Abstract
Aminoglycosides are polycationic antibiotics that have been shown to block a variety of cation channels. The inhibitory effect of externally applied aminoglycosides on P2X2 receptor currents was examined after heterologous expression in Xenopus laevis oocytes using the two-electrode voltage-clamp technique. All of the aminoglycosides tested inhibited the ATP-evoked responses with potencies ranging from 71 μM to 2 mM (IC50 values). The ranked order of potency was streptomycin > gentamicin > neomycin > paromomycin > kanamycin. The inhibition of P2X receptor currents was independent of the ATP concentration used for the activation, which is compatible with a noncompetitive mechanism. The inhibition was voltage-dependent and was reduced at more positive membrane potentials. To examine whether the current block was dependent on the receptor conformation, the aminoglycoside effect on a non-desensitizing P2X2-X1 receptor chimera was analyzed. The results from these measurements suggest that inhibition is caused by an open pore block that locks the P2X receptor chimera in an open nonconducting state from which the agonist dissociation is slow. We also demonstrate that the P2X2-X1 chimera can serve as a tool to directly test whether an antagonist acts competitively or not.
Keywords: Aminoglycoside antibiotics, P2X, Xenopus oocytes, Open pore block
Introduction
P2X receptors are cation-selective ligand-gated ion channels that open upon extracellular binding of ATP. To date, seven subtypes (P2X1–7) have been cloned that share a common topology: two transmembrane domains connected by a large extracellular loop. P2X receptors are expressed in cells from a wide variety of tissues, where they are involved in such diverse processes as exo- and endocrine secretion, platelet aggregation, inflammation, pain sensation, and cell proliferation. Functional receptors are oligomeric assemblies of three subunits [1, 2], a feature that was considered to be unique among all ion channel families until it was shown that acid-sensing ion channels belonging to the amiloride-sensitive Na+-channel/degenerin family are also trimers [3]. An important milestone achieved in the last year in the P2X receptor field was the report of the crystal structure of the zebrafish P2X4 receptor [4]. The 3-Ǻ-resolution X-ray structure of the closed-state P2X4 receptor enabled homology modeling of other P2X receptor family members for rational ligand design [5] and facilitated mutagenesis-based studies of structural rearrangements that accompany channel opening [6–9]. The seven subtypes of the P2X family show different sensitivities for several agonists and inhibitors, and some of them can undergo modulation by divalent metal cations (Zn2+, Mg2+, and Ca2+), protons [10, 11] or macromolecules such as ivermectin [12] and Cibacron Blue [13, 14]. All subtypes open within milliseconds of binding to extracellular ATP but show different desensitization rates. P2X1 and P2X3 receptors desensitize in tens or hundreds of milliseconds, whereas all other subtypes show no or less pronounced desensitization.
Aminoglycosides are polycationic and hydrophilic compounds that constitute a group of antibiotics extracted from Streptomyces (suffix -mycin) or Micromonospora (suffix -micin). Because of their positive charge, they bind to the bacterial 30S ribosomal subunit and thereby elicit their antibiotic effect by causing misreading of tRNA, inducing the incorrect synthesis of essential proteins such as membrane proteins. As a consequence, the bacterial membrane loses its integrity. Aminoglycosides are primarily used for the treatment of infections caused by aerobic Gram-negative bacteria such as Pseudomonas, Acinetobacter, or Enterobacter and also for some mycobacteria, such as Mycobacterium tuberculosis, which causes tuberculosis and was successfully treated for the first time with streptomycin in 1944 [15]. The clinical use of aminoglycosides is, however, limited by negative side effects such as oto- and nephrotoxicity [16, 17]. Aminoglycosides are applied systemically only under medical surveillance, i.e., while screening blood pressure and kidney function to minimize nephric damage [16, 17]. This toxicity is thought to arise from intracellular aminoglycoside accumulation and subsequent metabolism [18, 19]. Aminoglycosides have been shown to modulate synaptic transmission, most likely by blocking voltage-gated Ca2+ channels [20]. Other studies have demonstrated the inhibitory effects of aminoglycosides at mechanosensitive ion channels [21, 22], nicotinic ACh receptors [23, 24], and TRPV1 receptors [25]. At N-methyl D-aspartate (NMDA) glutamate receptors, aminoglycosides show inhibitory as well as stimulatory effects [26]. Because inner and outer hair cells and the type I spiral ganglion neurons of several species age-dependently express P2X receptors [27–29], a coherence with the ototoxicity of aminoglycosides is likely. Because outer hair cells (OHCs) have been shown to exhibit large inward current responses when challenged with extracellular ATP, Lin et al. [27] studied the effect of neomycin on these ATP responses in detail. They found that neomycin inhibits OHC ATP responses with an IC50 value of ∼90 μM. They also reported a less potent block by gentamicin and streptomycin. Later, it was shown that OHCs potentially express a variety of P2X and P2Y receptors, such as P2X1, P2X2, P2X4, P2X7, P2Y1, P2Y2, and P2Y4 [30–32]. Therefore, we decided to analyze the effects of streptomycin, gentamicin, neomycin, kanamycin, and paromomycin on homomeric P2X2 receptors.
P2X2 receptors were expressed in Xenopus laevis oocytes, and aminoglycoside effects were analyzed by the two-electrode voltage-clamp method. To further characterize their mechanism of action, we used a P2X2-X1 receptor chimera built from a P2X1 receptor subunit in which the N-terminal and first transmembrane domain was replaced by the corresponding domains from the P2X2 receptor [33, 34]. This chimera had unique properties, including the absence of desensitization, an apparent nanomolar affinity for ATP and an exceptionally slow deactivation time course after the removal of ATP [34]. In contrast to the results with OHCs, we found that streptomycin was the most potent inhibitor of the P2X2 receptor, followed by gentamicin and neomycin, suggesting that OHC ATP responses are not purely mediated by the activation of homomeric P2X2 receptors or that differences between species are the origin of this difference. Furthermore, we demonstrate that aminoglycosides locked the receptors in an open but nonconductive conformation. This characteristic may be used as a tool for the kinetic analyses of P2X receptors, e.g., when current inhibition without the prevention of agonist binding is desired.
Materials and methods
Cell preparation
Oocytes were obtained from mature female X. laevis toads by partially removing the ovary under general anesthesia with methane sulfonate salt (MS-222/tricaine, Sigma, Germany). Ovary lobes were digested by collagenase type IA (Sigma-Aldrich, Germany or Serva, Germany) treatment until the oocytes were separated and completely defolliculated (2 mg/mL collagenase, 2–3 h). One to three days before the experiments, the oocytes were injected with 41–50-nL aliquots of cRNA (2 ng/μL for rP2X2 and 0.5 μg/μL for rP2X2-X1) and incubated in Oocyte Ringer solution [ORi; 90 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, and 5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), pH 7.4 with NaOH] at 17–19°C until use.
cDNA constructs and cRNA synthesis
Oocyte expression plasmids encoding wild-type rat P2X subunits (rP2X1, rP2X2) from previous studies were used [1, 2]. A P2X2-X1 chimera construct consisting of the coding sequence of Met1-Val47 of the rP2X2 subunit was joined in frame with the coding sequence for 48Val-399Ser of the rP2X1 subunit, which has also been described previously [34]. The capped cRNAs were prepared from linearized templates using SP6 RNA polymerase (Ambion).
Electrophysiology
The current responses to ATP were measured 1–3 days after cRNA injection using the two-electrode voltage-clamp technique. Microelectrodes with resistances below 1 MΩ were pulled from borosilicate glass and filled with 3 M KCl solution. Two Ag/AgCl bath electrodes were used to minimize series resistance errors. Currents were recorded at a holding potential of −60 mV using a TEC-03X amplifier (NPI Electronics, Lambrecht, Germany), low-pass filtered at 100 Hz, and sampled at 400 Hz. All measurements were carried out at room temperature (20–22°C). Data analyses were performed with Origin 7.5 software (OriginLab Corp., Northampton, MA, USA) or GraphPad Prism5 software (GraphPad Software, Inc., La Jolla, CA, USA). Dose-inhibition curves were fitted by the four-parameter Hill equation:
 |
Data are presented as the mean±SE from n experiments. Error bars were omitted in the figures when they were smaller than the symbols used. A p value of p < 0.05 was defined as significant.
Drug application
The standard solution used to superfuse the oocytes during the voltage-clamp experiments contained 90 mM NaCl, 1 mM KCl, 2 mM MgCl2, and 5 mM HEPES and had a pH of 7.4 (Mg2+ Oocyte Ringer). Calcium salts were omitted to avoid activation of endogenous Ca2+-activated Cl– channels upon opening of the P2X receptors [35, 36] and to prevent P2X2 receptor inhibition by Ca2+ [37, 38]. Aminoglycoside-containing solutions were adjusted to a pH of 7.4 before use. A fast and reproducible solution exchange was achieved with a small-volume oocyte chamber (∼10 μL) combined with fast and continuous perfusion at a rate of ∼10 mL/min [39]. The average time constant of complete solution exchange analyzed in non-injected oocytes by switching from a solution containing 90 mM NaCl to a solution containing 0 mM NaCl and 90 mM KCl was τ = 0.916 ± 0.005 s. The solutions were switched by magnetic valves under the control of Cellworks software (NPI Electronics, Lambrecht, Germany). All of the reagents were purchased from Fluka (Germany) or Sigma-Aldrich (Taufkirchen, Germany). All of the aminoglycosides were added as sulfate salts.
Results
Aminoglycoside inhibition of P2X2 receptor currents
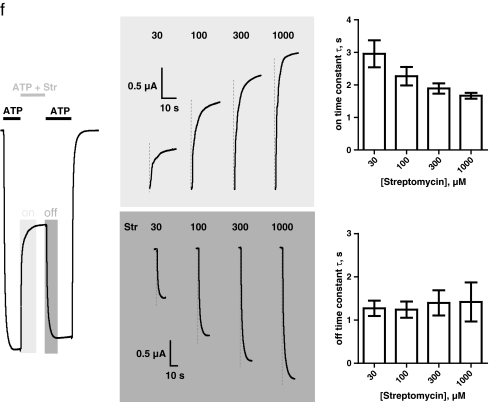
Concentration–inhibition curves for streptomycin, gentamicin, neomycin, paromomycin, and kanamycin were generated to determine the potencies of the aminoglycosides at rP2X2 receptors. To this end, receptor currents were recorded at half-maximum activating concentrations (EC50) of ATP (∼30 μM [40]) in the absence or in the presence of increasing aminoglycoside concentrations. Because all aminoglycosides were supplied as sulfate salts, the potential influence of sulfate on P2X2 receptor currents was tested separately by performing control experiments with identical protocols but with solutions in which the aminoglycoside sulfate was replaced by Na2SO4. No significant influence of sulfate in concentrations up to 3 mM could be detected (data not shown). After a stationary current was elicited by co-application of 30 μM ATP (black bars) and increasing concentrations of streptomycin (10–3,000 μM, gray bars), 30 μM ATP was applied until a new steady state was reached to check for reversibility of inhibition and potential receptor current rundown (Fig. 1a). The extent of inhibition was calculated from the ratio of the current in the absence and presence of aminoglycoside, respectively. Figure 1b shows the respective concentration–inhibition curves, demonstrating that all of the tested aminoglycosides inhibited the P2X2 receptor in the presence of 30 μM ATP. Nonlinear curve fits by the Hill equation yielded the IC50 values and Hill coefficients summarized in Table 1. The most potent inhibition was achieved by streptomycin (IC50 = 71 μM), and the weakest inhibition was seen with kanamycin (IC50 2 mM). The Hill coefficients of all inhibitors were in the range of nH = 0.77–0.81. In addition, we tested whether there was a difference in the inhibition values or time course of the onset of the aminoglycoside block of P2X2 receptors when we used aminoglycoside preincubation. Figure 1c illustrates that the presence of streptomycin before the co-application of ATP and streptomycin resulted in a current amplitude and onset time constant (Fig. 1d, e) similar to the absence of streptomycin before the co-application.
Fig. 1.
Concentration dependence of P2X2 receptor current inhibition by aminoglycosides. a The protocol for the assessment of the inhibitory potency of aminoglycosides from stationary current measurements. A representative original current trace is shown. After a stationary current was elicited by co-application of 30 μM ATP (black bars) and increasing concentrations of streptomycin (10–3,000 μM, gray bars), 30 μM ATP alone was applied until a new steady state was reached. The extent of inhibition was judged from the ratio of the maximal and minimal stationary current in the absence and presence of aminoglycoside, respectively. b Using the protocol shown in (a), concentration–inhibition curves were generated in the presence of incrementally larger concentrations of streptomycin (Str, filled squares), gentamicin (Gen, filled circles), neomycin (Neo, filled triangles), paromomycin (Par, open circles), and kanamycin (Kan, open squares). Continuous lines represent nonlinear curve fits by the Hill equation with a nonzero minimum. All IC50 values and Hill coefficients are given in Table 1. The P2X2 receptor currents were most potently blocked by streptomycin (with an IC50 value of 71 μM), and the weakest inhibition was induced by kanamycin (with an IC50 value of 2 mM). c–e Inhibition of P2X2 receptors by 100 μM streptomycin (Str) judged from different protocols. c The presence of 100 μM streptomycin (dark gray bar) before the co-application of 10 μM ATP and 100 μM streptomycin (light gray bar and area) exhibited a similar current amplitude and onset time constant compared with the absence of streptomycin [solely oocyte ringer solution (ORi) present] before the co-application of ATP and streptomycin. d, e Quantification of the onset time constant (d) as obtained from monoexponential curve fitting and the current amplitude (e) of the co-application of 10 μM ATP and 100 μM streptomycin from n = 7 experiments shown in panel (c). f Detailed analysis of the onset and offset time course of the aminoglycoside block of P2X2 receptors. A representative current trace (left panel) of experiments with increasing concentrations of streptomycin (Str, 30–1,000 μM) co-applied with 10 μM ATP is shown. Portions of the corresponding current traces illustrating the onset (light gray area) or offset (dark gray area) time course are shown in the middle panel (a vertical dashed gray line is depicted at each concentration to increase the visualization of slight differences). The time course was quantified by monoexponential curve fitting. The time constants of the onset (upper right panel) and offset (lower right panel) of the inhibition at different streptomycin concentrations are shown (means from n = 7 experiments). As tested by one-way analysis of variance (ANOVA), the mean onset time constants for increasing streptomycin concentrations (30 μM, τ = 3.0 ± 0.4 s; 100 μM, τ = 2.3 ± 0.3 s; 300 μM, τ = 1.9 ± 0.2 s; 1,000 μM, τ = 1.7 ± 0.1 s) decreased significantly (p = 0.021), but the offset time constants (30 μM, τ = 1.3 ± 0.2 s; 100 μM, τ = 1.2 ± 0.2 s; 300 μM, τ = 1.4 ± 0.3 s; 1,000 μM, τ = 1.4 ± 0.5 s) were not significantly different (p = 0.964)
Table 1.
Aminoglycoside inhibition values of P2X2 and P2X2-X1 receptor currents
| P2X2 | P2X2-X1 | |||
|---|---|---|---|---|
| IC50 [μM] | nH | IC50 [μM] | nH | |
| Streptomycin | 71 ± 20 | 0.79 ± 0.08 | 480 ± 52 | 0.83 ± 0.08 |
| Gentamicin | 290 ± 84 | 0.80 ± 0.09 | 311 ± 52 | 0.78 ± 0.07 |
| Neomycin | 306 ± 28 | 0.81 ± 0.07 | 314 ± 56 | 0.83 ± 0.09 |
| Paromomycin | 1,025 ± 113 | 0.79 ± 0.06 | 2,139 ± 208 | 0.77 ± 0.08 |
| Kanamycin | 2,024 ± 314 | 0.77 ± 0.06 | 1,693 ± 245 | 0.98 ± 0.13 |
IC50 values and Hill coefficients derived from nonlinear curve fits by the Hill equation with a nonzero minimum to the pooled data from n = 6–8 experiments for P2X2 and P2X2-X1 chimeric receptors expressed in Xenopus oocytes
All of the tested aminoglycosides exhibited fast onset and offset rates and a full reversibility of inhibition. A detailed quantification of the onset and offset time course of the streptomycin block of P2X2 receptors at different streptomycin concentrations is shown in Fig. 1f. Streptomycin exhibited onset time constants that decreased significantly with increasing concentrations (30–1,000 μM) of streptomycin (time constants τ = 3.0–1.7 s as obtained from monoexponential curve fitting). In contrast, the offset time constants were not significantly different at increasing streptomycin concentrations (time constants τ = 1.2–1.4 s; Fig. 1f).
Voltage and ATP dependence of aminoglycoside inhibition at P2X2 receptors
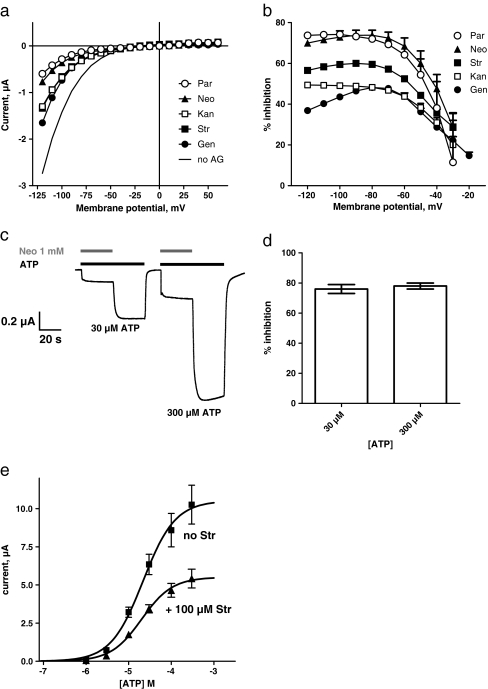
To examine the voltage dependency of the aminoglycoside block, we recorded the current-voltage dependencies between −120 mV and +60 mV in the absence and presence of aminoglycosides (Fig. 2a). We applied 30 μM ATP and the different aminoglycosides at concentrations close to their individual IC50 values. The aminoglycoside block showed a strong voltage dependence at membrane potentials between −80 and −20 mV and was saturated at potentials more negative than −80 mV (Fig. 2b). To test the effect of the ATP concentration on inhibition, the relative current inhibitions with 30 μM and 300 μM ATP were compared. Figure 2c, d demonstrate that the inhibition induced by 1 mM neomycin was virtually identical (∼80%) at both ATP concentrations, suggesting that the aminoglycoside inhibition of P2X2 receptors is independent of the agonist concentration used. A concentration–response analysis of ATP in the presence and absence of 100 μM streptomycin showed a ∼50% maximum depression, whereas the ATP EC50 value was unaffected by the presence of streptomycin (Fig. 2e).
Fig. 2.
Voltage and ATP dependence of aminoglycoside inhibition of P2X2 receptor currents. a Current-voltage (IV) dependencies recorded at 30 μM ATP (continuous line) and at 30 μM ATP in the presence of half-maximal inhibitory concentrations of gentamicin (Gen, filled circles), kanamycin (Kan, open squares), streptomycin (Str, filled squares), neomycin (Neo, filled triangles), and paromomycin (Par, open circles). b Voltage dependency of an aminoglycoside block of P2X2 receptor currents. The inhibition values of aminoglycosides (same symbols as in panel [a]) at different membrane potentials are shown (n = 7). The P2X receptor current inhibition was voltage-dependent and was greatly reduced at more positive membrane potentials. c Inhibition of P2X2 receptor currents by 1 mM neomycin (Neo, gray bar) in the presence of 30 μM and 300 μM ATP (black bar). Original current trace recorded from a single oocyte expressing P2X2 receptors. d Comparison of the inhibition by 1 mM neomycin (Neo) at 30 μM and 300 μM ATP (n = 6), which were not significantly different from each other. e ATP concentration–response curves were established by stimulating oocytes expressing the rP2X2 receptor with incrementally larger concentrations of ATP in the absence of streptomycin (filled square, EC50 = 21.7 ± 7.1 μM, and nH = 1.2 ± 0.3, n = 7) or the presence of 100 μM streptomycin (filled triangle, EC50 = 20.1 ± 4.9 μM, and nH = 1.2 ± 0.3, n = 6)
Aminoglycoside block of the P2X2-X1 chimera
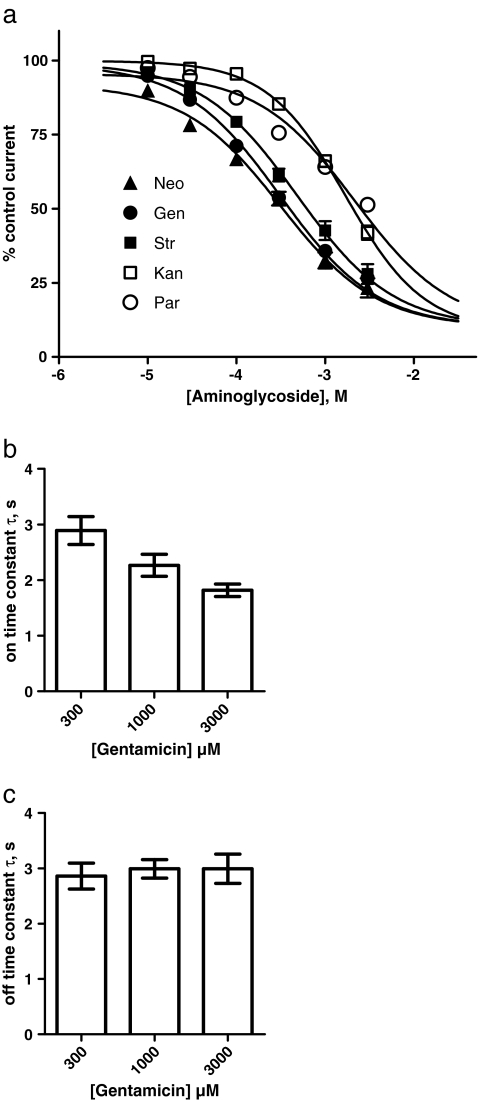
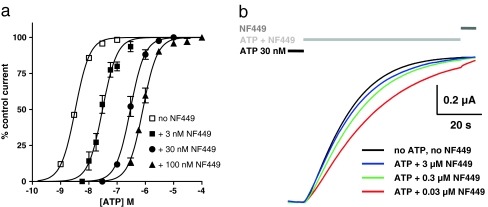
We have shown that aminoglycosides exhibit a noncompetitive mechanism of action at P2X2 receptors and that the inhibition is independent of the agonist (ATP) concentration. Based on these findings and combined with the voltage dependence of the P2X2 block, it can be assumed that aminoglycosides act on the open conformation of the receptor, causing an open pore block. This suggests that aminoglycoside inhibition also changes the equilibrium between the closed and open state in favor of the latter. To study this potential dependency between aminoglycoside inhibition and the receptor conformation, we used the non-desensitizing P2X2-X1 receptor chimera for which ATP dissociation is extremely slow. This is reflected by a deactivation time constant τ of ∼60 s after removal of extracellular ATP and an apparent affinity of ∼3 nM for ATP [34]. We first characterized the potencies of the various aminoglycosides to block P2X2-X1 chimeric receptor currents. To this end, a similar protocol to the one for the P2X2 receptor was used. The various aminoglycosides reversibly inhibited the chimera in a dose-dependent manner. Gentamicin and neomycin were the most potent inhibitors (IC50 = 311 μM and 314 μM, respectively), and paromomycin showed the lowest potency (IC50 = 2.1 mM; Fig. 3a, Table 1). The order of potency was gentamicin = neomycin > streptomycin > kanamycin > paromomycin, which is different from the order of the P2X2 receptor inhibitors. All Hill coefficients were in the range of nH = 0.78–0.98 (Table 1). As with P2X2, the onset of inhibition was fast, and the onset time constant decreased significantly from τ = 2.9 to 1.8 s with increasing concentrations (300–3,000 μM) of gentamicin (Fig. 3b), whereas the offset time constant was independent of the gentamicin concentration used (Fig. 3c). This finding provides direct evidence for the noncompetitive nature of the aminoglycoside block of the chimera: if aminoglycosides had to bind to the ATP binding site to inhibit, an onset of inhibition being not faster than the deactivation rate (i.e., ATP dissociation) would be observed; thus, the inhibition should not develop with a time constant smaller than 60 s. In fact, this behavior is observed with the suramin derivative NF449 [41], which acts in a classical competitive manner at the P2X2-X1 chimera: concentration–response curves in the presence of increasing NF449 concentrations are shifted to the right and show no depression of maximum currents (Fig. 4a). Consequently, the onset of inhibition in the presence of 30 nM ATP (ten times the EC50 concentration for ATP) occurs more quickly with increasing NF449 concentrations but never exceeds the rate of deactivation after ATP washout, even after application of an NF449 concentration four orders of magnitude larger than the IC50 value (3 μM versus 0.3 nM; Fig. 4b).
Fig. 3.
Concentration dependence of the P2X2-X1 chimera current inhibition by aminoglycosides. a Using the protocol shown in Fig. 1a, concentration–inhibition curves were generated in the presence of incrementally larger concentrations of streptomycin (Str, filled squares), gentamicin (Gen, filled circles), neomycin (Neo, filled triangles), paromomycin (Par, open circles), and kanamycin (Kan, open squares). The continuous lines represent nonlinear curve fits by the Hill equation with a nonzero minimum. All IC50 values and Hill coefficients are given in Table 1. The P2X2-X1 receptor currents were most potently blocked by gentamicin and neomycin, with IC50 values of 311 μM and 314 μM, respectively. The weakest inhibition was induced by paromomycin, with an IC50 value of 2.1 mM. b, c Quantification of the analysis of the onset and offset time courses of the aminoglycoside blocking of P2X2-X1 receptors. The time course was obtained from experiments similar to those shown in Fig. 1f and quantified by a monoexponential curve fit. The time constants of the onset (upper right panel) and offset (lower right panel) at the different gentamicin concentrations are shown (means from n = 4 experiments). As tested by ANOVA, the mean onset time constants for increasing streptomycin concentrations (300 μM, τ = 2.9 ± 0.3 s; 1,000 μM, τ = 2.3 ± 0.2 s; 3,000 μM, τ = 1.8 ± 0.1 s) decreased significantly (p = 0.023), but the offset time constants (300 μM, τ = 2.9 ± 0.2 s; 1,000 μM, τ = 3.0 ± 0.2 s; 3,000 μM, τ = 3.0 ± 0.3 s) were not significantly (p = 0.893) different
Fig. 4.
NF449 acts as a competitive inhibitor of the P2X2-X1 chimera. a ATP concentration–response curves for the rP2X2-X1 receptor in the absence (open squares) and presence of 3 (filled squares), 30 (filled circles) and 100 nM (filled triangles) NF449. A simultaneous fit yielded an NF449 KB value of 0.29 nM with a Hill coefficient nH = 1.7. b The slow deactivation time course after removal of 30 nM ATP (black line) and the slow onset of inhibition by 0.03 (red line), 0.3 (green line), and 3 μM (blue line) NF449 in the presence of 30 nM ATP (light gray bar)
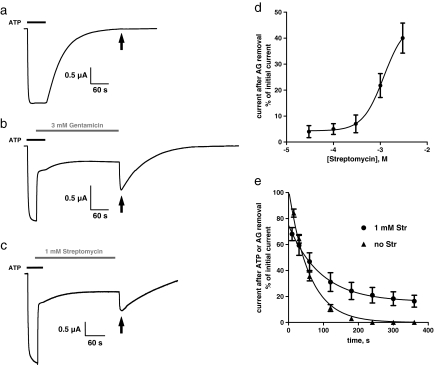
It is a well-described characteristic of ligand-gated ion channels that the agonist is more tightly bound in the open state, i.e., the open state has a higher affinity for the agonist than the closed state [42]. As mentioned above, the P2X2-X1 chimera deactivates slowly after ATP washout with a time constant of ∼60 s. If ATP dissociation occurs mainly from the closed state because ATP is more tightly bound to the open state, one should expect a significantly slower deactivation time course if the dwell-time duration in the open state is significantly increased, for example, by open-channel blocking. To test for the occurrence of receptors being locked in the open state by aminoglycosides, we recorded and compared the deactivation levels in the absence and presence of 3 mM gentamicin. After activation of the P2X2-X1 chimeric receptor with a saturating concentration of ATP (100 nM), gentamicin was co-applied for 30 s before gentamicin alone was applied for 240 s. Compared with the control experiment in the absence of gentamicin, in which the current decay was completed within a period of 240 s in ATP-free ORi solution (Fig. 5a), a significant inward current was activated after 240 s in the absence of ATP upon gentamicin washout (Fig. 5b, arrow). This inward current (also designated as a peak rebound current) of about half of the initial receptor current disappeared after an additional period of ∼180 s in the absence of ATP, indicating that the ATP dissociation from the receptor was not completed after 240 s while being blocked by gentamicin.
Fig. 5.
Aminoglycosides decrease the ATP dissociation time course of the P2X2-X1 chimera. Deactivation kinetics of P2X2-X1 receptor currents in the absence (a) and in the presence (gray bar) of 3 mM gentamicin (b) or 1 mM streptomycin (c) after activation by 100 nM ATP (black bar). Shown are representative original current traces recorded from single oocytes. The deactivation of the receptor-mediated current followed a monoexponential time course with a time constant τ of ∼60 s and was completed after 240 s in ATP-free solution (a). In the presence of 3 mM gentamicin (b) or 1 mM streptomycin (c), deactivation was incomplete after 240 s in ATP-free solution. After removal of gentamicin or streptomycin, the fast-developing inward current (arrow) in the absence of ATP indicates that aminoglycoside blocking significantly decelerated ATP dissociation. d Current amplitudes as a percentage of the initial ATP-elicited current after 240 s of application for increasing concentrations of streptomycin. The continuous line represents a nonlinear curve fit by the Hill equation to the pooled data points from n = 5 experiments. The curve has a slope of 1.9 ± 0.8 (nH) and exhibits a half-maximal current response at 1.19 ± 0.5 mM (judged as the response halfway between the bottom and the top). e Current amplitudes as a percentage of the initial ATP-elicited current in the absence of streptomycin (filled triangle) or in the presence of 1 mM streptomycin (filled circle) after different time periods (10, 30, 60, 120, 180, 240, 300, or 360 s) after removal of 100 nM ATP (see panel a, c). The continuous lines represent monoexponential curve fits to the pooled data points from n = 6 experiments. In the absence of streptomycin, the current decay after ATP removal showed a time constant of τ = 59 s. The presence of 1 mM streptomycin for increasing time periods caused a prolonged time constant of τ = 92 s
A virtually identical current trace was recorded when streptomycin was used instead of gentamicin (Fig. 5c). The dependence of the peak rebound current on the streptomycin concentration present for a fixed time of 240 s is shown in Fig. 5d. This relationship was best fit using a nonlinear, sigmoidal function, and it yielded a concentration of 1.1 mM streptomycin that activated the rebound current half-maximally after streptomycin washout (Fig. 5d). An analysis of the rebound current after streptomycin washout as a function of time is shown in Fig. 5e. The rebound current amplitudes that occurred subsequent to increasing the duration of streptomycin (1 mM) applications from 10 to 360 s were analyzed. The pooled data of n = 7 experiments were fitted by a monoexponential decay function and compared with the washout decay in the absence of ATP and streptomycin. As apparent from Fig. 5e, the presence of 1 mM streptomycin increased the time constant of the current decay from 59 to 92 s. This comparison gives an estimate of the extent to which ATP unbinding is delayed by holding the pore open.
Discussion
The clinical use of aminoglycosides is limited by negative side effects such as oto- and nephrotoxicity [16, 17]. Aminoglycosides have been shown to inhibit several membrane ion channels, including presynaptic voltage-gated Ca2+ channels [20], mechanosensitive ion channels [21, 22], nicotinic ACh receptors [23, 24], and TRPV1 receptors [25]. At NMDA receptors, aminoglycosides show inhibitory as well as stimulatory effects [26], but, in most of cases, the basic mechanism of action has yet to be discovered. Exceptions are the noncompetitive gentamicin block of ACh-evoked K+ currents in guinea pig outer hair cells, which is caused by impairing Ca2+ entry at the cholinergic receptor [18] and the competitive antagonism of gentamicin at αβγδ and α7 ACh receptors but has contrary effects on the desensitization rates of these receptors [24]. An open pore block of mechanosensitive ion channels in skeletal muscle fibers by neomycin and other aminoglycosides has been suggested [22].
Here, we report the inhibition of P2X2 and P2X2-X1 chimera receptor currents by different aminoglycosides and present an electrophysiological characterization of the basic mechanism underlying this inhibition.
All tested aminoglycoside antibiotics inhibited ATP-mediated currents of P2X2 receptors, and the order of potency was streptomycin > gentamicin > neomycin > paromomycin > kanamycin. These potencies differ considerably from what was observed in guinea pig cochlear outer hair cells by Lin et al. [27], who found that neomycin was significantly more effective than streptomycin and gentamicin (kanamycin and paromomycin were not tested) in inhibiting ATP-mediated currents. Later, outer hair cells were shown to express a variety of P2X receptors, including P2X1, P2X2, P2X4, and P2X7, as well as P2Y receptors such as P2Y1, P2Y2, and P2Y4 [30–32]. Therefore, it seems likely that the difference between the ranked orders of potency in OHCs and recombinant P2X2 receptors is due to the fact that ATP responses in OHCs are not exclusively mediated by P2X2 receptors. Another possibility is species differences, as there are known differences between species in P2X receptor expression in other compartments of the inner ear [28]. Aminoglycoside inhibition at recombinant P2X2 receptors showed a fast onset that was dependent on the aminoglycoside concentration applied and a fast offset that was independent of the aminoglycoside concentration applied. The concentration dependence of the onset and the independence of the offset (because the off-rate is dominated by the dissociation rate) of the aminoglycoside block are compatible with the mechanism of an open pore block. In addition, the inhibition was not dependent on the ATP concentration used, and the ATP concentration–response curve showed a pronounced maximum depression, but unaffected EC50 values, in the presence of aminoglycosides, both of which indicate a noncompetitive mechanism. Together, these results are strongly indicative of an open pore block of P2X2 receptors by aminoglycosides. Furthermore, the inhibition was voltage-dependent and greatly reduced at more positive potentials, which was also found by Lin et al. [27], who showed a voltage dependency of inhibition by neomycin for ATP-mediated currents in guinea pig outer hair cells. All of these properties are consistent with a model in which the inhibitor molecule physically plugs the ionic pore. Lin et al. [27] also proposed an open pore block mechanism for neomycin in ATP-mediated currents in guinea pig outer hair cells. This model of an open pore block is also consistent with aminoglycoside inhibition at other ion channels, such as the inhibition of mechanosensitive ion channels in skeletal muscle fibers by neomycin [22].
The slow activation and inactivation kinetics of the P2X2-X1 chimera available from previous studies [34] compared with the P2X2 receptor makes the chimera ideal for a quantitative investigation of the mechanism of antibiotic inhibition. Moreover, the P2X2-X1 chimera can serve as a model of the P2X1 receptor in terms of binding/gating and pore properties without the bias of fast desensitization [34, 43] because the pore region, the channel gate and the ATP binding site do not include residues of the N-terminal tail or the transmembrane domain I [4, 6–9].
Like the P2X2 receptor, the P2X2-X1 chimera was also inhibited by all of the tested aminoglycosides, but with an order and degree of potencies different from P2X2. The most potent aminoglycosides were gentamicin and neomycin, and the least potent was paromomycin. The ranked order of potency was gentamicin = neomycin > streptomycin > kanamycin > paromomycin. In addition to being used to determine the inhibitory potencies, the chimeric receptors were used to further elucidate the mechanism of the aminoglycoside block. Kinetic models commonly used for ligand-gated ion channels assume a higher ligand affinity for the open conformation [42]. This difference in affinity between the open and closed states causes ligand dissociation to occur mainly from the closed state. Because of the slow agonist dissociation, the P2X2-X1 chimera can serve as an ideal model to test this hypothesis. Removal of ATP in the absence of aminoglycosides led to a monoexponential current decay with a time constant τ of ∼60 s. After a time period of 240 s, virtually no receptor current was detectable. This was not the case when the receptors were blocked by gentamicin or streptomycin in the presence of ATP and subsequently after ATP washout; after a 240-s lasting period in ATP-free solution, the washout of gentamicin or streptomycin caused a significant rebound of receptor current. We interpreted this as a deceleration of ATP dissociation from the open state caused by the aminoglycoside apparently locking the receptors in the open (but nonconducting) conformation. The analysis of the current decay in the presence of streptomycin as a function of time demonstrates that the ATP unbinding was delayed ∼1.6-fold by 1 mM streptomycin.
Inhibition of P2X2 receptors by aminoglycosides appears to be noncompetitive, as shown by the maximum depression of the ATP concentration–response curve in the presence of streptomycin and because the inhibition was independent of the agonist (ATP) concentration. For the chimera, we could use a more direct approach to test whether inhibition was competitive or not. Because ATP dissociates very slowly from the chimera, fast receptor inhibition could only occur when the inhibition is not caused by aminoglycoside binding at the agonist binding site. In fact, the onset and offset of inhibition at the chimera was equally fast for the P2X2 receptor, directly demonstrating a mechanism independent of the availability of free agonist binding sites. Therefore, the P2X2-X1 chimera can serve as a tool for directly distinguishing between competitive and noncompetitive inhibition at the P2X1 receptor.
Acknowledgements
We thank Anja Becker and Janna Enderich for technical assistance, Ernst Bamberg for generous support, and Günther Schmalzing for critical review of the manuscript.
Footnotes
Jürgen Rettinger and Ralf Hausmann contributed equally to this work.
References
- 1.Nicke A, Bäumert HG, Rettinger J, et al. P2X1 and P2X3 receptors form stable trimers: a novel structural motif of ligand-gated ion channels. EMBO J. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aschrafi A, Sadtler S, Niculescu C, et al. Trimeric architecture of homomeric P2X2 and heteromeric P2X1+2 receptor subtypes. J Mol Biol. 2004;342:333–343. doi: 10.1016/j.jmb.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 3.Jasti J, Furukawa H, Gonzales EB, et al. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- 4.Kawate T, Michel JC, Birdsong WT, et al. Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature. 2009;460:592–598. doi: 10.1038/nature08198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jarvis MF. The neural–glial purinergic receptor ensemble in chronic pain states. Trends Neurosci. 2010;33:48–57. doi: 10.1016/j.tins.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 6.Cao L, Broomhead HE, Young MT, et al. Polar residues in the second transmembrane domain of the rat P2X2 receptor that affect spontaneous gating, unitary conductance, and rectification. J Neurosci. 2009;29:14257–14264. doi: 10.1523/JNEUROSCI.4403-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kracun S, Chaptal V, Abramson J, et al. Gated access to the pore of a P2X receptor: structural implications for closed–open transitions. J Biol Chem. 2010;285:10110–10121. doi: 10.1074/jbc.M109.089185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang R, Martz A, Gonin S, et al. A putative extracellular salt bridge at the subunit interface contributes to the ion channel function of the ATP-gated P2X2 receptor. J Biol Chem. 2010;285:15805–15815. doi: 10.1074/jbc.M110.101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keceli B, Kubo Y. Functional and structural identification of amino acid residues of the P2X2 receptor channel critical for the voltage- and [ATP]-dependent gating. J Physiol. 2009;587:5801–5818. doi: 10.1113/jphysiol.2009.182824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wildman SS, King BF, Burnstock G. Modulatory activity of extracellular H+ and Zn2+ on ATP-responses at rP2X1 and rP2X3 receptors. Br J Pharmacol. 1999;128:486–492. doi: 10.1038/sj.bjp.0702802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Virginio C, Church D, North RA, et al. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology. 1997;36:1285–1294. doi: 10.1016/S0028-3908(97)00141-X. [DOI] [PubMed] [Google Scholar]
- 12.Khakh BS, Proctor WR, Dunwiddie TV, et al. Allosteric control of gating and kinetics at P2X4 receptor channels. J Neurosci. 1999;19:7289–7299. doi: 10.1523/JNEUROSCI.19-17-07289.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KJ, Michel AD, Chessell IP, et al. Cibacron blue allosterically modulates the rat P2X4 receptor. Neuropharmacology. 1998;37:1579–1586. doi: 10.1016/S0028-3908(98)00153-1. [DOI] [PubMed] [Google Scholar]
- 14.North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- 15.Schatz A, Bugie E, Waksman SA. Streptomycin, a substance exhibiting antibiotic activity against gram-positive and gram-negative bacteria. 1944. Clin Orthop Relat Res. 2005;437:3–6. doi: 10.1097/01.blo.0000175887.98112.fe. [DOI] [PubMed] [Google Scholar]
- 16.Rybak LP, Whitworth CA. Ototoxicity: therapeutic opportunities. Drug Discov Today. 2005;10:1313–1321. doi: 10.1016/S1359-6446(05)03552-X. [DOI] [PubMed] [Google Scholar]
- 17.Selimoglu E. Aminoglycoside-induced ototoxicity. Curr Pharm Des. 2007;13:119–126. doi: 10.2174/138161207779313731. [DOI] [PubMed] [Google Scholar]
- 18.Blanchet C, Erostegui C, Sugasawa M, et al. Gentamicin blocks ACh-evoked K+ current in guinea-pig outer hair cells by impairing Ca2+ entry at the cholinergic receptor. J Physiol. 2000;525(Pt 3):641–654. doi: 10.1111/j.1469-7793.2000.t01-1-00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schacht J. Biochemical basis of aminoglycoside ototoxicity. Otolaryngol Clin North Am. 1993;26:845–856. [PubMed] [Google Scholar]
- 20.Vital BO, Prado-Franceschi J. The nature of neuromuscular block produced by neomycin and gentamicin. Arch Int Pharmacodyn Thér. 1969;179:78–85. [PubMed] [Google Scholar]
- 21.Ohmori H. Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol. 1985;359:189–217. doi: 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winegar BD, Haws CM, Lansman JB. Subconductance block of single mechanosensitive ion channels in skeletal muscle fibers by aminoglycoside antibiotics. J Gen Physiol. 1996;107:433–443. doi: 10.1085/jgp.107.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okamoto T, Sumikawa K. Antibiotics cause changes in the desensitization of ACh receptors expressed in Xenopus oocytes. Brain Res Mol Brain Res. 1991;9:165–168. doi: 10.1016/0169-328X(91)90144-M. [DOI] [PubMed] [Google Scholar]
- 24.Amici M, Eusebi F, Miledi R. Effects of the antibiotic gentamicin on nicotinic acetylcholine receptors. Neuropharmacology. 2005;49:627–637. doi: 10.1016/j.neuropharm.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Raisinghani M, Premkumar LS. Block of native and cloned vanilloid receptor 1 (TRPV1) by aminoglycoside antibiotics. Pain. 2005;113:123–133. doi: 10.1016/j.pain.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Masuko T, Kuno T, Kashiwagi K, et al. Stimulatory and inhibitory properties of aminoglycoside antibiotics at N-methyl-d-aspartate receptors. J Pharmacol Exp Ther. 1999;290:1026–1033. [PubMed] [Google Scholar]
- 27.Lin X, Hume RI, Nuttall AL. Voltage-dependent block by neomycin of the ATP-induced whole cell current of guinea-pig outer hair cells. J Neurophysiol. 1993;70:1593–1605. doi: 10.1152/jn.1993.70.4.1593. [DOI] [PubMed] [Google Scholar]
- 28.Ito K, Dulon D. Purinergic signaling in cochleovestibular hair cells and afferent neurons. Purinergic Signal. 2010;6:201–209. doi: 10.1007/s11302-010-9183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Telang RS, Paramananthasivam V, Vlajkovic SM, et al. Reduced P2x(2) receptor-mediated regulation of endocochlear potential in the ageing mouse cochlea. Purinergic Signal. 2010;6:263–272. doi: 10.1007/s11302-010-9195-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Housley GD, Greenwood D, Ashmore JF. Localization of cholinergic and purinergic receptors on outer hair cells isolated from the guinea-pig cochlea. Proc Biol Sci. 1992;249:265–273. doi: 10.1098/rspb.1992.0113. [DOI] [PubMed] [Google Scholar]
- 31.Szucs A, Szappanos H, Toth A, et al. Differential expression of purinergic receptor subtypes in the outer hair cells of the guinea pig. Hear Res. 2004;196:2–7. doi: 10.1016/j.heares.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Werner P, Seward EP, Buell GN, et al. Domains of P2X receptors involved in desensitization. Proc Natl Acad Sci USA. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rettinger J, Schmalzing G. Desensitization masks nanomolar potency of ATP at the P2X1 receptor. J Biol Chem. 2004;279:6426–6433. doi: 10.1074/jbc.M306987200. [DOI] [PubMed] [Google Scholar]
- 35.Miledi R, Parker I, Woodward RM. Membrane currents elicited by divalent cations in Xenopus oocytes. J Physiol. 1989;417:173–195. doi: 10.1113/jphysiol.1989.sp017796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber WM. Endogenous ion channels in oocytes of Xenopus laevis: recent developments. J Membr Biol. 1999;170:1–12. doi: 10.1007/s002329900532. [DOI] [PubMed] [Google Scholar]
- 37.Evans RJ, Lewis C, Buell G, et al. Pharmacological characterization of heterologously expressed ATP-gated cation channels (P2x purinoceptors) Mol Pharmacol. 1995;48:178–183. [PubMed] [Google Scholar]
- 38.Ding S, Sachs F. Inactivation of P2X2 purinoceptors by divalent cations. J Physiol. 2000;522:199–214. doi: 10.1111/j.1469-7793.2000.t01-1-00199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rettinger J, Schmalzing G. Activation and desensitization of the recombinant P2X1 receptor at nanomolar ATP concentrations. J Gen Physiol. 2003;121:451–461. doi: 10.1085/jgp.200208730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.International Union of Pharmacology. Khakh BS, Burnstock G, Kennedy C, et al. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol Rev. 2001;53:107–118. [PubMed] [Google Scholar]
- 41.Rettinger J, Braun K, Hochmann H, et al. Profiling at recombinant homomeric and heteromeric rat P2X receptors identifies the suramin analogue NF449 as a highly potent P2X1 receptor antagonist. Neuropharmacology. 2005;48:461–468. doi: 10.1016/j.neuropharm.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 42.Grosman C, Auerbach A. The dissociation of acetylcholine from open nicotinic receptor channels. Proc Natl Acad Sci USA. 2001;98:14102–14107. doi: 10.1073/pnas.251402498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hausmann R, Rettinger J, Gerevich Z, et al. The suramin analog 4, 4′, 4″, 4‴-(carbonylbis(imino-5,1,3-benzenetriylbis (carbonylimino)))tetra-kis-benzenesulfonic acid (NF110) potently blocks P2X3 receptors: subtype selectivity is determined by location of sulfonic acid groups. Mol Pharmacol. 2006;69:2058–2067. doi: 10.1124/mol.106.022665. [DOI] [PubMed] [Google Scholar]