Abstract
The p21-activated serine/threonine protein kinase Pak2/γ-PAK and the nonreceptor type of protein tyrosine kinase Syk are known to be activated when the cells are exposed to osmotic stress. The purpose of the present study was to examine whether Pak2 and Syk functionally cooperate in cellular signaling. Cotransfection studies revealed that Pak2 associates with Syk in COS cells. The constitutively active form of Cdc42 increases the association of Pak2 with Syk. Pak2 coexpressed with an inactive form of Cdc42 or kinase-inactive Pak2 interacts to a lesser extent with Syk, suggesting that Pak2-Syk association is enhanced by Pak2 activation. Interaction with Pak2 enhances the intrinsic kinase activity of Syk. This is supported by in vitro studies showing that Pak2 phosphorylates and activates Syk. Treatment of cells with sorbitol to induce hyperosmolarity results in the translocation of Pak2 and Syk to the region surrounding the nucleus and in dramatic enhancement of their association. Furthermore, cotransfection of Pak2 and Syk leads to the activation of c-Jun N-terminal kinase (JNK) under hyperosmotic conditions. Pak2 short interfering RNA suppresses sorbitol-mediated activation of endogenous Syk and JNK, thus identifying a novel pathway for JNK activation by Cdc42. These results demonstrate that Pak2 and Syk positively cooperate to regulate cellular responses to stress.
The p21-activated protein kinase (Pak) family is composed of three isoforms: Pak1 (α-PAK), Pak2 (γ-PAK or PAK I), and Pak3 (β-PAK) (3, 15). Pak family protein kinases contain a highly conserved kinase domain and an N-terminal regulatory domain consisting of proline-rich regions and the Cdc42/Rac binding and autoinhibitory domains. A variety of receptors generate signals that can activate Pak family protein kinases via distinct mechanisms. Association of the GTP-bound form of Cdc42/Rac reverses an autoinhibitory intramolecular interaction inducing enzymatic activation and autophosphorylation of Pak family protein kinases.
Pak2 is activated in response to hyperosmolarity, irradiation, UV light, and DNA-damaging chemotherapeutic drugs such as cytosine β-d-arabinofuranoside and cis-platinum(II)diammine dichloride (cisplatin) (24, 25). The activation of Pak2 in response to irradiation or cytosine β-d-arabinofuranoside is dependent on protein tyrosine kinases (PTKs) and phosphatidylinositol 3-kinase (PI 3-kinase) activity. Cleavage of Pak2 by caspase during Fas ligand-induced apoptosis results in the generation of an active 34-kDa C-terminal fragment of Pak2 that mediates morphological and biochemical changes seen in apoptosis (18, 28, 38). Caspase-mediated proteolytic activation of Pak2 is also observed in the cells stimulated with tumor necrosis factor alpha or C2 ceramide. These findings indicate that Pak2 is a proapoptotic effector. In contrast, Pak2 associates with the Nef protein of human immunodeficiency virus and is required for Nef-mediated antiapoptotic signaling (23, 39). Expression of wild-type (WT) Pak2 induces cytostasis in COS-7 and 293T cells, whereas kinase-inactive mutants of Pak2 do not alter cell division (7). Therefore, Pak2 seems to be involved in both cell death and survival pathways. This is supported by a study by Jakobi et al. with a cell line containing stably transfected Pak2 (11).
The nonreceptor type of PTK Syk is widely expressed in hematopoietic cells. Syk is essential for lymphocyte development and is critical for B-cell receptor or Fc receptor-mediated cell activation or integrin-mediated signaling (8, 21, 31, 37). Recent findings reveal that Syk is also expressed in endothelial and epithelial cells and has tumor-suppressive activity in breast cancer cells (4, 40). Induction of Syk results in the suppression of tumor growth and metastasis formation. Syk is composed of a C-terminal kinase domain and tandem N-terminal Src homology 2 (SH2) domains that bind to the phosphorylated immunoreceptor tyrosine-based activating motif (ITAM) of the immune receptor. Conformational changes induced by binding to phosphorylated ITAM and autophosphorylation increase the enzymatic activity of Syk (14, 17). Phosphorylation of tyrosines in the linker region, which separates the SH2 and kinase domains, creates docking sites for other signaling molecules to propagate the downstream signals (6, 13). Similar to the immune receptor stimulation, it was found that oxidative stress and osmotic stress induce the activation of Syk in avian B cells (34).
Pak2 interacts with nonreceptor PTK c-Abl (26). Tyrosine phosphorylation of Pak2 by c-Abl results in the suppression of Pak2 activity and causes accumulation and stabilization of Pak2 by inhibiting protein turnover through the proteasome degradation pathway. The experiments reported here have demonstrated that Pak2 complexes with another member of the nonreceptor type of PTK Syk. This association is enhanced by the activation of Pak2. Moreover, the association of Pak2 with Syk stimulates the kinase activity of Syk. In vitro, Pak2 phosphorylates Syk and stimulates Syk activity. Treatment of cells with sorbitol increases the formation of the Pak2-Syk complex, translocates both kinases from cytosol to the region surrounding the nucleus, and enhances Syk kinase activity. Furthermore, Pak2 and Syk functionally cooperate to activate the downstream c-Jun N-terminal kinase (JNK) in osmotic stress-mediated cellular signaling.
MATERIALS AND METHODS
Materials and antibodies.
Protein A-agarose, histone H2B, anti-Flag monoclonal antibody (MAb), and anti-α-tubulin were purchased from Sigma (St. Louis, Mo.). Antiphosphotyrosine (anti-pTyr) MAb (4G10), anti-JNK antibody, and anti-Syk MAb were obtained from Upstate Biotechnology (Lake Placid, N.Y.). Antihemagglutinin epitope (anti-HA) (Y-11), anti-Syk, and anti-Pak2/γ-PAK antibodies were from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-phospho-SAPK/JNK, anti-phospho-Syk (Tyr525/526), and anti-Syk antibodies were from Cell Signaling Technology (Beverly, Mass.). FuGENE 6 transfection reagent was from Roche Molecular Biochemicals (Indianapolis, Ind.). The HA-tagged WT Pak2, HA-Pak2 K278R (kinase inactive), HA-Pak2 T402A (dominant negative), and HA-Cdc42L61 (constitutively active) and HA-Cdc42N17 (constitutively inactive) (provided by J. S. Gutkind, National Institutes of Health, Bethesda, Md.) cDNAs were subcloned into the expression vector pcDNA3.1+ (7, 26). Flag-Cdc42L61 cDNA was generated by PCR. Human Syk cDNA (a gift from B. Mueller-Hilke, Deutsches RheumaForschungs-Zentrum, Berlin, Germany) was subcloned into pApuro expression vector. The Flag-tagged kinase-inactive form of human Syk cDNA was created by replacement of Lys402 by Arg (K402R) by using a PCR-based method. The cDNA of CAKβ/Pyk2 (a gift from T. Sasaki, Sapporo Medical University, Sapporo, Japan) was subcloned into pSVL expression vector. The expression constructs pSVL-Lyn and pApuro-T7-Btk were kindly provided by R. P. Siraganian (NIH) and T. Kurosaki (Kansai Medical School, Osaka, Japan), respectively. [γ-32P]ATP from NEN Life Science Products (Boston, Mass.) and Val5-angiotensin II from Calbiochem (San Diego, Calif.) were used for in vitro studies. Glutathione S-transferase (GST)-Cdc42 was expressed in Escherichia coli and purified as described previously (10). Sorbitol was purchased from Wako (Osaka, Japan), and 30% hydrogen peroxide was from Santoku (Tokyo, Japan).
Cell culture and transient transfection.
COS-7 cells were maintained as monolayer cultures in Dulbecco's modified Eagle's medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum and 100 U of penicillin per ml at 37°C. For transient transfection, 6 μl of FuGENE 6 reagent and 2 to 3 μg of cDNA were added to 105 of COS-7 cells seeded in six-well plate, according to the manufacturer's instructions. The amount of transfected cDNA was normalized by the empty vector. In some experiments, cells were incubated with Dulbecco's modified Eagle's medium containing 400 mM sorbitol for 30 min or with 100 μM of H2O2 for 10 min at 37°C. Ramos B cells were maintained in RPMI 1640 medium (Sigma) supplemented with 10% heat-inactivated fetal bovine serum and 100 U of penicillin per ml at 37°C. Ramos B cells expressing simian virus 40 T antigen (Ramos-T cells) were kindly provided by H. Band (Harvard Medical School, Boston, Mass.) and were maintained in the same manner as the parental Ramos B cells. For transient transfection, 10 μg of cDNA was transfected into 107 Ramos-T cells by electroporation (310 V, 950 μF). At 48 h after transfection, cells were utilized for the experiments.
Immunoprecipitation and immunoblotting.
At 48 h after transfection, cells were washed twice with ice-cold phosphate-buffered saline (PBS) and solubilized in lysis buffer (1% Nonidet P-40, 50 mM Tris [pH 7.4], 150 mM NaCl, 10 mM EDTA, 1 mM NaF, 1 mM Na3VO4, and protease inhibitors [1 mM phenylmethylsulfonyl fluoride and 2 μg of aprotinin per ml]) (29, 30). The cell lysates were precleared by centrifugation, and the resultant supernatants were incubated with 1 μg of each primary antibody prebound to protein A-agarose. Aliquots from the first centrifugation are referred to as detergent-soluble lysate. After incubation for 1 h at 4°C, the beads were washed four times with lysis buffer. The immunoprecipitated proteins were eluted by heat treatment at 100°C for 5 min in 2× sample buffer, separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and electrophoretically transferred to polyvinylidene difluoride membranes (Millipore, Bedford, Mass.). The membranes were blocked with 5% milk in TBST (25 mM Tris [pH 8.0], 150 mM NaCl, and 0.1% Tween 20) for 1 h at room temperature. The blots were incubated with the indicated primary antibodies for 1 h at 4°C. The membranes were washed four times with TBST and incubated with horseradish peroxidase-conjugated secondary antibodies for 30 min at room temperature. After extensive washing with TBST, proteins were visualized by enhanced chemiluminescence (Perkin-Elmer Life Science, Boston, Mass.). For preparation of total cell lysates, cells were washed twice with PBS and directly analyzed by SDS-PAGE after addition of 2× sample buffer. Densitometric analysis of immunoblotting was performed with NIH Image software.
In vitro protein kinase assay.
The washed anti-Syk immunoprecipitates from the differentially transfected cells were incubated in 40 μl of kinase buffer (30 mM HEPES [pH 7.5], 10 mM MgCl2, 2 mM MnCl2, 4 μM ATP, 4 μCi of [γ-32P]ATP) and 10 μg of the substrate histone H2B for the indicated times at room temperature (32). Reactions were terminated by heat treatment at 100°C for 5 min in 2× sample buffer, and the proteins were separated by SDS-PAGE. The gels were treated with 1 N KOH for 1 h at 56°C to remove phosphoserine and most of the phosphothreonine. After gel drying, radiolabeled proteins were visualized by autoradiography.
Expression and purification of Syk from insect cells.
Syk cDNA was subcloned into the baculovirus expression vector pAcG2T by PCR, using the 5′ primer GGATCCATGGCAGACAGTG (underlining indicates the BamHI site) and the 3′ primer GAATTCTTAATTAACCACATCGTAGTAG (the EcoRI site is underlined). The PCR product obtained with Pfu DNA polymerase was subcloned into the BamHI (5′) and EcoRI (3′) sites of pAcG2T (BD Biosciences, Lexington, Ky.) to make Syk in frame with the upstream GST tag. Transfection of insect cells (TN5B-4) and establishment of the recombinant virus were performed with the BaculoGold Transfection kit (BD Biosciences), and overexpression clones were identified. GST-Syk was purified on glutathione-Sepharose 4B (Amersham, Piscataway, N.J.) and eluted with glutathione.
Phosphorylation of Syk and Pak2 in vitro.
GST-Pak2 was obtained by expression of the cDNA in insect cells (TN5B-4) and purified by binding to glutathione-Sepharose 4B. GST-Pak2 was eluted with glutathione, and Pak2 was obtained by cleavage with thrombin as described previously (10). Cdc42 was preloaded with 0.18 mM GTPγS prior to addition of Pak2 (10). Phosphorylation was carried out in 30-μl reaction mixtures containing GST-Syk (0.5 μg) and Pak2 (0.5 to 1.0 μg) in 20 mM Tris (pH 7.4)-5 mM MnCl2-5 mM MgCl2-30 mM 2-mercaptoethanol with 0.2 mM [γ-32P]ATP (specific activity, 2,000 to 4,000 cpm/pmol) and 2 μg of leupeptin and aprotinin per ml. Incubation was at 30°C for 30 min. Reactions were terminated by the addition of 5 μl of 100 mM ATP and SDS sample buffer and analyzed by SDS-PAGE on 10% gels. The radiolabeled proteins were detected by autoradiography with X-ray film or a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). The 32P incorporated was quantified by counting proteins excised from the gel in a liquid scintillation counter.
To determine the protein kinase activity of Syk, reactions were carried out as described above except that Val5-angiotensin II (1 mM) and bovine serum albumin (0.2 mg/ml) were added. After termination of the reaction with 5 μl of 100 mM ATP, an aliquot (3 to 5 μl) of each sample was analyzed by thin-layer electrophoresis and an aliquot of 20 μl was analyzed by precipitation on Whatman P81 phosphocellulose filter paper as described previously (36). 32P incorporation into Syk precipitated in the filter paper was quantified by liquid scintillation counting. 32P incorporation when analyzed by thin-layer electrophoresis was quantified with the ImageQuant software of the PhosphorImager.
Phosphoamino acid analysis.
GST-Syk and Pak2 phosphorylated with [γ-32P]ATP were analyzed by SDS-PAGE as described above. Phosphoamino acid analysis was carried out on phosphorylated proteins excised from the gel as described previously (35).
Immunofluorescence microscopy.
COS-7 cells transiently transfected with different cDNAs were fixed with 4% paraformaldehyde in PBS for 10 min. Cells were washed twice with PBS, permeabilized with 0.2% Triton X-100 in PBS for 10 min, washed three times with PBS, and blocked with 3% bovine serum albumin in PBS, all at room temperature. For double staining, cells were primed with anti-HA MAb and rabbit polyclonal anti-Syk antibody for 1 h at room temperature, washed three times with 0.5% Triton X-100 in PBS, and then incubated with secondary antibody (Cy3-labeled goat anti-mouse immunoglobulin G or fluorescein isothiocyanate-labeled goat anti-rabbit immunoglobulin G) for 30 min. The cells were washed as described above, mounted by using a SlowFade antifade kit (Molecular Probes, Eugene, Oreg.), and analyzed by confocal microscopy with LSM 5 Pascal and LSM 510 META instruments (Carl Zeiss, Jena, Germany) (20).
RNA interference.
Two sets of synthetic oligonucleotides for the sense and antisense target sequences of the human Pak2-coding sequences including bp 17 to 35 and 472 to 490 with stem-loop sequence to form the small hairpin RNA (5′-GATCCGCTGGAAGATAAGCCTCCAGTTCAAGAGACTGGAGGCTTATCTTCCAGTTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAACTGGAAGATAAGCCTCCAGTCTCTTGAACTGGAGGCTTATCTTCCAGCG-3′ [bp 17 to 35] and 5′-GATCCGTGCCAAGGGAACAGAAGCATTCAAGAGATGCTTCTGTTCCCTTGGCATTTTTTGGAAA-3′ and 5′-AGCTTTTCCAAAAAATGCCAAGGGAACAGAAGCATCTCTTGAATGCTTCTGTTCCCTTGGCACG-3′ [bp 472 to 490]) were phosphorylated with T4 kinase (Takara, Tokyo, Japan), annealed, and ligated into BamHI- and HindIII-cleaved backbone of pSilencer 2.1-U6 (Ambion, Austin, Tex.). Both sequences are specific for Pak2. Ten micrograms of the empty vector, pSilencer-Pak2 short interfering RNA (siRNA) (bp 17 to 35), or pSilencer-Pak2 siRNA (bp 472 to 490) was transiently transfected to Ramos-T cells (107) by electroporation (310 V, 950 μF). At 48 h after transfection, cells were utilized for the experiments.
RESULTS
Activation of Pak2 induces the association with Syk.
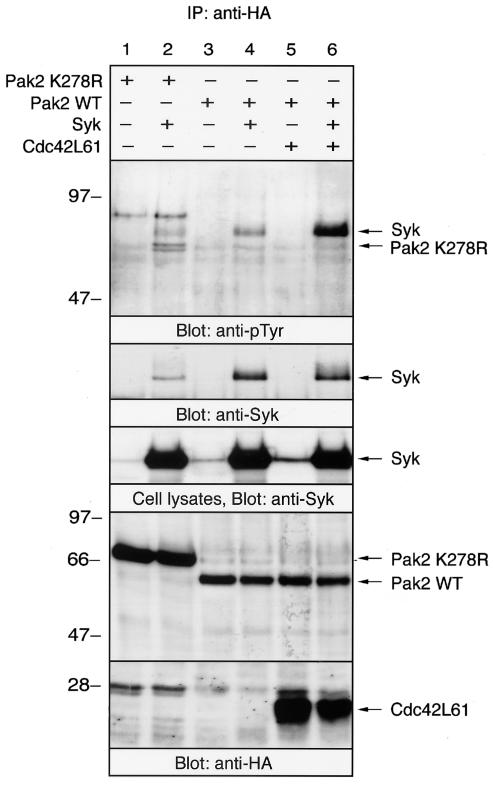
The p21-activated protein kinase Pak2 and Syk are known to be activated when the cells are exposed to osmotic stress (24, 34). To examine whether Pak2 and Syk cooperate in cellular signaling, HA-tagged Pak2 and Syk cDNAs were transiently transfected into COS-7 cells. Pak2 was immunoprecipitated by anti-HA antibody, and the immunoprecipitates were analyzed by immunoblotting with anti-pTyr MAb (Fig. 1, top panel). Tyrosine-phosphorylated proteins migrating with the molecular weight of Syk were obtained in the anti-HA immunoprecipitates of Pak2, and the level of phosphorylation of Syk was significantly increased with constitutively active Cdc42. Immunoblotting experiments confirmed that Syk coimmunoprecipitated with WT Pak2, but only a low level of Syk was associated with the kinase-inactive mutant (K278R) (Fig. 1, second panel). This association of Pak2 with Syk was enhanced by coexpression with Cdc42L61, the constitutively active form of Cdc42 (Fig. 1, lane 6).
FIG. 1.
Association of Pak2 with Syk. The cDNAs encoding WT HA-Pak2, the kinase-inactive mutant Pak2 K278R, Syk, and HA-Cdc42L61 were transiently cotransfected into COS-7 cells. After 48 h of transfection, cells were solubilized with lysis buffer and Pak2 was immunoprecipitated (IP) with anti-HA antibody. Immunoprecipitated proteins and detergent-soluble lysates were separated by SDS-PAGE, electrophoretically transferred to polyvinylidene difluoride membranes and then analyzed by immunoblotting with anti-pTyr MAb and anti-Syk and anti-HA (Pak2 and Cdc42) antibodies. Positions of molecular mass markers are shown on the left in kilodaltons.
Quantitative analysis revealed that Cdc42L61 increased the amount of Syk protein (1.2-fold) and the extent of tyrosine phosphorylation of Syk (4.2-fold) in the immunoprecipitates of Pak2 (Fig. 1, top and second panels, lane 4 versus lane 6). In the immunoprecipitates of Pak2 K278R, the amounts of coprecipitated Syk and its tyrosine phosphorylation were decreased (0.3- and 0.4-fold, respectively) compared to those of WT Pak2 (Fig. 1, top and second panels, lane 2 versus lane 4). HA-Cdc42 was also precipitated with anti-HA antibody; however, Cdc42 alone could not precipitate Syk (data not shown). In addition, a different HA-tagged protein, HA-Cbl, could not precipitate significant amounts of Syk, suggesting that Pak2 specifically associates with Syk in COS cells (data not shown). Immunoblotting of the detergent-soluble lysates indicated that equal amounts of Syk were expressed in the cells transfected with the different cDNAs (Fig. 1, third panel). As reported previously (26), the expression level of WT Pak2 was much lower than that of the kinase-inactive mutant K278R, because WT Pak2 can be down-regulated by the proteasome degradation pathway (Fig. 1, bottom panel). In addition, the migration of K278R was slower than that of the WT, as reported previously (7, 26). These results demonstrated that the association of Pak2 with Syk is enhanced by the activation of Pak2.
Cotransfection of Pak2 with Syk elicited slight tyrosine phosphorylation of K278R (Fig. 1, top panel). Tyrosine phosphorylation of WT Pak2 was observed only when the membrane was exposed for longer times (data not shown).
The dominant-negative form of Cdc42 inhibits the association of Pak2 with Syk.
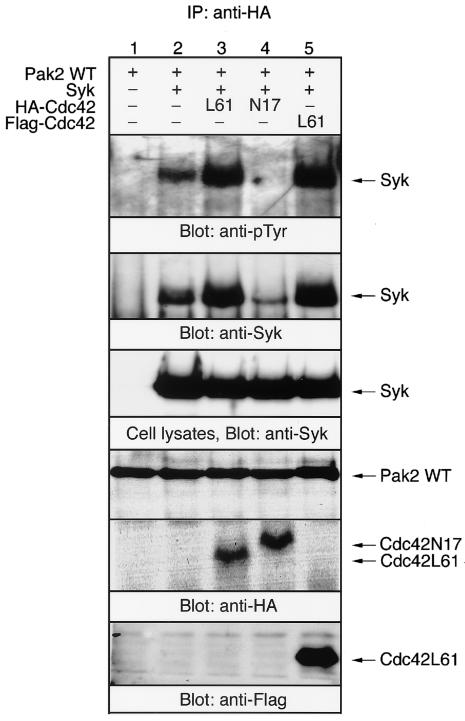
The serine/threonine kinase activity of Pak family protein kinases is stimulated by binding of activated GTP bound to Cdc42/Rac (3). To examine the functional interaction between Pak2 and Syk, the cDNAs of HA-tagged WT Pak2 and Syk were cotransfected into COS-7 cells along with either constitutively active Cdc42L61 or inactive Cdc42N17 (Fig. 2). The expression of Cdc42L61 increased the association of Pak2 with Syk, as shown in Fig. 1. On the other hand, expression of inactive Cdc42N17 blocked the coprecipitation of Syk with Pak2 (Fig. 2, lane 4). It is suggested that overexpression of the dominant-negative form of Cdc42 inhibited the activation of Pak2 by endogenous Cdc42 and the subsequent interaction with Syk (Fig. 2, lane 2). The amounts of Syk protein expressed in cells transfected with different cDNAs were similar (Fig. 2, third panel). To confirm that the increase in the coprecipitation of Syk with HA-Pak2 was not due to the same epitope tag on Cdc42, we tested Cdc42L61 with a different epitope tag. As shown (Fig. 2, lane 5), cotransfection with Flag-tagged Cdc42L61 also resulted in a similar increase in the coprecipitation of Syk with Pak2. These findings suggested that Cdc42 functionally regulates the association of Pak2 with Syk in vivo.
FIG. 2.
Cdc42 regulates the association of Pak2 with Syk. COS-7 cells were transiently transfected with the cDNAs for WT HA-Pak2, Syk, HA-Cdc42L61, HA-Cdc42N17, and Flag-Cdc42L61, as indicated. At 48 h after transfection, cells were solubilized with lysis buffer and Pak2 was immunoprecipitated (IP) with anti-HA antibody. Immunoprecipitated proteins and detergent-soluble lysates were analyzed by immunoblotting with anti-pTyr, anti-Syk, anti-HA (Pak2 and Cdc42), and anti-Flag (Cdc42) antibodies.
Association with Pak2 induces the activation of Syk.
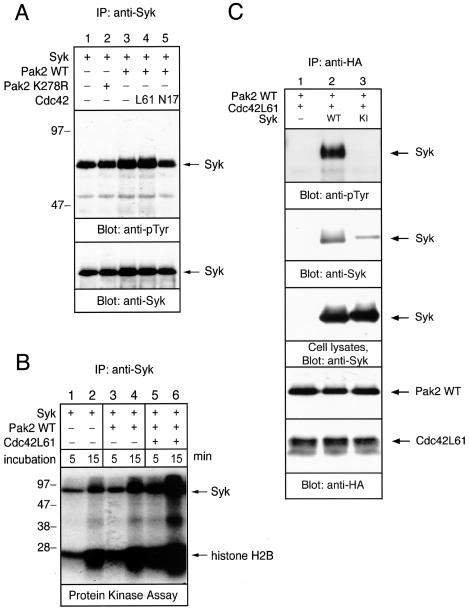
Since activation of Pak2 resulted in its association with Syk, we examined the effects of Pak2 association on Syk kinase activity. Tyrosine phosphorylation and the intrinsic kinase activity of Syk were measured by immunoblotting with anti-pTyr and by a Syk activity assay in vitro (Fig. 3A and B, respectively). Addition of WT Pak2 slightly elevated the tyrosine phosphorylation of Syk. This Pak2-mediated enhancement of tyrosine phosphorylation of Syk was enhanced further by cotransfection of WT Pak2 and Cdc42L61. Expression of the kinase-inactive Pak2 K278R had no effect on Syk phosphorylation. Coexpression with Cdc42N17, which inhibited the association of Pak2 with Syk, suppressed tyrosine phosphorylation of Syk (Fig. 3A, lane 5). To examine the intrinsic kinase activity of Syk, anti-Syk immunoprecipitates were subjected to an in vitro protein kinase assay (Fig. 3B). The samples were separated by SDS-PAGE, and gels were incubated with KOH to remove phosphoserine and most phosphothreonine. Coexpression with Pak2 elevated autophosphorylation of Syk and phosphorylation of exogenous substrate histone H2B. There was no detectable signal in an in vitro kinase assay with Pak2 immunoprecipitated by anti-HA after the KOH treatment (data not shown). This ruled out the possibility that phosphorylation of H2B histone was due to the coprecipitated Pak2. Also, we could not detect Pak2 in the Syk immunoprecipitates by immunoblotting (data not shown). Moreover, activation of Pak2 by Cdc42L61 significantly enhanced the kinase activity of Syk (Fig. 3B). Cdc42N17 showed a negative effect on the kinase activity of Syk (data not shown). Thus, this result indicated that association of the active form of Pak2 enhances tyrosine phosphorylation and the intrinsic kinase activity of Syk. It suggested that association with Pak2 stimulates the kinase activity of Syk.
FIG. 3.
Pak2 activates Syk. (A and B) COS-7 cells were transfected with Syk, Pak2, and Cdc42 cDNAs, as indicated. Cells were solubilized with the lysis buffer and Syk was immunoprecipitated (IP) with anti-Syk antibody. Each immunoprecipitate was split, analyzed by immunoblotting, and assayed for protein kinase activity. (A) Anti-Syk immunoprecipitates were separated by SDS-8% PAGE and analyzed by immunoblotting with anti-pTyr and anti-Syk antibodies. Positions of molecular mass markers are shown on the left in kilodaltons. (B) Anti-Syk immunoprecipitates were assayed for protein kinase activity. Samples were incubated with 4 μCi of [γ-32P]ATP and 10 μg of the exogenous substrate histone H2B for the indicated times and then separated by SDS-15% PAGE. The gel was treated with 1 N KOH for 1 h at 56°C, and radiolabeled proteins were visualized by autoradiography. (C) COS-7 cells were transfected with Pak2, Cdc42L61, WT Syk, and Flag-tagged kinase-inactive (KI) Syk cDNAs, as indicated. Cells were solubilized with the lysis buffer, and Pak2 was immunoprecipitated with anti-HA antibody. The immunoprecipitated proteins and detergent-soluble lysates were analyzed by immunoblotting with anti-pTyr MAb and anti-Syk and anti-HA (Pak2 and Cdc42) antibodies.
We then tested whether the kinase activity of Syk is required for the interaction between Pak2 and Syk. Coprecipitation of Pak2 with the kinase-inactive form of Syk was less than that of WT Syk (Fig. 3C). Therefore, the association of Pak2 with Syk requires the kinase activity of Syk.
Phosphorylation and activation of Syk by Pak2 in vitro.
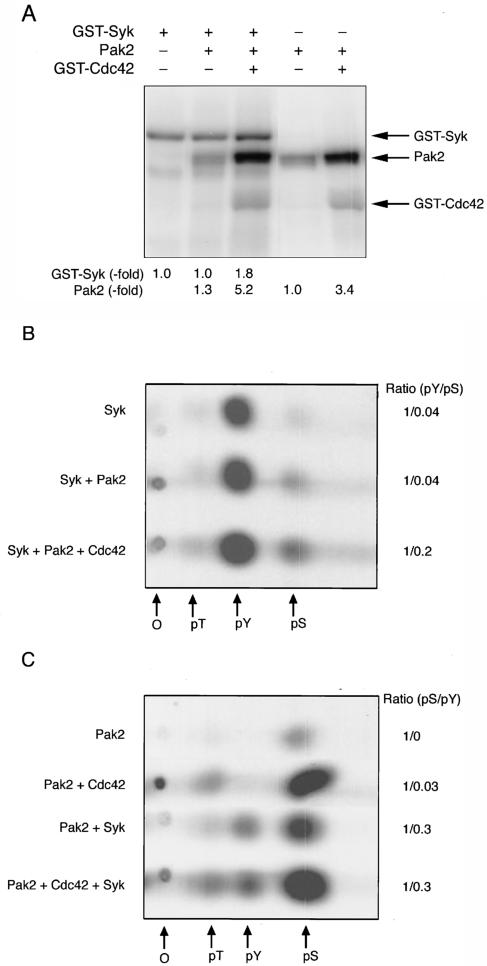
The association of Pak2 and Syk, with the resultant enhancement of autophosphorylation and activity of Syk when Syk was coexpressed with Pak2 and Cdc42, suggested that Syk could be a substrate for Pak2. To examine this, purified GST-Syk expressed in insect cells was incubated with purified Pak2 and [γ-32P]ATP in the presence of Mg2+ and Mn2+. Phosphorylation of GST-Syk was increased 1.8-fold when phosphorylation was by Pak2 activated with Cdc42 (GTPγS) (Fig. 4A). As expected, phosphoamino acid analysis of autophosphorylated Syk showed that the phosphorylation was exclusively on tyrosine (Fig. 4B). There was little effect on phosphorylation with inactive Pak2. When activated Pak2 was added to the assay mixture, there was additional phosphorylation of Syk on both serine and tyrosine. Quantification of the data showed that the phosphotyrosine/phosphoserine ratio decreased fivefold with activated Pak2. Taken together, the data indicated that there was a substantial increase in tyrosine phosphorylation when Syk was phosphorylated on serine by Pak2.
FIG. 4.
Phosphorylation of Syk and Pak2 in vitro. GST-Syk (0.5 μg) was incubated with Pak2 and [γ-32P]ATP as described in Materials and Methods. (A) Samples were analyzed by SDS-PAGE, and the radiolabeled proteins were identified by autoradiography. (B) Phosphoamino acid analysis of Syk phosphorylated by Pak2. (C) Phosphoamino acid analysis of Pak2 phosphorylated by Syk. O, origin; pT, pY, and pS, phosphorylated threonine, tyrosine, and serine, respectively. The autoradiograms are shown.
Pak2 was also phosphorylated by Syk (Fig. 4A). There was a 1.3-fold increase in phosphorylation of inactive Pak2 and a 1.5-fold increase in Cdc42-activated Pak2. Pak2 was autophosphorylated only on serine in the absence of Cdc42 and on serine and threonine in the presence of Cdc42 (Fig. 4C). Addition of Syk resulted in phosphorylation of tyrosine in both cases. Phosphorylation of tyrosine did not result in activation of Pak2, as shown by a lack of phosphate in the activation loop threonine in inactive Pak2 (Fig. 4B). In fact, the ratio of phosphoserine to phosphotyrosine was similar with both active and inactive Pak2.
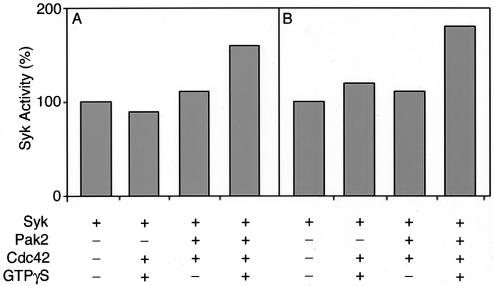
The effects of phosphorylation of Syk by Pak2 were examined by using Val5-angiotensin II as the substrate. As shown in Fig. 5, phosphorylation of angiotensin was increased when Syk was incubated with Cdc42-activated Pak2. An increase of 1.6-fold was observed when 32P incorporation into Syk was analyzed by thin-layer electrophoresis (Fig. 5A). This was consistent with the results obtained by precipitation of phosphorylated Syk on filter paper (Fig. 5B).
FIG. 5.
Stimulation of Syk tyrosine kinase activity by Pak2. GST-Syk and Pak2 were incubated with [γ-32P]ATP with angiotensin as a substrate. Samples were analyzed by thin-layer electrophoresis (A) and by precipitation on P81 phosphocellulose paper (B) as described in Materials and Methods. 32P incorporated into angiotensin was quantified with a PhosphorImager (A) or by liquid scintillation counting (B).
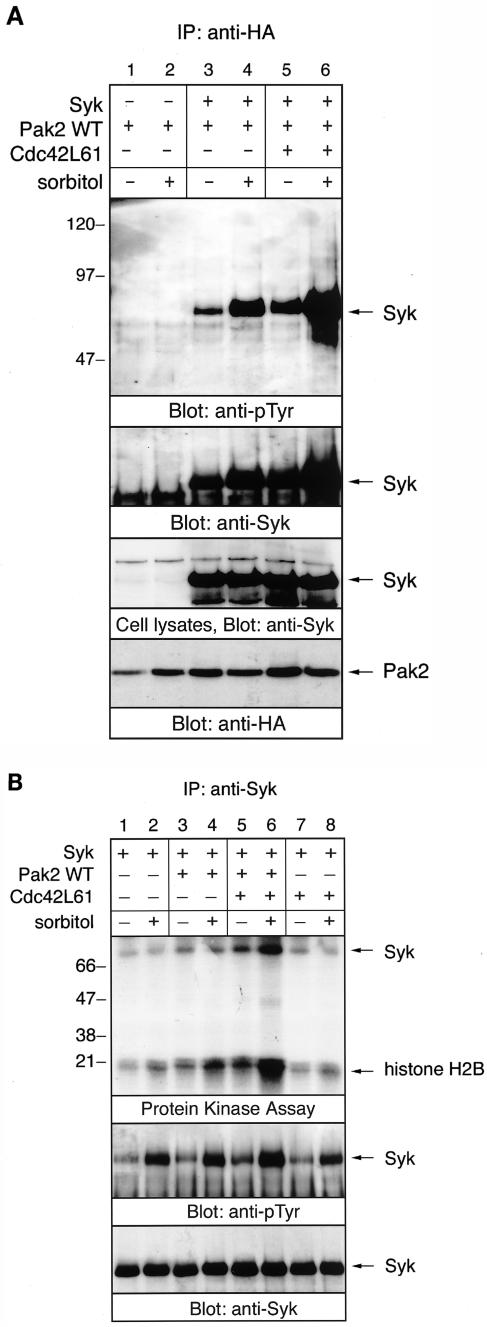
Hyperosmotic stress promotes the association of Pak2 with Syk.
We have demonstrated that treatment of avian B cells with sorbitol activates Syk, although the receptor for the osmotic stress has not yet been identified (22). Sorbitol also results in the translocation and subsequent activation of the protein kinase activity of Pak2 in the particulate fraction by association with Cdc42 (24). Therefore, we examined whether the interaction between Pak2 and Syk was affected by osmotic stress. Sorbitol stimulation resulted in an increase in the association of Pak2 with Syk (Fig. 6A, lanes 3 and 4). This association was dramatically increased in cells transfected with Cdc42L61 (Fig. 6A, lanes 5 and 6). Equal amounts of Syk were expressed in cells transfected with different cDNAs (Fig. 6A, third panel). This result indicated that hyperosmotic stress and Cdc42 synergistically promote the association of Pak2 with Syk.
FIG.6.
Hyperosmolarity promotes the association of Pak2 with Syk and Pak2-mediated activation of Syk. COS-7 cells transfected with the combination of Syk, WT Pak2, and Cdc42 cDNAs were incubated in medium with or without 400 mM sorbitol. Cell lysates were immunoprecipitated (IP) with either anti-HA or anti-Syk antibody. (A) Proteins immunoprecipitated with anti-HA antibody, and detergent-soluble lysates were analyzed by immunoblotting with anti-pTyr, anti-Syk, and anti-HA (Pak2) antibodies. Positions of molecular mass markers are shown on the left in kilodaltons. (B) Anti-Syk immunoprecipitates were split and analyzed for protein kinase assay (top panel) or by immunoblotting with anti-pTyr and anti-Syk antibodies (middle and lower panels, respectively). For the protein kinase assay, anti-Syk immunoprecipitates were incubated with 4 μCi of [γ-32P]ATP and 10 μg of exogenous substrate histone H2B for 10 min at room temperature. Radiolabeled proteins were visualized by autoradiography.
We then tested the effect of osmotic stress on Pak2-mediated activation of Syk (Fig. 6B). In unstimulated cells, coexpression of Pak2 elevated the kinase activity of Syk, and addition of Cdc42 further stimulated Pak2-mediated activation of Syk (Fig. 6B, upper panel, lanes 1, 3, and 5). Cdc42L61 alone induced the kinase activity of Syk to a lesser extent, perhaps by activating endogenous Pak kinases (Fig. 6B, lane 7). Stimulation of cells with sorbitol increased the kinase activity of Syk, and cotransfection of WT Pak2 enhanced the sorbitol-mediated activation of Syk (Fig. 6B, lanes 1 to 4). Addition of Cdc42L61 could further stimulate sorbitol-mediated activation of Syk (Fig. 6B, lanes 5 and 6). This result demonstrated that hyperosmolarity increases the association of Pak2 with Syk and Pak2-mediated activation of Syk. A similar effect was observed when cells were treated with 400 mM sucrose (data not shown).
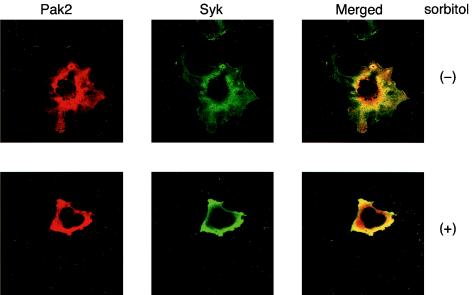
Hyperosmotic stress induces the association of Pak2 with Syk in the perinuclear region.
Endogenous Pak2 is present in both the cytosolic and particulate fractions in mouse fibroblast 3T3-L1 cells. Moreover, recent findings demonstrate that localization of Pak2 in the endoplasmic reticulum is required for the induction of cytostasis (7). Therefore, we examined the intracellular localization of Pak2 and Syk in COS-7 cells. Immunohistochemical analysis using confocal microscopy confirmed the association of Pak2 and Syk (Fig. 7). Pak2 and Syk were concentrated in a region near the nucleus and were also located elsewhere in the cytosolic region, but not in the nucleus (Fig. 7, top panels). Sorbitol treatment resulted in shrinkage of the cells and an increase in the association of Pak2 with Syk in the perinuclear region (Fig. 7, bottom panels).
FIG. 7.
Hyperosmolarity promotes the association of Pak2 with Syk in the perinuclear region. COS-7 cells were transfected with Pak2 and Syk cDNAs. The intracellular localization of Pak2 and Syk was analyzed by immunohistochemical analysis with anti-HA and anti-Syk antibodies. The upper panels show the localization of Pak2 (left) and Syk (middle) in unstimulated cells and a merged image (right). The lower panels show the localization of the molecules in sorbitol-stimulated cells.
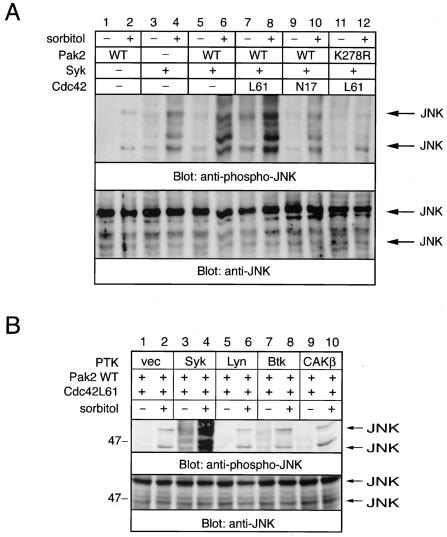
Pak2 and Syk functionally cooperate to enhance sorbitol-mediated activation of JNK.
It was previously demonstrated that Syk and Rac1 lead to synergistic JNK activation in T cells (9). In addition, Syk is critical for antigen-mediated activation of JNK in avian B cells (12). Therefore, we examined the effects of Pak2 and Syk in sorbitol-mediated activation of JNK (Fig. 8). As shown by immunoblotting with antibody to phospho-JNK, sorbitol-mediated activation of JNK was enhanced in the cells transfected with Syk. The JNK activity was dramatically enhanced in cells coexpressing Syk, Pak2, and Cdc42L61 (Fig. 8A). Osmotic stress had no effect on activation of extracellular signal-regulated kinase (ERK) and p38 kinase in all transfected cells (data not shown). Furthermore, we tested whether the different kinds of nonreceptor PTKs cooperate with Pak2 to activate JNK. Dramatic activation of JNK was observed only in cells cotransfected with Syk PTK and not in those transfected with Lyn, Btk, or CAKβ/Pyk2 (Fig. 8B). Thus, hyperosmolarity dramatically increased the association of Pak2 with Syk, leading to the activation of Syk and thereby downstream JNK phosphorylation.
FIG. 8.
Pak2 and Syk cooperate in hyperosmolarity-induced activation of JNK. COS-7 cells were transfected with the combination of Syk, Pak2 (WT and K278R), and Cdc42 cDNAs as indicated (A) or Pak2, Cdc42L61, and the various PTK cDNAs were cotransfected into COS-7 cells (B). Cells were incubated in medium with or without the addition of 400 mM sorbitol. Total cell lysates were analyzed by immunoblotting with anti-phospho-JNK and anti-JNK antibodies. Positions of molecular mass markers are shown on the left in kilodaltons.
Oxidative stress induces the association of Pak2 with Syk.
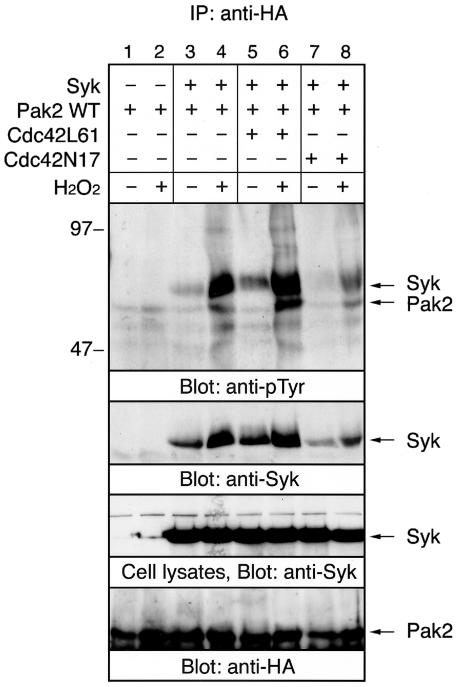
Besides hyperosmolarity, we tested the effect of a different kind of cellular stress on the association of Pak2 with Syk. Syk is required for H2O2-mediated activation of Akt and PI 3-kinase (34). Therefore, we examined whether treatment of cells with H2O2 induces the association of Pak2 with Syk. Treatment of cells with 100 μM H2O2 for 10 min induced the association of Pak2 with Syk and tyrosine phosphorylation of Pak2 by Syk (Fig. 9). There was no apparent precipitation of Syk with the antibody to HA when the cells were transfected with Syk alone (data not shown). This association was also regulated by the activity of Cdc42. Therefore, this result indicated that Pak2 and Syk functionally cooperated in both osmotic and oxidative stress-mediated signaling in vivo.
FIG. 9.
Oxidative stress induces the association of Pak2 with Syk. COS-7 cells transfected with Syk, Pak2, Cdc42L61, and Cdc42N17 cDNAs were incubated with or without 100 μM H2O2. Cell lysates were immunoprecipitated (IP) with anti-HA antibody. Immunoprecipitates and detergent-soluble cell lysates were analyzed by immunoblotting with anti-pTyr, anti-Syk, and anti-HA antibodies. Positions of molecular mass markers are shown on the left in kilodaltons.
Direct involvement of Pak2-Syk interaction in physiological hyperosmolar responses.
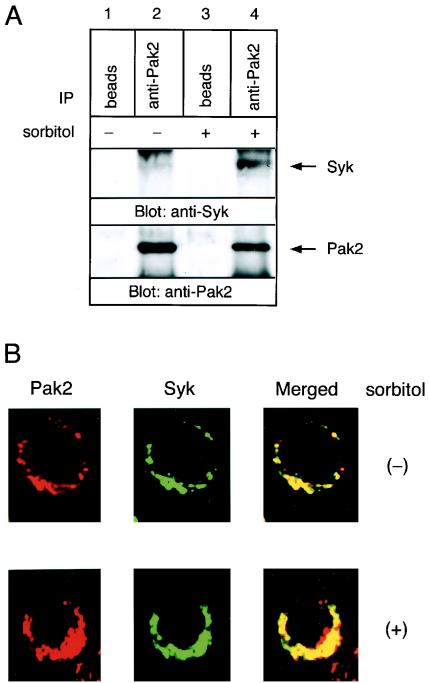
Based on these findings, we tested whether osmotic stress can induce the interaction between the endogenous Pak2 and Syk. Sorbitol treatment of Ramos B cells resulted in an increase in the endogenous association of Pak2 with Syk (Fig. 10A). These results further emphasize the biological significance of the interaction of the two molecules. Immunohistochemical analysis using confocal microscopy confirmed the association of Pak2 and Syk in B cells in the perinuclear region, which was enhanced in response to sorbitol (Fig. 10B).
FIG. 10.
Hyperosmolarity promotes the endogenous interaction of Pak2 with Syk in B cells. (A) Ramos cells (20 × 106) were incubated in medium with or without 400 mM sorbitol. Cell lysates were immunoprecipitated (IP) with anti-Pak2 antibody or beads alone. The immunoprecipitated proteins were analyzed by immunoblotting with anti-Syk and anti-Pak2 antibodies. (B) The intracellular localization of endogenous Pak2 and Syk in B cells was analyzed immunohistochemically with anti-Pak2 and anti-Syk antibodies. The upper panels show the localization of Pak2 (left) and Syk (middle) in unstimulated cells and a merged image (right). The lower panels show the localization of the molecules in sorbitol-stimulated cells.
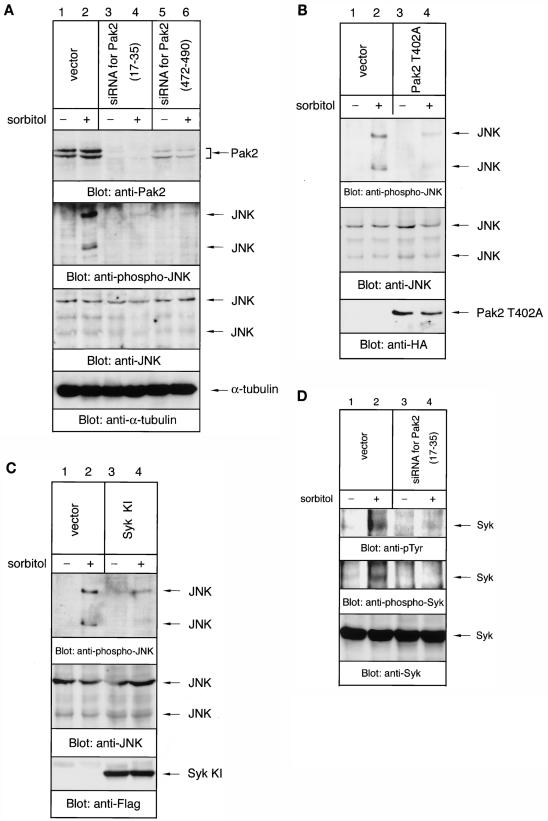
To examine the involvement of the proposed pathway in the hyperosmolar response, we designed two kinds of siRNAs specific for Pak2. Transfection of these expression constructs resulted in the lowering of the expression level of endogenous Pak2 and sorbitol-mediated activation of JNK in B cells (Fig. 11A). Transfection of Pak2 siRNA did not suppress the expression of JNK and α-tubulin (Fig. 11A). Densitometric analysis revealed that protein expression was suppressed by 90 or 70% with the Pak2(17-35) or Pak2(472-490) siRNA, respectively. This demonstrated that Pak2 is essential for hyperosmolarity-induced activation of JNK in B cells. In addition to the siRNA, overexpression of the dominant-negative form of Pak2 (T402A) resulted in the suppression of sorbitol-mediated activation of JNK (Fig. 11B) (7). Moreover, overexpression of the kinase-inactive form of Syk suppressed sorbitol-mediated activation of JNK (Fig. 11C). Thus, these results suggest that both Pak2 and its associating protein Syk are critical in B cells for hyperosmolarity-mediated physiological responses. Finally, the effect of Pak2 siRNA on hyperosmolarity-induced activation of endogenous Syk was examined (Fig. 11D). As shown, suppression of Pak2 directly inhibits phosphorylation of Syk. Phosphorylation of Tyr525 and Tyr526 in the activation loop of Syk is correlated with the activation of Syk (17, 41). These results demonstrated that Pak2 is required for the activation of Syk by sorbitol and that the Pak2-Syk pathway is involved in the hyperosmolar response. The data identify a new pathway for activation of the stress-activated protein kinase JNK through Cdc42.
FIG. 11.
Suppression of Pak2 reduces hyperosmolarity-induced activation of endogenous Syk and JNK in B cells. At 48 h after transfection, cells were incubated in serum-free medium without or with 400 mM sorbitol for 30 min. Equal amounts of the cell lysates were analyzed by immunoblotting with anti-Pak2, anti-phospho-JNK, anti-JNK, anti-α-tubulin, and anti-HA, anti-Flag, anti-pTyr, anti-phospho-Syk (Tyr525/526), and anti-Syk antibodies, as indicated. (A) Ramos cells were transiently transfected with the pSilencer vector, pSilencer-siRNA for Pak2(17-35) or for Pak2(472-490). (B) Ramos cells were transiently transfected with pcDNA3.1+ vector or the dominant-negative form of Pak2 (T402A). (C) Ramos cells were transiently transfected with pcDNA3.1+ vector or the kinase-inactive form of Syk (Syk KI). (D) Ramos cells were transiently transfected with pSilencer vector or pSilencer-siRNA for Pak2(17-35).
DISCUSSION
Activation of Pak2 by binding to the activated form of Cdc42 or in response to hyperosmotic stress synergistically promotes the association and enzymatic activation of Syk. Syk is a substrate of Pak2 in vitro and is activated by phosphorylation. Our finding is the first report that Syk is up-regulated by the serine/threonine kinase Pak2. It remains unknown which domain of Syk is responsible for the interaction with Pak2. In immune receptor signaling, activation of Syk requires conformational alteration from a closed inactive structure to an open active structure by binding of the SH2 domains to phosphorylated ITAM (14). It is suggested that phosphorylation of Syk on serine or threonine residues by Pak2 induces the conformational change to activate Syk kinase activity. In formyl methionyl-leucyl-phenylalanine receptor-mediated signaling, cyclic AMP-dependent protein kinase negatively regulates formyl methionyl-leucyl-phenylalanine-mediated activation of Syk (2). Syk is a substrate of cyclic AMP-dependent protein kinase in vitro (2). It is suggested that Pak2 may phosphorylate different a serine/threonine residue(s) on Syk, resulting in enzymatic activation.
We have demonstrated that Pak2 is upstream of Syk in cellular signaling and that Pak2 and Syk cooperate in the hyperosmotic stress-mediated activation of JNK. Activation of JNK leads to the activation of various transcription factors and expression of specific genes (5). We utilized sorbitol-mediated activation of JNK as a parameter of Syk kinase activity in cellular signaling. Our results show that sorbitol-mediated activation of endogenous JNK is enhanced by the overexpression of Syk (Fig. 8). Expression of the kinase-inactive form of Syk suppresses sorbitol-mediated activation of JNK (Fig. 11). Furthermore, coexpression of WT Pak2 and Cdc42 with Syk dramatically stimulates JNK activation, as a result of the association of Pak2 and Syk, and elevates Syk kinase activity. The suppression of Pak2 by the specific siRNA results in the inhibition of sorbitol-mediated activation of both Syk and JNK (Fig. 11). We have not tested whether caspase 3 cleavage products of Pak2 could associate with Syk and stimulate its kinase activity. In our experimental system, osmotic stress did not cause any increase in the caspase cleavage of Pak2 in COS-7 cells (data not shown). It remains unknown whether the extreme stress that results in cleavage and activation of Pak2 could induce the association of Pak2 with Syk to stimulate JNK activity. Collectively, Syk could be responsible for osmotic stress-mediated JNK activation in mammalian cells, cooperating with Pak2 and Rho family GTP binding proteins. Activation of Syk in response to osmotic stress requires the interaction with Pak2.
Pak2 has been shown to have cytostatic properties, as shown by microinjection of Pak2 into early frog embryos (27) and overexpression of Pak2 in COS-7 and HEK 293T cells (7). Activation of Pak2 by moderate cell stress inhibits cell division, resulting in inhibition of cell growth, whereas high levels of stress or irreparable damage results in cell death. Both Pak2 and Syk are activated in response to oxidative stress. Syk activation by moderate oxidative stress leads to the activation of the PI 3-kinase-Akt survival pathway (34). On the other hand, extreme oxidative stress induces Syk-dependent phospholipase C-γ activation, which accelerates oxidative stress-mediated apoptosis (34).
Both Pak2 and Syk were originally isolated as cytosolic kinases. Various cellular ligands induce the translocation of these enzymes to the perinuclear region. Active Pak2, but not the inactive enzyme, is tightly associated with the endoplasmic reticulum, and the amount of Pak2 associated with the endoplasmic reticulum is significantly enhanced in cells that are stressed and/or not dividing (7). In COS-7 cells, Pak2 and Syk are primarily cytosolic, and sorbitol treatment increases translocation of both kinases to the perinuclear region (Fig. 7). Unlike that of c-Abl, tyrosine phosphorylation of Pak2 by Syk did not result in an accumulation of inactive Pak2 or inhibit its protein turnover through the proteasome degradation pathway (Fig. 1). It is suggested that Pak2 and Syk transmit the activating signals to the downstream molecules from the perinuclear region. In immune receptor signaling, Syk translocates to the plasma membrane to interact with receptors in the glycolipid-enriched microdomain (33). Syk phosphorylates Vav, a guanine nucleotide exchange factor of Rac1, to regulate calcium mobilization and transcription of cytokine messages (1, 6, 19). In T cells, activation of Rac1 and subsequently Pak1 requires a Syk family PTK (16). Our findings demonstrate that there is a distinct distribution and function of Syk that is different from that established in the immune cells.
It is interesting that although both ERK and JNK are downstream of Syk in the immune receptor signaling, only JNK is activated in nonhematopoietic cells (12). In breast cancer cells, induction of Syk protein results in a suppression of tumor cell proliferation resulting from the abnormalities of cell division (14). Perhaps Pak2 and Syk cooperate to stimulate the transcription and translation of both proapoptotic and antiapoptotic proteins which are downstream of JNK.
Acknowledgments
This study was supported in part by research funding from the Uehara Memorial Foundation and the Hyogo Science and Technology Association; the 21st Century COE Program and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology of Japan; and a grant from the National Institutes of Health (GM26738) to J.A.T.
REFERENCES
- 1.Arudchandran, R., M. J. Brown, M. J. Peirce, J. S. Song, J. Zhang, R. P. Siraganian, U. Blank, and J. Rivera. 2000. The Src homology 2 domain of Vav is required for its compartmentation to the plasma membrane and activation of c-Jun NH(2)-terminal kinase 1. J. Exp. Med. 191:47-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asahi, M., Y. Tanaka, S. Qin, M. Tsubokawa, K. Sada, Y. Minami, and H. Yamamura. 1995. Cyclic AMP-elevating agents negatively regulate the activation of p72syk in N-formyl-methionyl-leucyl-phenylalanine receptor signaling. Biochem. Biophys. Res. Commun. 212:887-893. [DOI] [PubMed] [Google Scholar]
- 3.Bagrodia, S., and R. A. Cerione. 1999. Pak to the future. Trends Cell Biol. 9:350-355. [DOI] [PubMed] [Google Scholar]
- 4.Coopman, P. J., M. T. Do, M. Barth, E. T. Bowden, A. J. Hayes, E. Basyuk, J. K. Blancato, P. R. Vezza, S. W. McLeskey, P. H. Mangeat, and S. C. Mueller. 2000. The Syk tyrosine kinase suppresses malignant growth of human breast cancer cells. Nature 406:742-747. [DOI] [PubMed] [Google Scholar]
- 5.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 6.Deckert, M., S. Tartare-Deckert, C. Couture, T. Mustelin, and A. Altman. 1996. Functional and physical interactions of Syk family kinases with the Vav proto-oncogene product. Immunity 5:591-604. [DOI] [PubMed] [Google Scholar]
- 7.Huang, Z., J. Ling, and J. A. Traugh. 2003. Localization of p21-activated protein kinase gamma-PAK/Pak2 in the endoplasmic reticulum is required for induction of cytostasis. J. Biol. Chem. 278:13101-13109. [DOI] [PubMed] [Google Scholar]
- 8.Inoue, O., K. Suzuki-Inoue, W. L. Dean, J. Frampton, and S. P. Watson. 2003. Integrin alpha2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J. Cell Biol. 160:769-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacinto, E., G. Werlen, and M. Karin. 1998. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity 8:31-41. [DOI] [PubMed] [Google Scholar]
- 10.Jakobi, R., C. J. Chen, P. T. Tuazon, and J. A. Traugh. 1996. Molecular cloning and sequencing of the cytostatic G protein-activated protein kinase PAK I. J. Biol. Chem. 271:6206-6211. [DOI] [PubMed] [Google Scholar]
- 11.Jakobi, R., E. Moertl, and M. A. Koeppel. 2001. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J. Biol. Chem. 276:16624-16634. [DOI] [PubMed] [Google Scholar]
- 12.Jiang, A., A. Craxton, T. Kurosaki, and E. A. Clark. 1998. Different protein tyrosine kinases are required for B cell antigen receptor-mediated activation of extracellular signal-regulated kinase, c-Jun NH2-terminal kinase 1, and p38 mitogen-activated protein kinase. J. Exp. Med. 188:1297-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keshvara, L. M., C. C. Isaacson, T. M. Yankee, R. Sarac, M. L. Harrison, and R. L. Geahlen. 1998. Syk- and Lyn-dependent phosphorylation of Syk on multiple tyrosines following B cell activation includes a site that negatively regulates signaling. J. Immunol. 161:5276-5283. [PubMed] [Google Scholar]
- 14.Kimura, T., H. Sakamoto, E. Appella, and R. P. Siraganian. 1996. Conformational changes induced in the protein tyrosine kinase p72syk by tyrosine phosphorylation or by binding of phosphorylated immunoreceptor tyrosine-based activation motif peptides. Mol. Cell. Biol. 16:1471-1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knaus, U. G., and G. M. Bokoch. 1998. The p21Rac/Cdc42-activated kinases (PAKs). Int. J. Biochem. Cell Biol. 30:857-862. [DOI] [PubMed] [Google Scholar]
- 16.Ku, G. M., D. Yablonski, E. Manser, L. Lim, and A. Weiss. 2001. A PAK1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J. 20:457-465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurosaki, T., S. A. Johnson, L. Pao, K. Sada, H. Yamamura, and J. C. Cambier. 1995. Role of the Syk autophosphorylation site and SH2 domains in B cell antigen receptor signaling. J. Exp. Med. 182:1815-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, N., H. MacDonald, C. Reinhard, R. Halenbeck, A. Roulston, T. Shi, and L. T. Williams. 1997. Activation of hPAK65 by caspase cleavage induces some of the morphological and biochemical changes of apoptosis. Proc. Natl. Acad. Sci. USA 94:13642-13647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manetz, T. S., C. Gonzalez-Espinosa, R. Arudchandran, S. Xirasagar, V. Tybulewicz, and J. Rivera. 2001. Vav1 regulates phospholipase Cγ activation and calcium responses in mast cells. Mol. Cell. Biol. 21:3763-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitsui, N., R. Inatome, S. Takahashi, Y. Goshima, H. Yamamura, and S. Yanagi. 2002. Involvement of Fes/Fps tyrosine kinase in semaphorin 3A signaling. EMBO J. 21:3274-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poole, A., J. M. Gibbins, M. Turner, M. J. van Vugt, J. G. van de Winkel, T. Saito, V. L. Tybulewicz, and S. P. Watson. 1997. The Fc receptor gamma-chain and the tyrosine kinase Syk are essential for activation of mouse platelets by collagen. EMBO J. 16:2333-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin, S., Y. Minami, M. Hibi, T. Kurosaki, and H. Yamamura. 1997. Syk-dependent and -independent signaling cascades in B cells elicited by osmotic and oxidative stress. J. Biol. Chem. 272:2098-2103. [DOI] [PubMed] [Google Scholar]
- 23.Renkema, G. H., A. Manninen, and K. Saksela. 2001. Human immunodeficiency virus type 1 Nef selectively associates with a catalytically active subpopulation of p21-activated kinase 2 (PAK2) independently of PAK2 binding to Nck or beta-PIX. J. Virol. 75:2154-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roig, J., Z. Huang, C. Lytle, and J. A. Traugh. 2000. p21-activated protein kinase gamma-PAK is translocated and activated in response to hyperosmolarity. Implication of Cdc42 and phosphoinositide 3-kinase in a two-step mechanism for gamma-PAK activation. J. Biol. Chem. 275:16933-16940. [DOI] [PubMed] [Google Scholar]
- 25.Roig, J., and J. A. Traugh. 1999. p21-activated protein kinase gamma-PAK is activated by ionizing radiation and other DNA-damaging agents. Similarities and differences to alpha-PAK. J. Biol. Chem. 274:31119-31122. [DOI] [PubMed] [Google Scholar]
- 26.Roig, J., P. T. Tuazon, P. A. Zipfel, A. M. Pendergast, and J. A. Traugh. 2000. Functional interaction between c-Abl and the p21-activated protein kinase gamma-PAK. Proc. Natl. Acad. Sci. USA 97:14346-14351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rooney, R. D., P. T. Tuazon, W. E. Meek, E. J. Carroll, J. J. Hagen, E. L. Gump, C. A. Monnig, T. Lugo, and J. A. Traugh. 1996. Cleavage arrest of early frog embryos by the G protein-activated protein kinase PAK I. J. Biol. Chem. 271:21498-21504. [DOI] [PubMed] [Google Scholar]
- 28.Rudel, T., and G. M. Bokoch. 1997. Membrane and morphological changes in apoptotic cells regulated by caspase-mediated activation of PAK2. Science 276:1571-1574. [DOI] [PubMed] [Google Scholar]
- 29.Sada, K., S. M. S. Miah, K. Maeno, S. Kyo, X. Qu, and H. Yamamura. 2002. Regulation of Fcepsilon RI-mediated degranulation by an adaptor protein 3BP2 in rat basophilic leukemia RBL-2H3 cells. Blood 100:2138-2144. [DOI] [PubMed] [Google Scholar]
- 30.Sada, K., Y. Minami, and H. Yamamura. 1997. Relocation of Syk protein-tyrosine kinase to the actin filament network and subsequent association with Fak. Eur. J. Biochem. 248:827-833. [DOI] [PubMed] [Google Scholar]
- 31.Sada, K., T. Takano, S. Yanagi, and H. Yamamura. 2001. Structure and function of Syk protein-tyrosine kinase. J. Biochem. (Tokyo) 130:177-186. [DOI] [PubMed] [Google Scholar]
- 32.Sada, K., J. Zhang, and R. P. Siraganian. 2000. Point mutation of a tyrosine in the linker region of Syk results in a gain of function. J. Immunol. 164:338-344. [DOI] [PubMed] [Google Scholar]
- 33.Sada, K., J. Zhang, and R. P. Siraganian. 2001. SH2 domain-mediated targeting, but not localization, of Syk in the plasma membrane is critical for FcepsilonRI signaling. Blood 97:1352-1359. [DOI] [PubMed] [Google Scholar]
- 34.Takano, T., K. Sada, and H. Yamamura. 2002. Role of protein-tyrosine kinase Syk in oxidative stress signaling in B cells. Antioxid. Redox Signal. 4:533-540. [DOI] [PubMed] [Google Scholar]
- 35.Tuazon, P. T., M. Chinwah, and J. A. Traugh. 1998. Autophosphorylation and protein kinase activity of p21-activated protein kinase gamma-PAK are differentially affected by magnesium and manganese. Biochemistry 37:17024-17029. [DOI] [PubMed] [Google Scholar]
- 36.Tuazon, P. T., W. C. Spanos, E. L. Gump, C. A. Monnig, and J. A. Traugh. 1997. Determinants for substrate phosphorylation by p21-activated protein kinase (gamma-PAK). Biochemistry 36:16059-16064. [DOI] [PubMed] [Google Scholar]
- 37.Turner, M., E. Schweighoffer, F. Colucci, J. P. Di Santo, and V. L. Tybulewicz. 2000. Tyrosine kinase SYK: essential functions for immunoreceptor signalling. Immunol. Today 21:148-154. [DOI] [PubMed] [Google Scholar]
- 38.Walter, B. N., Z. Huang, R. Jakobi, P. T. Tuazon, E. S. Alnemri, G. Litwack, and J. A. Traugh. 1998. Cleavage and activation of p21-activated protein kinase gamma-PAK by CPP32 (caspase 3). Effects of autophosphorylation on activity. J. Biol. Chem. 273:28733-28739. [DOI] [PubMed] [Google Scholar]
- 39.Wolf, D., V. Witte, B. Laffert, K. Blume, E. Stromer, S. Trapp, P. d'Aloja, A. Schurmann, and A. S. Baur. 2001. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 7:1217-1224. [DOI] [PubMed] [Google Scholar]
- 40.Yanagi, S., R. Inatome, J. Ding, H. Kitaguchi, V. L. Tybulewicz, and H. Yamamura. 2001. Syk expression in endothelial cells and their morphologic defects in embryonic Syk-deficient mice. Blood 98:2869-2871. [DOI] [PubMed] [Google Scholar]
- 41.Zhang, J., M. L. Billingsley, R. L. Kincaid, and R. P. Siraganian. 2000. Phosphorylation of Syk activation loop tyrosines is essential for Syk function. An in vivo study using a specific anti-Syk activation loop phosphotyrosine antibody. J. Biol. Chem. 275:35442-35447. [DOI] [PubMed] [Google Scholar]