 7), thromboxane B2 (50 μM) (lane 8), leukotriene B4 (1 μM) (lane 9), arachidonic acid (50 μM) (lane 10), or diethylmaleate (100 μM) (lane 11). Total RNA fractions were analyzed by RNA blot analysis as for panel A.
We then examined which prostaglandins could strongly activate Nrf2 by using the peritoneal macrophage system. Of arachidonic acid and the arachidonic acid metabolites, including PGA1, PGB2, PGD2, PGE2, PGF1α, 15d-PGJ2, thromboxane B2, and leukotriene B4, only PGA1, 15d-PGJ2, and PGD2 markedly induced the expression of Nrf2 target genes (Fig. 3B). PGA1 and 15d-PGJ2 are classified as electrophilic cyPGs. Since PGD2 is readily metabolized to 15d-PGJ2 (38), 15d-PGJ2 is suggested to be the most important cyPG in the regulation of Nrf2.
cyPGs activate Nrf2 in macrophages and hepatocytes.
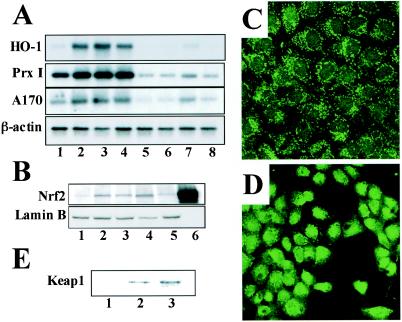
Subsequently, we examined the requirement for Nrf2 in the induction of antioxidant genes, using primary cultures of peritoneal macrophages from Nrf2-deficient mice. While HO-1, PrxI, and A170 mRNAs were all induced in wild-type macrophages by the addition of 5-μM 15d-PGJ2 to the culture medium (Fig. 4A, lane 2), the induction was not observed in nrf2−/− macrophages (lane 6). The result was reproducible with 10-μM 15d-PGJ2 (lanes 3 and 7), but in this case a weak induction was observed in the nrf2−/− macrophages (lane 7), suggesting the presence of a minor complementary pathway to Nrf2. Consistent with these results, immunoblot analysis with anti-Nrf2 antibody revealed that 15d-PGJ2 (Fig. 4B, lane 3) and PGA1 (lane 4), but not PGE2 (lane 5), induced the nuclear accumulation of Nrf2.
FIG. 4.
cyPGs activate Nrf2 in macrophages and hepatocytes. (A) Peritoneal macrophages derived from either wild-type mice (lanes 1 to 4) or nrf2−/− mice (lanes 5 to 8) were treated with 5 μM (lanes 2 and 6) or 10 μM (lanes 3 and 7) 15d-PGJ2 or with 100 μM diethylmaleate (lanes 4 and 8). Untreated controls are shown in lanes 1 and 5. Total RNAs were analyzed by RNA blotting analysis, using cDNAs for HO-1, PrxI, or A170 as probes. A β-actin cDNA probe was used for a loading control. (B) Nuclear extracts were prepared from macrophages untreated (lane 1) or treated with diethylmaleate (100 μM) (lane 2), 15d-PGJ2 (5 μM) (lane 3), PGA1 (100 μM) (lane 4), or PGE2 (100 μM) (lane 5). The extracts were immunoblotted with anti-Nrf2 antibody or anti-lamin B antibody. Recombinant Nrf2 in 293T cells was also loaded as a control (lane 6). (C and D) RL34 cells were either untreated (C) or treated with 10 μM 15d-PGJ2 for 4 h (D), and expression of Nrf2 were examined immunocytochemically with the anti-Nrf2 antibody. Subcellular localization of Nrf2 was analyzed by confocal microscopy. (E) RL34 cells were treated with dimethyl sulfoxide (lane 1), biotinylated Δ12-PGJ2 (lane 2), or biotinylated 15d-PGJ2 (lane 3). Keap1-PGJ2 complexes were precipitated from the cell extracts with avidin beads and probed with the anti-Keap1 antibody.
Immunocytochemical analysis of the rat hepatocyte cell line RL34, using the anti-Nrf2 antibody, further demonstrated that under normal culture conditions Nrf2 is retained by Keap1 in the cytoplasm (Fig. 4C). However, after the addition of 15d-PGJ2, Nrf2 is liberated from Keap1 and translocates to and accumulates within the nucleus (Fig. 4D).
Recent studies indicate that certain Nrf2 inducers, such as Michael reaction acceptors, directly bind to reactive cysteine residues in Keap1, thereby liberating Nrf2 (9). To elucidate whether 15d-PGJ2 binds directly to Keap1, we examined the binding of biotin-tagged 15d-PGJ2 to Keap1 in RL34 cells. A pull-down analysis with avidin beads followed by probing with anti-Keap1 antibody demonstrated that Keap1 could be detected in precipitates from biotinylated 15d-PGJ2-treated or biotinylated Δ12-PGJ2-treated cells but not in the control immunoprecipitates (Fig. 4E). This result suggests that 15d-PGJ2 directly binds to Keap1, most probably through covalent linkage, allowing Nrf2 to be released from Keap1.
COX-2 inhibitor affects the inflammatory cell infiltration.
In order to elucidate the relationship between the accumulation of 15d-PGJ2 and activation of Nrf2, we examined the time course of PrxI gene expression during the carrageenan-induced pleurisy. Immunoblot analyses with anti-PrxI antibody revealed that the expression of PrxI was induced at as early as 2 h of pleurisy (Fig. 5A, lanes 3 to 5). In contrast, the induction of PrxI during pleurisy was largely abolished in nrf2−/− mice, and this difference was prominent at 2 to 24 h (lanes 4, 7, and 10) but became relatively small after 48 h of pleurisy (lanes 13 and 16).
FIG. 5.
PrxI expression at the early phase of carrageenan-induced pleurisy depends on both 15d-PGJ2 and Nrf2. (A) Both the COX-2 inhibitor NS-398 and nrf2 gene disruption affect PrxI expression during carrageenan-induced pleurisy. Whole-cell extracts of pleural inflammatory cells from wild-type mice (lanes 1, 3, 6, 9, 12, and 15), nrf2−/− mice (lanes 2, 4, 7, 10, 13, and 16), or NS-398-treated wild-type mice (lanes 5, 8, 11, 14, and 17) at the indicated time points of pleurisy were examined by immunoblotting with anti-PrxI antibody. (B) The expression of PrxI, 15d-PGJ2, F4/80, HO-1, COX-2, and hematopoietic PGDS in pleural inflammatory cells at 24 h of pleurisy was analyzed immunohistochemically with the corresponding antibodies as indicated. Arrows indicate macrophages, while arrowheads represent neutrophils. (C and D) Administration of NS-398 affects neutrophil (C) and macrophage (D) numbers during carrageenan-induced pleurisy. The number of pleural inflammatory cells was microscopically counted, and the means from four experiments are presented with standard errors of the mean. *, significantly different from time-matched NS398-untreated mice (P < 0.05). a, significantly different from carrageenan-untreated wild-type mice at the 0-h time point (P < 0.05). b, significantly different from carrageenan-untreated nrf2−/− mice at the 0-h time point (P < 0.05). (E) Effect of indomethacin on pleural inflammatory cells. Cell counts were taken at 24 h of pleurisy. The means from four experiments are presented with standard errors of the mean. *, significantly different from untreated control mice (P < 0.05).
COX-2 is known to regulate PG synthesis in carrageenan pleurisy. To examine the role of COX-2 in the inducible expression of PrxI, we examined the in vivo effect of NS-398, a specific inhibitor of COX-2 (42), on PrxI expression. Peritoneal injection of NS-398 1 h before carrageenan treatment significantly attenuated the expression of PrxI in macrophages when tested by immunoblotting (Fig. 5A) and of 15d-PGJ2 when tested by immunocytochemistry (data not shown). NS-398 caused a reduction of PrxI expression especially at 2 to 24 h (Fig. 5A, lanes 5, 8, and 11), but the effect was not as prominent after 48 h of pleurisy (lanes 14 and 17). These results suggest a possible scenario in which COX-2 mediates the accumulation of 15d-PGJ2 and 15d-PGJ2 in turn activates Nrf2 and regulates the expression of PrxI as well as the other antioxidant genes.
Immunohistochemical analysis demonstrated that PrxI expression was specifically observed in macrophages, but not in neutrophils, of pleural lavage fluid (Fig. 5B). The expression profile of PrxI clearly overlapped with those of 15d-PGJ2 and macrophage-specific F4/80 antigen (Fig. 5B). The expression of HO-1 was also detected exclusively in pleural macrophages (Fig. 5B), in very good agreement with previous analysis of the rat pleurisy model. Furthermore, COX-2 and hematopoietic PGDS, two major rate-limiting enzymes for 15d-PGJ2 synthesis, were highly expressed in the macrophages (Fig. 5B), supporting our contention that 15d-PGJ2 was synthesized mainly in the macrophages.
It should be noted that NS-398 treatment caused a persistence of neutrophil infiltration at 24 h of pleurisy (Fig. 5C) and a delay in macrophage recruitment (Fig. 5D) in pleural lavage fluid. This is in contrast to the case for untreated mice, where the macrophage number was high at 48 h and decreased at 72 h of pleurisy. It can be observed that in the NS-398-treated mice the macrophage number continued to climb even at 72 h of pleurisy (Fig. 5D). Also, in the NS-398-treated mice, the macrophage number was rather low at the 24- and 48-h time points, indicating a delay in macrophage recruitment. The persistence of neutrophil infiltration and the delay of macrophage recruitment were also observed in the COX-1/COX-2 dual inhibitor indomethacin (Fig. 5E). Thus, inflammatory cell infiltration in COX-2-inhibited mice closely reflects that observed in Nrf2-deficient mice, further supporting our contention that 15d-PGJ2 (and COX-2) acts as a regulator of the Nrf2 pathway and the expression of antioxidant genes.
Replacement of 15d-PGJ2 into the intrapleural space.
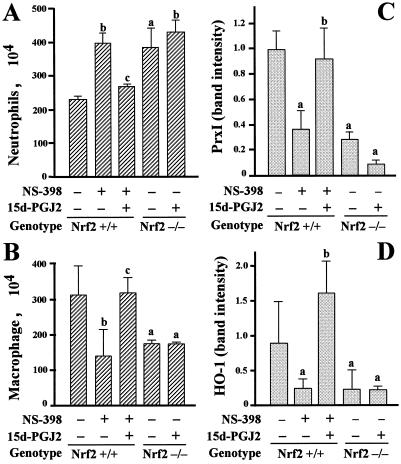
Finally, we wished to test directly the significance of 15d-PGJ2 accumulation in the development of and recovery from carrageenan-induced pleurisy. To this end, we administered 15d-PGJ2 into the intrapleural cavity and examined changes in inflammatory cell infiltration at 24 h of pleurisy. Consistent with the results shown above, administration of NS-398 to mice with pleurisy increased the neutrophil number at 24 h (Fig. 6A, bar 2). We found that simultaneous injection of 15d-PGJ2 with carrageenan into the pleural space reversed the increase of neutrophil infiltration in NS-398-treated mice (bar 3). The important finding here is that the administration of 15d-PGJ2 into the pleural space of Nrf2-null mice did not affect the accumulation of neutrophils (compare bars 4 and 5).
FIG. 6.
Intrapleural injection of 15d-PGJ2 reverses the decrease of PrxI expression and persistence of neutrophils in NS-398-treated mice but not in Nrf2-deficient mice. (A and B) Numbers of neutrophils (A) and macrophages (B) at 24 h of pleurisy in wild-type (nrf2+/+) mice (bars 1), NS-398-treated nrf2+/+ mice (bars 2), NS-398-treated nrf2+/+ mice administrated 15d-PGJ2 (bars 3), nrf2−/− mice (bars 4), and nrf2−/− mice administrated NS-398 (bars 5). Neutrophils and macrophages were counted microscopically, and the means and standard deviations of triplicates are shown. a, P < 0.05 compared with untreated control mice; b, P < 0.01 compared with untreated control mice; c, P < 0.01 compared with NS-398-treated wild-type mice. (C, D) RT-PCR analysis of PrxI (C) and HO-1 (D) mRNAs in pleural inflammatory cells in mice. Intensities of RT-PCR bands were quantified by densitometric analysis, and the means of triplicates are presented. a, P < 0.01 compared with untreated control group; b, P < 0.01 compared with NS-398-treated wild-type mice.
We found the opposite result for macrophages recruitment during pleurisy. While the NS-398 treatment provoked a delay in the macrophage recruitment (Fig. 6B, bar 2), this was reversed by the simultaneous administration of 15d-PGJ2 (bar 3). Nrf2 must therefore mediate this effect of 15d-PGJ2, as 15d-PGJ2 did not help to reverse the delay in macrophage recruitment in the Nrf2-deficient mice (compare bars 4 and 5).
To determine changes in the antioxidant gene expression, we carried out RT-PCR analyses with pleural inflammatory cells and specific primers for PrxI (Fig. 6C) and HO-1 (Fig. 6D). The expression of PrxI and HO-1 showed a pattern of changes similar to that for macrophage number. The NS-398-treatment produced a decrease in PrxI and HO-1 gene expression (bar 2). On the other hand, the administration of 15d-PGJ2 to the NS-398-treated mice reversed the PrxI and HO-1 mRNA expression level (bar 3) to that for wild-type control mice (bar 1). The expression levels of PrxI and HO-1 mRNAs in the Nrf2-deficient mice did not change with 15d-PGJ2 treatment (compare bars 4 and 5). These results thus argue that 15d-PGJ2 transduces the inflammatory signals to Nrf2 and that Nrf2 transcriptionally regulates both the antioxidant gene expression in pleural macrophages and inflammatory cell recruitment.
DISCUSSION
The cyPG 15d-PGJ2 is emerging as a probable regulator of acute inflammation (13, 43-45). By exploiting carrageenan-induced pleurisy as a model system for acute inflammation, this study examined the relationship between 15d-PGJ2 and Nrf2. We found that during carrageenan-induced pleurisy, 15d-PGJ2 accumulates in pleural inflammatory cells and the accumulation is confined to macrophages. The results of this study unveil a number of significant correlations between accumulation of 15d-PGJ2 and activation of Nrf2 during carrageenan-induced pleurisy. First, in Nrf2-deficient mutant mice, the accumulation of neutrophils during inflammation persisted and macrophage recruitment was delayed to the late phases of pleurisy. Second, the COX-2 inhibitor NS-398 and the COX-1/COX-2 dual inhibitor indomethacin, which attenuate the accumulation of 15d-PGJ2, affected the acute inflammatory response in a manner similar to a deficiency in Nrf2. NS-398 repressed the expression of PrxI in pleural macrophages and caused a persistence of neutrophil accumulation with a concomitant delay in macrophage recruitment. Third, the administration of 15d-PGJ2 into the pleural space reversed both the decrease of PrxI expression and persistence of neutrophils in NS-398-treated mice, whereas this treatment did not reverse a similar phenotype in the Nrf2-deficient mice, arguing strongly that 15d-PGJ2 functions to activate Nrf2. Fourth, our data show that in peritoneal macrophages and hepatocytes, 15d-PGJ2 directly bound to Keap1 and thereby liberated Nrf2. Taken together, these results demonstrate that Nrf2 regulates the acute inflammatory response by orchestrating the recruitment of inflammatory cells and regulating the expression of anti-oxidative stress genes downstream of 15d-PGJ2.
Increasing lines of evidence (8, 44, 45) suggest that in addition to establishing acute inflammation, COX-2 also contributes to the resolution phase of inflammation through 15d-PGJ2. 15d-PGJ2 can function as both an activator and a repressor of signal-transducing transcription factors. The possible contributions made by 15d-PGJ2 to the acute inflammatory response are summarized in Fig. 7. It has been reported that 15d-PGJ2 inhibits NF-κB activation by covalently binding to IκB kinase β or the p50 subunit of NF-κB (3, 36). In addition, 15d-PGJ2 has been reported to serve as a natural ligand of PPARγ (21, 34). Activated PPARγ regulates the synthesis of proinflammatory cytokines and the induction of nitric oxide synthetase in activated monocytes by negatively interacting with AP-1, NF-κB, or STAT. Thus, 15d-PGJ2 may have an impact upon multiple mechanisms during the resolution of inflammation. The results of this study demonstrate that 15d-PGJ2 can act as a potent anti-inflammatory agent by exploiting the Nrf2-Keap1 pathway, a previously unrecognized alternative pathway in the cascades downstream of 15d-PGJ2.
FIG. 7.
Network of 15d-PGJ2 and the Nrf2-Keap1 pathway in carrageenan-induced pleurisy. 15d-PGJ2 is generated in pleural macrophages during carrageenan-induced pleurisy and is known to directly inhibit NF-κB activity and also to act as a ligand for PPARγ. This study shows that 15d-PGJ2 activates the Nrf2-Keap1 pathway through covalent binding to Keap1. Nrf2 induces HO-1 and PrxI expression as well as other ARE-regulated genes in macrophages (green). The upregulation of PrxI appears to inhibit NF-κB, TNF-α, and the β-tautomerase activity of MIF, thus modulating the inflammation process. On the other hand, HO-1 is known to inhibit the expression of TNF-α through CO production.
The possible involvement of Nrf2 in inflammation has been alluded to in some earlier reports. For instance, antirheumatic gold(I) compounds markedly activate Nrf2 (24). We found that female Nrf2 knockout mice frequently developed severe glomerulonephritis (46). Braun et al. recently reported that Nrf2 regulates inflammation during healing of skin wounds (2). The expression of several inflammatory cytokines was shown to persist during the healing of a skin wound in Nrf2-deficient mice, such that interleukin-1β levels in the wound remained elevated to day 13, most likely due to the persistence of macrophages at the site of the wound. Finally, ARE battery genes are activated by laminar shear stress, which acts as an anti-inflammatory signal in endothelial cells (6). Indeed, in endothelial cells, overexpression of Nrf2 inhibited the tumor necrosis factor alpha (TNF-α)-mediated induction of vascular cell adhesion molecule-1 gene expression, which is important for monocyte recruitment during inflammation response. Our present observations, along with those cited above, suggest that Nrf2 is important for regulating the process of acute inflammation.
We demonstrated in this study that the intracellular accumulation of 15d-PGJ2 occurs during carrageenan-induced pleurisy in the mouse (Fig. 2). This observation is consistent with the previous analysis in rat carrageenan pleurisy, where 15d-PGJ2 accumulates with two peaks at low nanomolar levels in the pleural exudates. As the 15d-PGJ2 concentration used in this study is higher than that detected in pleural exudates, it might be challenging to hypothesize that 15d-PGJ2 works as an endogenous regulator of acute inflammation. However, Narumiya et al. previously reported that exogenously added Δ12-PGJ2 accumulates in the cells in a protein-bound form and is resistant to cell extraction, suggesting that intracellular sequestration of these cyPGs is most probably due to the Michael adduction to the protein (27). Furthermore, Keap1 localizes in the perinuclear cytoskeleton close to COX-2 and PGDS. Therefore, it is likely that the local concentration of cyPGs accumulates to a level sufficient for Nrf2 activation during pleurisy.
It was reported recently that submicromolar concentrations of 15d-PGJ2 can activate HO-1 and contribute to the anti-inflammatory effect of this reagent (25). As NF-κB inhibition requires a concentration of 15d-PGJ2 in the micromolar range in cell culture (36), this observation suggests that an in vivo anti-inflammatory effect of 15d-PGJ2 may be more relevant to the activation of Nrf2.
Under normal conditions, Nrf2 activity is suppressed primarily by its compartmentalization to the cytosol by Keap1 and consequent rapid degradation by the proteasome (reference 20 and references therein). Recently it was demonstrated that electrophiles classified as Michael reaction acceptors directly bound to Keap1 and dissociated Nrf2 from Keap1 (9). We have demonstrated in this study that 15d-PGJ2 and Δ12-PGJ2, both of which contain a reactive α,β-unsaturated carbonyl group in their cyclopentane ring, directly bind to Keap1 and activate Nrf2. These results are consistent with our contention that cyPGs act as endogenous activators of the Nrf2-Keap1 pathway in macrophages, thereby regulating the recruitment of inflammatory cells.
NS-398-treated wild-type mice and nrf2-null mice displayed similar phenotypes in pleurisy, i.e., a persistence of neutrophil residence and a delay in macrophage recruitment. These results suggest that COX-2 products negatively regulate the accumulation of neutrophils in the intrapleural space, through either enhancing the phagocytosis of neutrophils by macrophages or decreasing the neutrophil infiltration. The administration of 15d-PGJ2 into the pleural space successfully reversed the phenotype in NS-398-treated mice, indicating that 15d-PGJ2 works downstream of COX-2 activation. Consistent with this observation, we demonstrated in this study that the actual inducible accumulation of 15d-PGJ2 occurred in macrophages during carrageenan pleurisy (Fig. 2). Since the introduction of 15d-PGJ2 into the pleural spaces of Nrf2-deficient mice had no effect, Nrf2 must be a downstream mediator of 15d-PGJ2 activity in macrophages. Thus, we conclude that 15d-PGJ2 regulates acute inflammation through regulating the function of macrophages (Fig. 7).
It remains to be elucidated to how Nrf2 regulates acute inflammation. We speculate that a battery of Nrf2 target genes cooperatively function to repress proinflammatory signals, such as those of TNF-α or interleukin-1β. HO-1 and PrxI are the Nrf2 target genes that are likely to influence the inflammatory process (Fig. 7) (16). Recently, Gong et al. have demonstrated that 15d-PGJ2 can activate HO-1 via a stress-responsive element and by an Nrf2-mediated mechanism (14). In the carrageenan-induced pleurisy model, it has been shown that elevation of HO-1 expression can suppress, whereas inhibition of HO-1 activity can exacerbate, the inflammatory response (43, 45). Indeed, HO-1 can inhibit the expression of TNF-α most probably through the generation of carbon monoxide (30). With respect to PrxI, its human counterpart PAG was reported to directly bind to and negatively regulate β-tautomerase activity of MIF, which is one of the central regulators of inflammation (22). Although the physiological significance of the β-tautomerase activity of MIF is unclear at present, we nonetheless expect that the repression of MIF activity by PrxI may play important roles in the regulation of inflammation. Furthermore, the overexpression of PrxI removed H2O2 (23), suggesting that PrxI can repress TNF-α signaling by the removal of H2O2 (Fig. 7). These observations suggest that Nrf2 may have multiple downstream targets that regulate the acute inflammation process.
In the rat carrageenan-induced pleurisy model, accumulation of 15d-PGJ2 in the late phase of pleurisy was associated with resolution of the inflammation (13). However, the true role of 15d-PGJ2 during the early phase of pleurisy remained largely unknown. Our results imply that in the early phase, accumulation of 15d-PGJ2 activates Nrf2 and regulates the inflammation process through the induction of target gene expression, including that of HO-1 and PrxI. Whereas COX-2 has been reported to accelerate inflammation in the early phase of pleurisy through the induction of PGE2, our present analyses suggest that COX-2 also can suppress the early phase of inflammation through the production of 15d-PGJ2. These results are consistent with the present model that the inflammation process is balanced by an acceleration and deceleration of integrating signaling pathways (28). Understanding of how the signals are integrated to establish and resolve the acute inflammation process provides important clues to facilitate the development of effective treatments for chronic inflammation.
Acknowledgments
We thank S. Taketani for providing the polyclonal antibody against rat HO-1. We also thank Y. Katoh, A. Kobayashi, M. Kobayashi, S. Nishimura, K. I. Tong, and N. Wakabayashi for discussion and advice.
This work was supported in part by grants from JST-ERATO; the Ministry of Education, Culture, Sports, Science and Technology; the Ministry of Health, Labor and Welfare; and the Naito foundation.
REFERENCES
- 1.Aoki, Y., H. Sato, N. Nishimura, S. Takahashi, K. Itoh, and M. Yamamoto. 2001. Accelerated DNA adduct formation in the lung of the Nrf2 knockout mouse exposed to diesel exhaust. Toxicol. Appl. Pharmacol. 173:154-160. [DOI] [PubMed] [Google Scholar]
- 2.Braun, S., C. Hanselmann, M. G. Gassmann, U. auf dem Keller, C. Born-Berclaz, K. Chan, Y. W. Kan, and S. Werner. 2002. Nrf2 transcription factor, a novel target of keratinocyte growth factor action which regulates gene expression and inflammation in the healing skin wound. Mol. Cell. Biol. 22:5492-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cernuda-Morollon, E., E. Pineda-Molina, F. J. Canada, and D. Perez-Sala. 2001. 15-Deoxy-Δ12,14-prostaglandin J2 inhibition of NF-κB-DNA binding through covalent modification of the p50 subunit. J. Biol. Chem. 276:35530-35536. [DOI] [PubMed] [Google Scholar]
- 4.Chan, K., and Y. W. Kan. 1999. Nrf2 is essential for protection against acute pulmonary injury in mice. Proc. Natl. Acad. Sci. USA 96:12731-12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, K., R. Lu, J. C. Chang, and Y. W. Kan. 1996. NRF2, a member of the NFE2 family of transcription factors, is not essential for murine erythropoiesis, growth, and development. Proc. Natl. Acad. Sci. USA 93:13943-13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, X. L., S. E. Varner, A. S. Rao, J. Y. Grey, S. Thomas, C. K. Cook, M. A. Wasserman, R. M. Medford, A. K. Jaiswal, and C. Kunsch. 2003. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J. Biol. Chem. 278:703-711. [DOI] [PubMed] [Google Scholar]
- 7.Cho, H. Y., A. E. Jedlicka, S. P. Reddy, T. W. Kensler, M. Yamamoto, L. Y. Zhang, and S. R. Kleeberger. 2002. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell. Mol. Biol. 26:175-182. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzocrea, S., N. S. Wayman, E. Mazzon, L. Dugo, R. Di Paola, I. Serraino, D. Britti, P. K. Chatterjee, A. P. Caputi, and C. Thiemermann. 2002. The cyclopentenone prostaglandin 15-deoxy-Δ12,14-prostaglandin J2 attenuates the development of acute and chronic inflammation. Mol. Pharmacol. 61:997-1007. [DOI] [PubMed] [Google Scholar]
- 9.Dinkova-Kostova, A. T., W. D. Holtzclaw, R. N. Cole, K. Itoh, N. Wakabayashi, Y. Katoh, M. Yamamoto, and P. Talalay. 2002. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 99:11908-11913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Di Rosa, M., J. P. Giroud, and D. A. Willoughby. 1971. Studies on the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J. Pathol. 104:15-29. [DOI] [PubMed] [Google Scholar]
- 11.Enomoto, A., K. Itoh, E. Nagayoshi, J. Haruta, T. Kimura, T. O'Connor, T. Harada, and M. Yamamoto. 2001. High sensitivity of Nrf2 knockout mice to acetaminophen hepatotoxicity associated with decreased expression of ARE-regulated drug metabolizing enzymes and antioxidant genes. Toxicol. Sci. 59:169-177. [DOI] [PubMed] [Google Scholar]
- 12.Fukushima, M. 1992. Biological activities and mechanisms of action of PGJ2 and related compounds: an update. Prostaglandins Leukot. Essential Fatty Acids 47:1-12. [DOI] [PubMed] [Google Scholar]
- 13.Gilroy, D. W., P. R. Colville-Nash, D. Willis, J. Chivers, M. J. Paul-Clark, and D. A. Willoughby. 1999. Inducible cyclooxygenase may have anti-inflammatory properties. Nat. Med. 5:698-701. [DOI] [PubMed] [Google Scholar]
- 14.Gong, P., D. Stewart, B. Hu, N. Li, J. Cook, A. Nel, and J. Alam. 2002. Activation of the mouse heme oxygenase-1 gene by 15-deoxy-Δ12,14-prostaglandin J2 is mediated by the stress response elements and transcription factor Nrf2. Antioxid. Redox Signal. 4:249-257. [DOI] [PubMed] [Google Scholar]
- 15.Ishii, T., K. Itoh, and M. Yamamoto. 2002. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 348:182-190. [DOI] [PubMed] [Google Scholar]
- 16.Ishii, T., K. Itoh, S. Takahashi, H. Sato, T. Yanagawa, Y. Katoh, S. Bannai, and M. Yamamoto. 2000. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 275:16023-16029. [DOI] [PubMed] [Google Scholar]
- 17.Ishii, T., M. Yamada, H. Sato, M. Matsue, S. Taketani, K. Nakayama, Y. Sugita, and S. Bannai. 1993. Cloning and characterization of a 23-kDa stress-induced mouse peritoneal macrophage protein. J. Biol. Chem. 268:18633-18636. [PubMed] [Google Scholar]
- 18.Itoh, K., T. Chiba, S. Takahashi, T. Ishii, K. Igarashi, Y. Katoh, T. Oyake, N. Hayashi, K. Satoh, I. Hatayama, M. Yamamoto, and Y. Nabeshima. 1997. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 236:313-322. [DOI] [PubMed] [Google Scholar]
- 19.Itoh, K., N. Wakabayashi, Y. Katoh, T. Ishii, K. Igarashi, J. D. Engel, and M. Yamamoto. 1999. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 13:76-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Itoh, K., N. Wakabayashi, Y. Katoh, T. Ishii, T. O'Connor, and M. Yamamoto. 2003. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 8:379-381. [DOI] [PubMed] [Google Scholar]
- 21.Jiang, C., A. T. Ting, and B. Seed. 1998. PPAR-γ agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82-86. [DOI] [PubMed] [Google Scholar]
- 22.Jung, H., T. Kim, H. Z. Chae, K. T. Kim, and H. Ha. 2001. Regulation of macrophage migration inhibitory factor and thiol-specific antioxidant protein PAG by direct interaction. J. Biol. Chem. 276:15504-15510. [DOI] [PubMed] [Google Scholar]
- 23.Kang, S. W., H. Z. Chae, M. S. Seo, K. Kim, I. C. Baines, and S. G. Rhee. 1998. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-α. J. Biol. Chem. 273:6297-6302. [DOI] [PubMed] [Google Scholar]
- 24.Kataoka, K., H. Handa, and M. Nishizawa. 2001. Induction of cellular anti-oxidative stress genes through heterodimeric transcription factor Nrf2/small Maf by antirheumatic gold(I) compounds. J. Biol. Chem. 276:34074-34081. [DOI] [PubMed] [Google Scholar]
- 25.Lee, T. S., H. L. Tsai, and L. Y. Chau. 2003. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-Δ12,14-prostaglandin J2. J. Biol. Chem. 278:19325-19330. [DOI] [PubMed] [Google Scholar]
- 26.Metz, C. N., and R. Bucala. 1997. Role of macrophage migration inhibitory factor in the regulation of the immune response. Adv. Immunol. 66:197-223. [DOI] [PubMed] [Google Scholar]
- 27.Narumiya, S., K. Ohno, M. Fukushima, and M. Fujiwara. 1987. Site and mechanism of growth inhibition by prostaglandins. III. Distribution and binding of prostaglandin A2 and Δ12-prostaglandin J2 in nuclei. J. Pharmacol. Exp. Ther. 242:306-311. [PubMed] [Google Scholar]
- 28.Nathan, C. 2002. Points of control in inflammation. Nature 420:846-852. [DOI] [PubMed] [Google Scholar]
- 29.Negishi, M., T. Koizumi, and A. Ichikawa. 1995. Biological actions of delta 12-prostaglandin J2. J. Lipid Mediat. Cell Signal. 12:443-448. [DOI] [PubMed] [Google Scholar]
- 30.Otterbein, L. E., F. H. Bach, J. Alam, M. Soares, H. Tao Lu, M. Wysk, R. J. Davis, R. A. Flavell, and A. M. Choi. 2000. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 6:422-428. [DOI] [PubMed] [Google Scholar]
- 31.Prestera, T., Y. Zhang, S. R. Spencer, C. A. Wilczak, and P. Talalay. 1993. The electrophile counterattack response: protection against neoplasia and toxicity. Adv. Enzyme Regul. 33:281-296. [DOI] [PubMed] [Google Scholar]
- 32.Primiano, T., T. R. Sutter, and T. W. Kensler. 1997. Antioxidant-inducible genes. Adv. Pharmacol. 38:293-328. [DOI] [PubMed] [Google Scholar]
- 33.Ramos-Gomez, M., M. K. Kwak, P. M. Dolan, K. Itoh, M. Yamamoto, P. Talalay, and T. W. Kensler. 2001. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc. Natl. Acad. Sci. USA 98:3410-3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ricote, M., A. C. Li, T. M. Willson, C. J. Kelly, and C. K. Glass. 1998. The peroxisome proliferator-activated receptor-γ is a negative regulator of macrophage activation. Nature 391:79-82. [DOI] [PubMed] [Google Scholar]
- 35.Roger, T., J. David, M. P. Glauser, and T. Calandra. 2001. MIF regulates innate immune responses through modulation of Toll-like receptor 4. Nature 414:920-924. [DOI] [PubMed] [Google Scholar]
- 36.Rossi, A., P. Kapahi, G. Natoli, T. Takahashi, Y. Chen, M. Karin, and M. G. Santoro. 2000. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature 403:103-108. [DOI] [PubMed] [Google Scholar]
- 37.Seibert, K., Y. Zhang, K. Leahy, S. Hauser, J. Masferrer, W. Perkins, L. Lee, and P. Isakson. 1994. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc. Natl. Acad. Sci. USA 91:12013-12017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shibata, T., M. Kondo, T. Osawa, N. Shibata, M. Kobayashi, and K. Uchida. 2002. 15-Deoxy-Δ12,14-prostaglandin J2: a prostaglandin D2 metabolite generated during inflammatory processes. J. Biol. Chem. 277:10459-10466. [DOI] [PubMed] [Google Scholar]
- 39.Talalay. P., M. J. De Long, and H. J. Prochaska. 1988. Identification of a common chemical signal regulating the induction of enzymes that protect against chemical carcinogenesis. Proc. Natl. Acad. Sci. USA 85:8261-8265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vane, J. R. 1971. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat. New Biol. 231:232-235. [DOI] [PubMed] [Google Scholar]
- 41.Venugopal, R., and A. K. Jaiswal. 1996. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. USA 93:14960-14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warner, T. D., F. Giuliano, I. Vojnovic, A. Bukasa, J. A. Mitchell, and J. R. Vane. 1999. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc. Natl. Acad. Sci. USA 96:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willis, D., A. R. Moore, R. Frederick, and D. A. Willoughby. 1996. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat. Med. 2:87-90. [DOI] [PubMed] [Google Scholar]
- 44.Willoughby, D. A., A. R. Moore, and P. R. Colville-Nash. 2000. Cyclopentenone prostaglandins—new allies in the war on inflammation. Nat. Med. 6:137-138. [DOI] [PubMed] [Google Scholar]
- 45.Willoughby, D. A., A. R. Moore, P. R. Colville-Nash, and D. Gilroy. 2000. Resolution of inflammation. Int. J. Immunopharmacol. 22:1131-1135. [DOI] [PubMed] [Google Scholar]
- 46.Yoh, K., K. Itoh, A. Enomoto, A. Hirayama, N. Yamaguchi, M. Kobayashi, N. Morito, A. Koyama, M. Yamamoto, and S. Takahashi. 2001. Nrf2-deficient female mice develop lupus-like autoimmune nephritis. Kidney Int. 60:1343-1353. [DOI] [PubMed] [Google Scholar]