Abstract
Background & Aims
The μ opioid receptor (μOR) undergoes rapid endocytosis following acute stimulation with opioids and most opiates, but not with morphine. We investigated whether prolonged activation of μOR affects morphine’s ability to induce receptor endocytosis in enteric neurons.
Methods
We compared the effects of morphine, a poor μOR-internalizing opiate, and [D-Ala2, MePhe4,Gly-ol5] enkephalin (DAMGO), a potent μOR-internalizing agonist, on μOR trafficking in enteric neurons and on the expression of dynamin and β-arrestin immunoreactivity in the ileum of guinea pigs rendered tolerant by chronic administration of morphine.
Results
Morphine (100 µM) strongly induced endocytosis of μOR in tolerant but not naïve neurons (55.7%±9.3% vs. 24.2%±7.3%, P<0.001) whereas DAMGO (10 µM) strongly induced internalization of μOR in neurons from tolerant and naïve animals (63.6%±8.4% and 66.5%±3.6%). Morphine- or DAMGO-induced μOR endocytosis resulted from direct interactions between the ligand and the μOR, because endocytosis was not affected by tetrodotoxin, a blocker of endogenous neurotransmitter release. Ligand-induced μOR internalization was inhibited by pretreatment with the dynamin inhibitor, dynasore. Chronic morphine administration resulted in a significant increase in dynamin and translocation of dynamin immunoreactivity from the intracellular pool to the plasma membrane, but did not affect β arrestin immunoreactivity.
Conclusion
Chronic activation of μORs increases the ability of morphine to induce μOR endocytosis in enteric neurons, which depends on the level and cellular localization of dynamin, a regulatory protein that has an important role in receptor-mediated signal transduction in cells.
Keywords: G-protein coupled receptors, opioid peptides, opiate drugs, tolerant and naïve animals
μ opioid receptors (μORs) are G protein coupled receptors (GPCRs) abundantly expressed throughout the body, which mediate a variety of biological effects ranging from analgesia, stress response, immune processes, and inflammation1–5. They are activated by native opioid peptides and are the preferred targets of alkaloids drugs, the most efficacious and potent analgesics used in humans for pain treatment6, 7. In the gastrointestinal tract, μORs are localized to functionally distinct enteric neurons and immune cells and they affect motility and secretion 5, 8–11. μORs mediate opioid bowel dysfunction, a condition characterized by severe impairment of gastrointestinal motility and abdominal pain, which develops in patients receiving long-term opiate treatment for chronic pain 12–14, and have been proposed to serve as regulatory modulators of gut inflammatory processes 15.
μOR activation initiates a cascade of events including phosphorylation, receptor endocytosis, intracellular sorting and recycling resulting in desensitization and resensitization, important regulatory processes that control signaling and cellular response5, 6, 16–18. Receptor endocytosis contributes to the regulation of receptor mediated functions by removing receptors from the cell surface and participating to the attenuation and the recovery of cellular response18–20. μOR endocytosis is of particular interest because it is differentially regulated by native opioids and opiate drugs. Opioids such as enkephalins and endomorphins as well as several opiates like etorphine and fentanyl induce rapid and pronounced μOR internalization in cell lines and in neurons, including enteric neurons via a clathrin-mediated mechanism 21–27. By contrast, morphine and heroin differ in their inefficiency to trigger receptor endocytosis in multiple cell types, though they activate μOR to induce analgesia, tolerance and constipation21–26. The resistance of morphine-activated μORs to undergo internalization has gained considerable attention because morphine is a drug of clinical relevance given its widespread use for pain control and following surgery and its higher propensity to induce opioid tolerance compared to other opiates 28 highly efficient in triggering receptor internalization.
Whether the ability of morphine to induce μOR endocytosis is affected by prolonged receptor activation is not known. Chronic stimulation of μOR induces a variety of intracellular adaptations including changes in the expression of proteins implicated in receptor trafficking in regions of the brain expressing μORs and in cell lines29–31. In this study, we tested the hypothesis that prolonged μOR activation affects morphine ability to induce receptor endocytosis in enteric neurons. To test this hypothesis, we investigated the effect of morphine, a poor internalizing agonist, and D-Ala2-N-Me-Phe4-Glycol5-enkephalin (DAMGO), an opioid analog with high endocytic efficacy, on μOR internalization in guinea pig enteric neurons following chronic systemic administration of morphine. The guinea pig was chosen as animal model because ligand-μOR trafficking has been well characterized in this species enteric neurons in vivo and in vitro 24, 25, 32, 33 and it has been widely used for functional studies to characterize opioids and opiates effects in the gut 34. In order to study the possible mechanisms underlying receptor translocation following chronic exposure to morphine, we analyzed the expression of dynamin and β-arrestin, intracellular proteins that regulate receptor trafficking17, 18, 26. The cytosolic GTPase, dynamin, plays a role in receptor-mediated internalization via clathrin-coated vesicles and mediates early endosome formation, and it is required for μOR endocytosis. β arrestins interact with G-protein receptor kinase-phosphorylated receptors and uncouple receptors from G proteins inducing acute desensitization, and serve as adaptor proteins to link the receptor to endosome thus facilitating dynamin-dependent clathrin-mediated endocytosis.
Materials and Methods
Experimental animals
Animal care and procedures were in accordance with the National Institutes of Health recommendations for the humane use of animals and were approved by the Animal Use Committee of UCLA and VAGLAHS. Male albino, Porcellus guinea pigs (Simonsen, 150–250 g; San Diego, Ca) received s.c. injections of saline or morphine twice a day for 7 days. The morphine doses were progressively increased using an established regimen 29 utilized for studying chronic opiate effects as follows: day 1–2, 10 mg/kg; day 3–4, 20 mg/kg; day 5–6, 40 mg/kg; day 7, 80 mg/kg. Differences have been reported on the effect of opiates chronically administered intermittently or continuously28. We chose the intermittent injections of morphine since this is the regimen that has previously been reported to induce changes in trafficking intracellular proteins in the brain and cell lines 29. The distal ileum was rapidly removed 2 hours after the last injection. The following drugs were used: Morphine sulfate (ELKINS-SINN, INC. Cherry Hill, NJ 08003), tetrodotoxin (TTX) (EMD, Biosciences, CA, USA), d-Phe-Cys-Tyr-d-Trp-Orn-Yhr-NH2 (CTOP) (Bachem, CA, USA), and dynasore (Tocris Biosciences, Ellisville, MO, USA). Drugs were dissolved in 0.9% sodium chloride, with the exception of dynasore that was dissolved in 0.4% DMSO.
Organotypic cultures of the ileum and immunohistochemistry
Guinea pigs were killed with an overdose of sodium pentobarbital (100 mg/kg; i.p.). A total of 52 animals were used. Organotypic cultures of the distal ileum were prepared as described 24. Tissue was incubated in medium alone or containing saturated concentrations of morphine (100 µM) or DAMGO (10 µM) for 1 hour at 4°C to allow ligand-receptor binding, transferred to ligand-free medium at 37°C for 30 minutes to allow receptor internalization and fixed in 0.1 M phosphate buffer (PB), 4% paraformaldehyde, pH 7.4 for 2 hours at room temperature and washed. 24. In some experiments, tissues were incubated with the μOR selective antagonist, CTOP for 1 hour at 4°C before ligand incubation, preincubated for 90 minutes in Krebs containing TTX 10−7 M at 37°C to stop endogenous release of neurotransmitters, or with dynasore, a cell permeable inhibitor of dynamin 35 to inhibit dynamin-dependent endocytosis (80–120 µM in 0.4% DMSO) 30 min before and during ligand stimulation. DMSO (0.4%) alone was used to exclude an effect of vehicle on receptor internalization.
Whole mounts of the longitudinal muscle with attached the myenteric plexus were processed for immunohistochemistry as previously described 10 using a well characterized rabbit polyclonal, affinity-purified μOR antibody (Incstar Science, Technology and Research, Stillwater, MN; 1:3,000) directed to the C-terminus region of rat μ-OR (384–398) (48 hours at 4°C) and Alexa Fluor 488 affinity-purified donkey anti-rabbit (InVitrogen Molecular Probes, Eugene, Oregon, USA) (1:1,000) (2 hours at room temperature)24.
Confocal microscopy and quantification of μOR immunoreactivity
μOR immunoreactivity distribution was analyzed with a Zeiss 510 laser scanning confocal microscope with an 100x PlanApo 1.4 na objective (Carl Zeiss Inc., Thornwood, NY), as described32, 36. Images of 512×512 pixels were collected at a magnification zoom of 1.5x. Typically, 10–20 optical sections were taken at z-axis 0.5–0.75 µm intervals through the cells. Images were processed and labeled using Adobe Photoshop 7.0 (Adobe Systems, Mountain View, CA). The level of μOR internalization was quantified using NIH Image J software with single confocal images including the nucleus and a large area of cytoplasm 32, 36. For each data point, internalization was quantified for 70–80 neurons, i.e. 10 neurons from each of 7–8 animals per group. Fluorescence density values are shown as mean ± S.E.M. We compared means using unpaired two-way ANOVA test. Significance was attained with the nominal alpha value of 0.05.
Dynamin and β-arrestin expression
Strips of longitudinal muscle-myenteric plexus preparations (LMMPs) were homogenized in 1 ml of ice-cold lysis buffer (5mM Pipes/Tris Buffer, pH 7.4 with 2mM EDTA and EGTA) and centrifuged at 14,000 rpm for 20 minutes at 4°C. The supernatant proteins were size separated by SDS-PAGE on a 8–12% polyacrylamide gel and transferred onto Immobilion membranes. Membranes were incubated with 1:1,000 mouse antibody to dynamin 2 (Transduction Laboratories, Ohio, USA; dynamin 2 is ubiquitous and shares high homology with dynamin 1, more specific for the central nervous system) or 1:75,000 rabbit antiserum to β arrestin 1 (kindly provided by Robert Lefkowitz, Durham, USA; β-arrestin 1 shares high homology with β-arrestin 2), followed by horseradish peroxidase-conjugated anti-mouse or anti-rabbit immunoglobulin at 1:1,000 dilution for 1 hour at room temperature.
Since our pilot study showed increased expression of dynamin in chronically treated animals compared to controls, we used cellular fractionation to determine whether dynamin intracellular distribution was affected by chronic morphine. The homogenates from LMMPs were centrifuged (1,000g, 10 minutes, 4°C) to remove cellular debris and nuclei. The supernatant obtained was centrifuged at 12,000 g for 30 minutes at 4°C to remove the crude synaptosomal pellet or “mitochondrial” fraction (P2); then the resulting supernatants (S2) were pelleted at 100,000 g (Ty65 rotor) for 60 minutes at 4°C. Both the crude microsomal pellet or “synaptic plasma membranes” (P3) and the soluble supernatant fraction (S3) were removed for SDS/PAGE and immunoblotting analysis. Protein concentration was determined with Lowry analysis. Protein extracts (homogenates, P3 and S3) were fractionated by SDS-PAGE on a 8–12% polyacrylamide gel and transferred onto Immobilion membranes. Membranes were incubated with mouse anti-dynamin2 antibody as described above. Immunoreactive bands were detected using Western Blotting ECL reagent (Amersham Pharmacia, Piscataway, NJ). Autoradiograms were scanned using the GS-710 Calibrated Imaging Densitometer (Bio-Rad), and the labeled bands were quantified using the Quantity software program (Bio-Rad). The obtained results are representative of 4 experiments.
Electrically induced neurogenic contractions of the ileum in vitro
To determine the effects of chronic morphine treatment on neurogenic contractions of the ileum, we used LMMPs of the ileum from animals chronically treated with morphine or saline (6 animals each group) prepared as described33. Isometric contractions were recorded with a force-displacement transducer. Following a 45-minutes equilibration period, strips were stimulated with square wave pulses (0.5 ms) of supramaximal amplitude (60V) at a frequency of 0.1Hz. Concentration response-curves for the inhibitory effects of morphine (1 nM-1 µM) on electrically induced contractions in preparations from animals treated with saline or chronic morphine were constructed in a non-cumulative fashion. Strips were washed for three 5-min periods following each agonist administration, and contractions were allowed to return to baseline before any subsequent pharmacological treatment. For statistical analysis, we used ANOVA followed by post-hoc Fisher test.
Gastrointestinal transit
We measured the aboral gastrointestinal transit of a non-absorbable tracer fluorescein isothiocyanate-labeled dextran with an average molecular mass of 70 kDa (FD70) 37 in 12 animals (6 chronically treated with morphine and 6 with saline). Overnight fasted animals received 200 µl of FD70 dissolved in distilled water by oral gavage and were killed 90 minutes later. The gastrointestinal tract from stomach to distal colon was excised and divided into 15 segments: stomach, small intestine (divided into 10 segments of equal length), cecum, and colon (three segments of equal length). The luminal content of each segment was collected, suspended in 1 ml of distilled water, vortexed and centrifuged. The fluorescent signal in each segment was quantified in a multiwell fluorescent plate reader (Microplate Fluorescence Reader Flx 800, Bio-Tek). The wavelengths for detecting FD70 were 490 nm for excitation and 520 nm for emission; the slit width was 2.5 nm, and the integrated time was 1.0 second. The transit of FD70 along the gastrointestinal tract was summarized by calculating the geometric center (GC) for the FD70 distribution using the following equation: GC = sum of the products of the fraction of the marker in each segment times the segment number38.
Results
Effect of morphine and DAMGO on μOR cellular distribution in enteric neurons
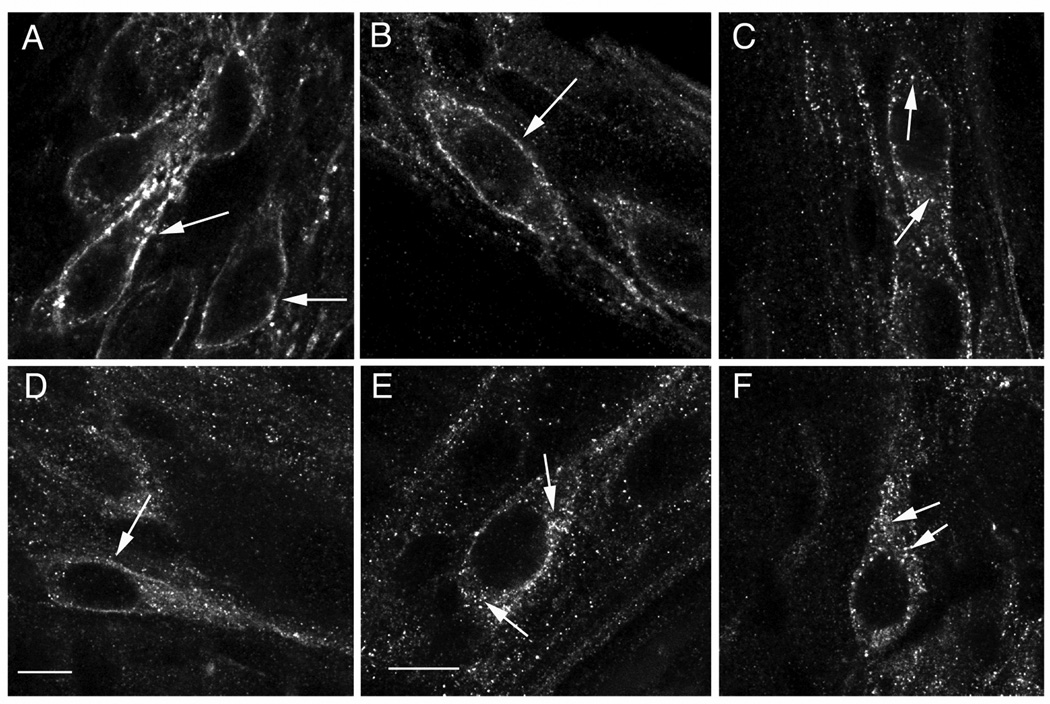
In naïve animals (chronic administration of saline), μOR immunoreactivity was mostly at the plasma membrane of unstimulated enteric neurons (Fig. 1 A) and in enteric neurons stimulated with morphine (Fig. 1 B). By contrast, DAMGO stimulation induced a pronounced translocation of μOR immunoreactivity into the cytoplasm as indicated by the punctate pattern of immunofluorescence inside the cell surface and around the nucleus and in neuronal processes, consistent with receptor internalization (Fig. 1 C). The effects of morphine and DAMGO on μOR immunoreactivity are similar to what we have previously shown in neurons from normal animals24, 25, confirming that intermittent injections of saline do not affect receptor distribution at the cellular level. In animals chronically treated with morphine, μOR immunoreactivity was mostly at the plasma membrane of unstimulated enteric neurons with low level of punctuate immunofluorescence in the cytoplasm (Fig. 1D). Interestingly, both morphine and DAMGO induced a pronounced redistribution of μOR immunoreactivity in the cytoplasm with the punctuate appearance consistent with receptor internalization (Figs. 1E and 1 F).
Fig. 1.
μOR immunoreactivity in enteric neurons from animals chronically treated with saline (naïve) (A–C) or morphine (tolerant) (D–F). μOR immunoreactivity is predominantly at the cell surface (arrows) of enteric neurons from unstimulated (A) and morphine stimulated (B) tissue from naive animals. Low level of μOR internalization is observed in unstimulated enteric neurons from animals chronically treated with morphine (D). μOR internalization is observed in DAMGO-stimulated neurons from saline treated animals (C) as well as in morphine-stimulated (E) and DAMGO-stimulated (F) neurons from animals chronically treated with morphine. Arrows in A,B and D point to μOR immunoreactivity on cell surface, whereas in C, E and F they point to examples of μOR immunoreactivity in endosomes inside the cytoplasm. Calibration bars: 4µm in A and D; 5 µm in B, C, D, E and F.
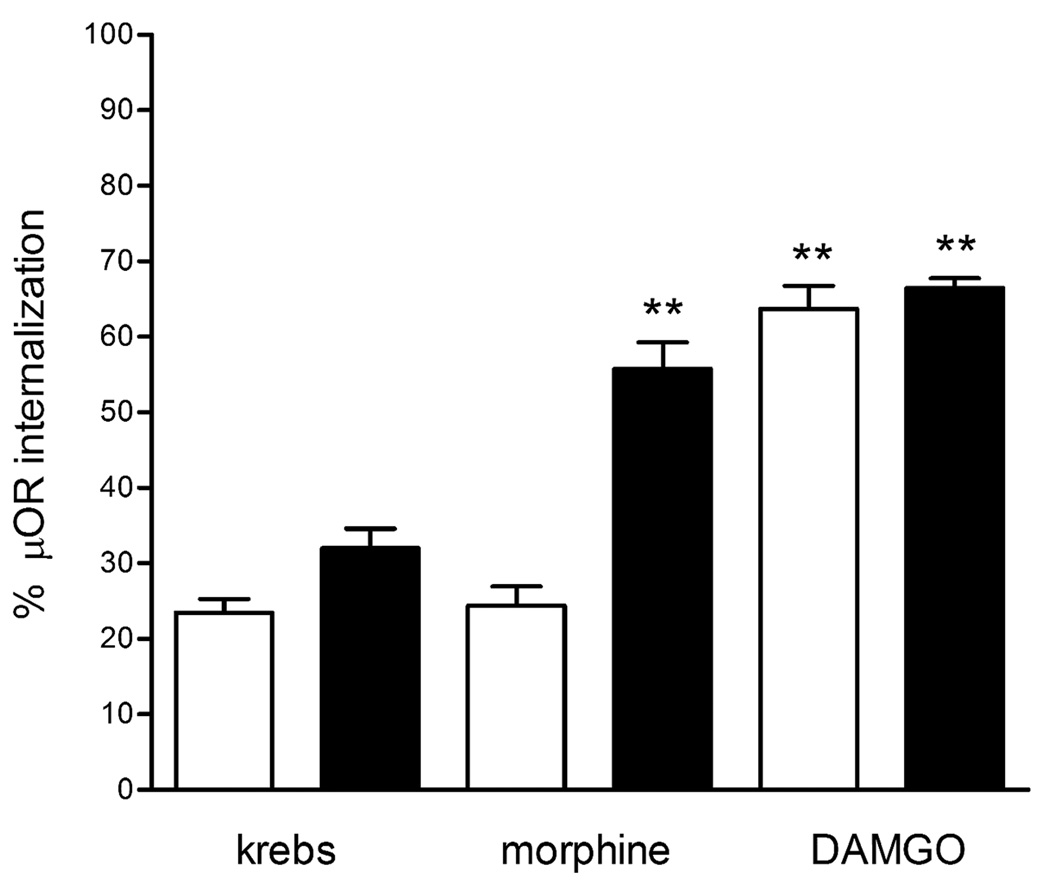
The proportion of μOR immunoreactivity in the cytoplasm in unstimulated neurons from animals chronically treated with morphine was slightly higher than in naïve animals (Fig. 2), but within the range previously observed in enteric neurons from normal animals32. The levels of μOR endocytosis induced by acute stimulation with morphine in neurons from animals chronically treated with morphine were significantly higher compared to neurons form saline-treated animals (55.7±9.3% vs. 24.2±7.3%, p<0.001) (Fig. 2), indicating that prolonged μOR activation increases morphine efficiency in inducing μOR endocytosis. DAMGO stimulation induced comparable levels of μOR internalization in neurons from animals chronically exposed to morphine or saline (63.6±8.4% vs. 66.5±3.6%) (Fig.2), suggesting that μORs remain equally functional in neurons from naïve and tolerant animals.
Fig. 2.
Levels of μOR immunoreactivity in the cytoplasm of enteric neurons from animals treated with saline (white bars; naïve) and animals chronically exposed to morphine (black bars; tolerant) incubated with medium (Krebs; unstimulated), morphine or DAMGO. Fluorescence density values are expressed as mean±SEM of measures taken from 10 neurons per experiment (70–80 neurons from 7–8 experiments). ** p<0.001 vs. unstimulated (Krebs) neurons and morphine stimulated naïve neurons.
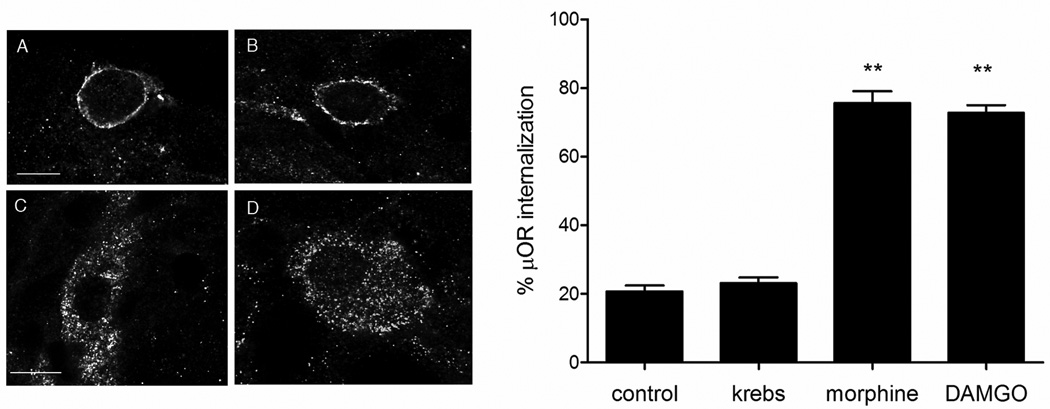
μOR endocytosis induced by either morphine or DAMGO in neurons from chronic morphine animals was not affected by the sodium channel blocker TTX treatment (Fig.3) prior each ligand stimulation to prevent depolarization, confirming that μOR translocation is due to a direct interaction of morphine or DAMGO with μOR and not to endogenous opioids released in response to chronic receptor activation. There was no internalization in distal ileum exposed only to TTX and immediately fixed or following exposure to Krebs alone.
Fig. 3.
μOR immunoreactivity in neurons from animals chronically treated with morphine that were pretreated in organotypic cultures with TTX and then fixed immediately (A) or after exposure to Krebs (B), morphine (C) or DAMGO (D). μOR is internalized in morphine- and DAMGO-stimulated neurons (C and D, respectively), but not in control and Krebs treated (A and B, respectively), indicating that blockade of endogenous transmitter release with TTX does not prevent μOR internalization in vitro. Calibration bars: 6 µm in A and B; 5 µm in C and D. Graphic on the right shows the quantification of μOR internalization in control (corresponding to A), exposure to Krebs solution (corresponding to B) and exposure to either morphine or DAMGO in enteric neurons from tolerant animals pretreated with TTX to block transmitter release (corresponding to C and D, respectively). P<0.01 compared to control and Krebs.
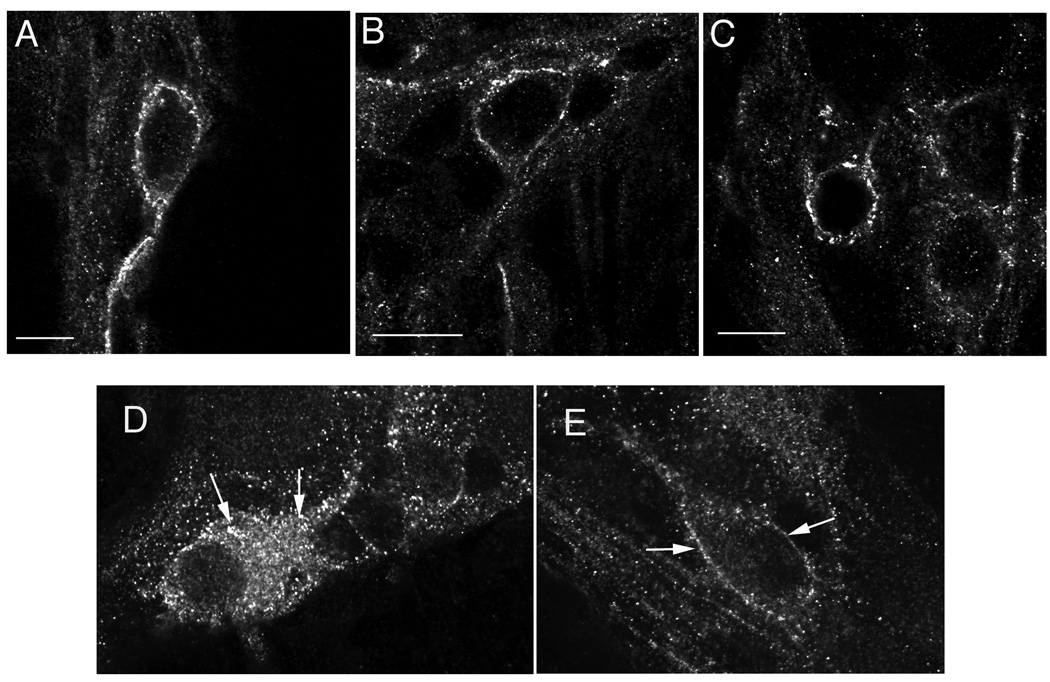
In organotypic cultures of distal ileum of chronic morphine animals that were pretreated with CTOP (Fig. 4), μOR was detected at plasma membrane as observed in saline unstimulated animals, confirming specificity of ligand-induced μOR internalization.
Fig. 4.
A–C show specificity of μOR endocytosis in response to ligand stimulation in tolerant animals. μOR is predominantly at the cell surface in unstimulated neurons (A), and it remains at the plasma membrane in neurons from tolerant animals, which were treated with the selective μOR antagonist CTOP before exposure to either morphine (B) or DAMGO (C). D and E show the effect of morphine on μOR internalization in tolerant neurons in the absence (D) or presence (E) of the dynamin inhibitor, dynasore. D: μOR in internalized in a neuron from a tolerant animal stimulated with morphine. E: μOR is predominantly at the cell surface in a neuron of the ileum from a tolerant animal stimulated with morphine in the presence of the dynamin inhibitor, dynasore. Arrows in D point to endosomes and in E to the cell membrane. Calibration bars: 4 µm in A; 6 µm in B; 5 µm in C; 10 µm in D, E.
Morphine-induced μOR internalization (Fig. 4 D) was inhibited by the pretreatment with the dynamin inhibitor, dynasore (Fig. 4 E) whereas it was not affected by DMSO alone (not shown). Dynasore did not affect μOR distribution in morphine-activated neurons from control animals and in unstimulated neurons. Dynasore inhibited the DAMGO-induced μOR internalization in controls and tolerant animals (not shown). These findings support that μOR endocytosis in enteric neurons in control and tolerant animals is dynamin-dependent and are consistent with the hypothesis that the increased efficiency of morphine to induce μOR internalization in enteric neurons from tolerant animals is mediated by dynamin.
Effect of chronic morphine on dynamin and β-arrestin expression
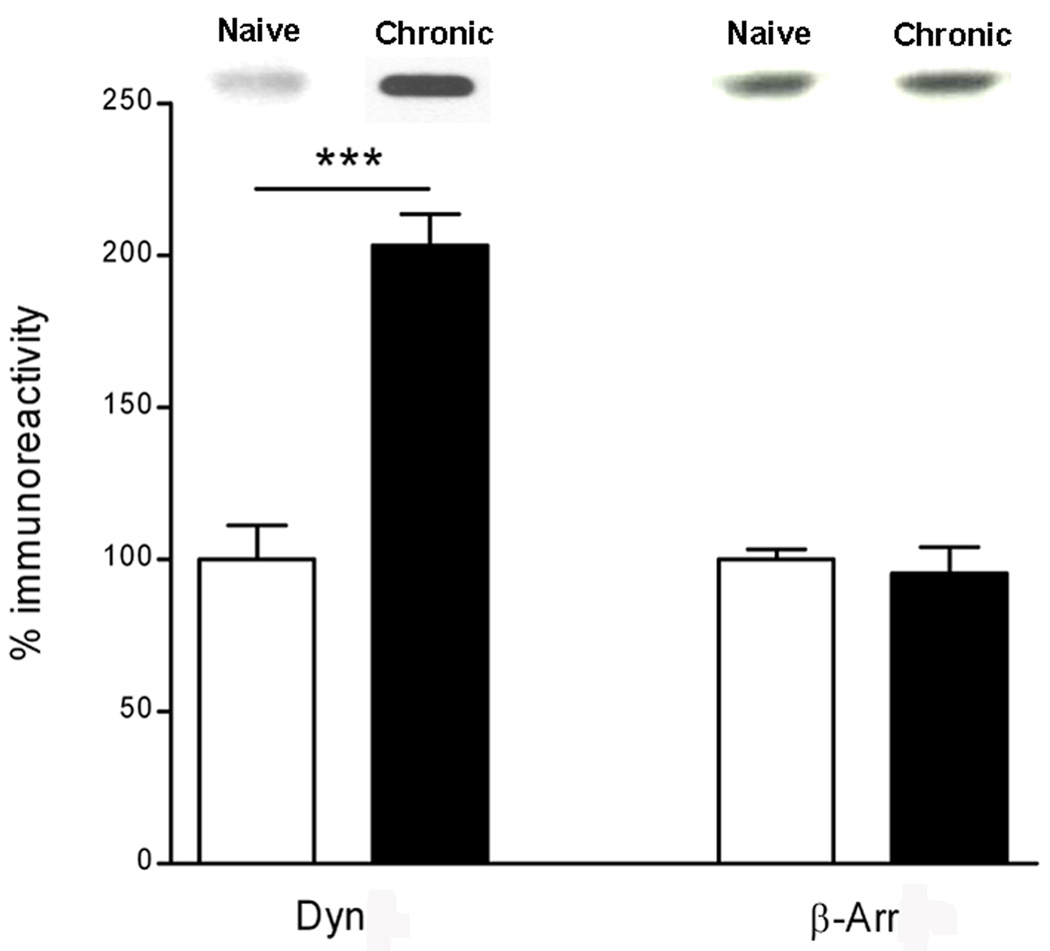
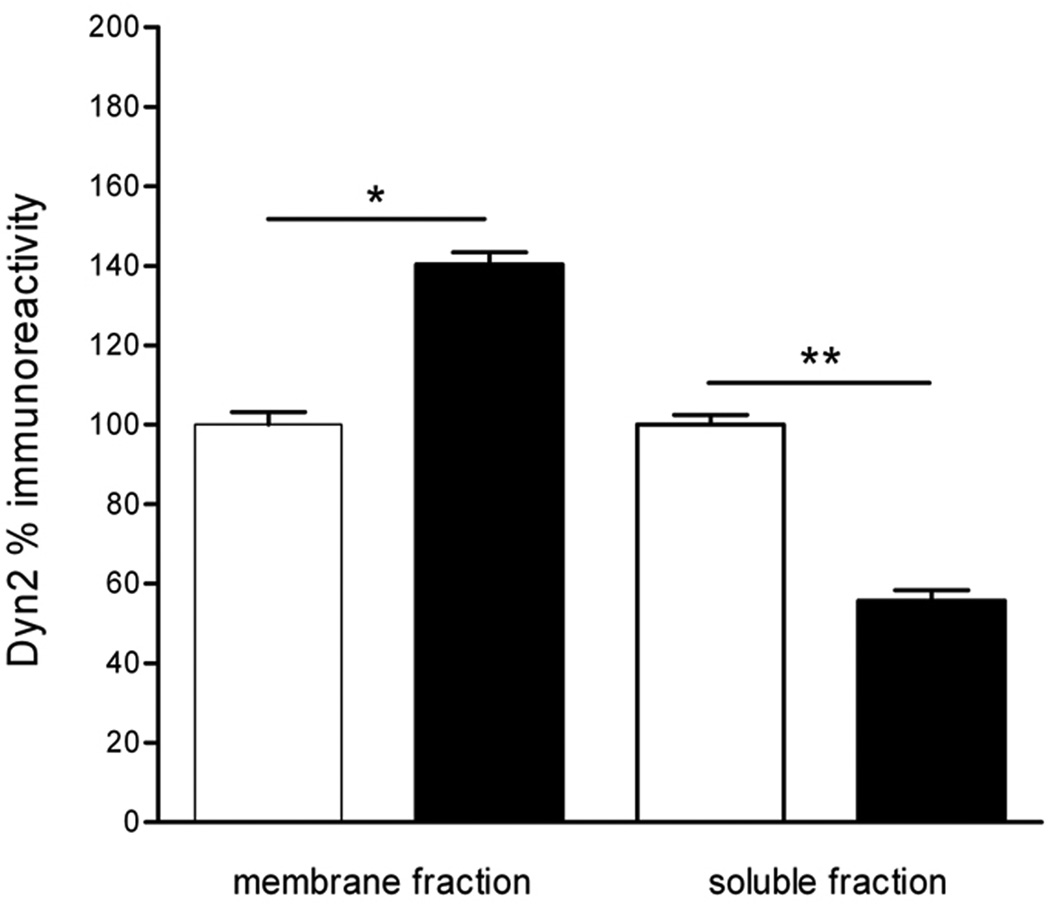
Chronic morphine treatment significantly increased the levels of dynamin 2 immunoreactivity (65%), whereas it did not affect the level of β-arrestin 1 immunoreactivity in LMMPs compared to saline (Fig 5). Since dynamin 2 levels were affected by chronic morphine treatment, we evaluated dynamin cellular distribution by cell fractionation of LMMPs. There was a significant increase of dynamin 2 immunoreactivity in the membrane fraction in ileum specimens from morphine chronically treated animals compared to saline treated, consistent with a redistribution of dynamin 2 from the cytoplasm to the membrane. (Fig.6).
Fig. 5.
Quantification of dynamin 2 (Dyn) and β-arrestin 1 (β-Arr) immunoreactivity in LMMPs of the guinea pig distal ileum from animals treated with saline (white bars; naive) and animals chronically treated with morphine (black bras; chronic) measured by Western Blot. Results represent the % increase of dynamin or β-arrestin compared to tissue from naïve animals. ***p<0.001 compared to control (saline). Representative immunoblots are shown on top of the histograms.
Fig. 6.
Distribution of dynamin 2 immunoreactivity in subcellular fractionation of LMMPs of distal ileum from saline-treated (white bars) animals and animals chronically treated with morphine (black bars). *p<0.05 and ** p<0.01 compared to control (saline). Values are expressed as % of dynamin immunoreactivity in control (saline).
Effect of morphine on neurogenic cholinergic response and gastrointestinal transit
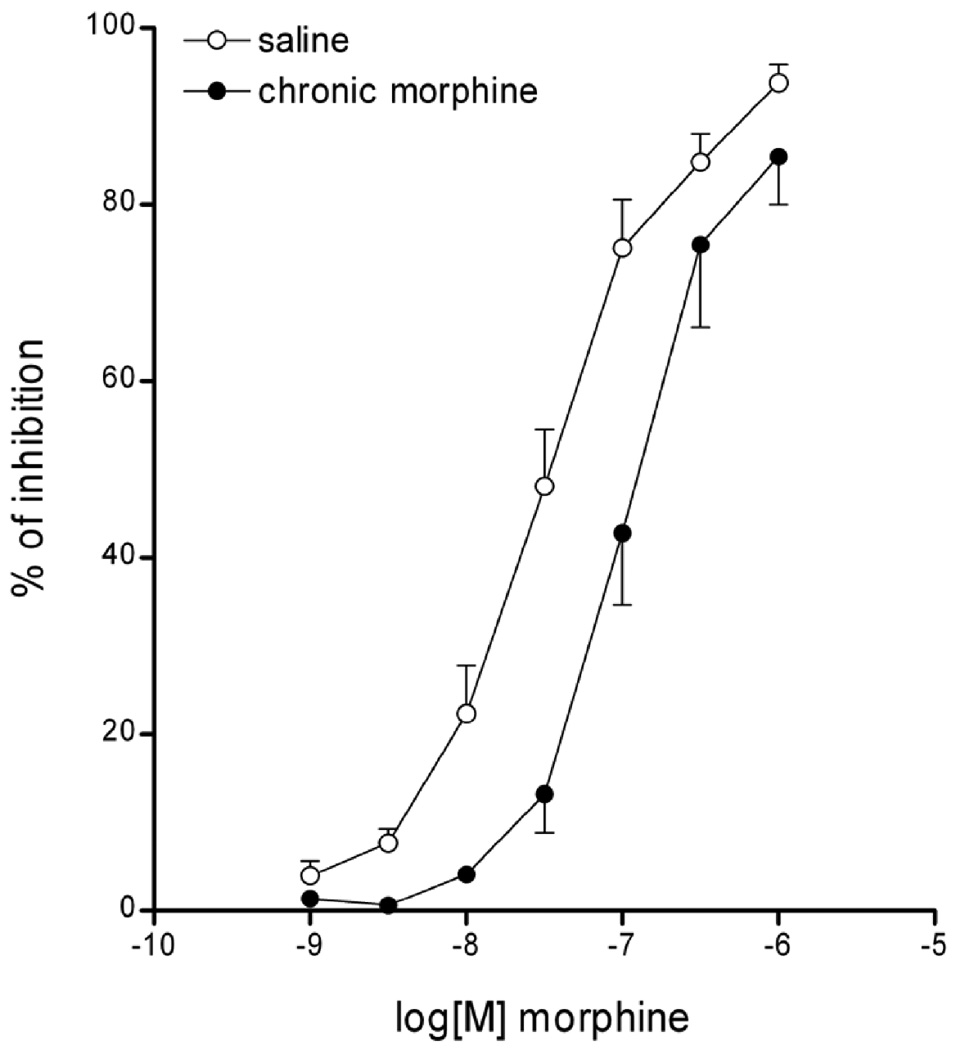
Morphine inhibited in a concentration-dependent manner the electrically induced twitch contractions of LMMPs in saline and chronic morphine treated ilea (Fig.7). The morphine inhibitory curve in ilea from animals chronically treated with morphine was shifted to the right in a parallel manner without reduction of the maximum response, indicative of a reduced response to the same concentrations of morphine compared to controls, which is consistent with the animals being tolerant33, 39.
Fig. 7.
Morphine inhibition of electrically-induced twitch contractions of LMMPs from guinea pigs chronically treated with saline (open circles) or chronic morphine (black circles). Morphine (1nM-1µM) induces a dose-dependent inhibition of muscle twitch. In animals chronically treated with morphine, the muscle twitch inhibitory curve induced by morphine was shifted to the right in a parallel manner without reduction of the maximum response, indicative of tolerance.
There was a marked delay in gastrointestinal transit in morphine chronically treated animals compared to controls (GC 3.82±0.5 vs. GC 9.81±0.18, p<0.05), further confirming the effect of chronic morphine treatment on gastrointestinal motility.
Discussion
This study shows that 1) morphine stimulation induces pronounced μOR endocytosis in enteric neurons from tolerant, but not naïve animals, which results from direct effect of ligand on μOR and 2) morphine enhanced efficiency to induce μOR endocytosis is likely due to the increased levels and translocation of dynamin resulting from prolonged μOR activation. In our experiments, the development of tolerance was supported by the reduced inhibitory effect of morphine on cholinergic nerve-mediated muscle twitch and by a marked delay in gastrointestinal transit, which is consistent with reduced cellular responsiveness to opiate stimulation, typical side effect of prolonged μOR activation.
The inefficiency of certain opiates like morphine to induce μOR internalization in many cell types including neurons and cell lines has been a puzzling finding that is still not fully understood. Morphine’s poor efficacy in promoting μOR endocytosis has been attributed to different factors, including its partial agonist properties, the lower degree and slower kinetics of receptor phosphorylation compared to other opiates, or the inability of morphine to induce desensitization 26, 27, 40–43. However, these hypotheses have not been substantiated. Indeed, morphine has been shown to have similar signaling efficacy as other opiates 26, 44, 45, and to induce phosphorylation and desensitization in heterologous cells and in neurons of the locus ceruleus in brain slices without promoting internalization 43, 44, 46, 47. Other studies have shown that overexpression of intracellular proteins regulating receptor trafficking like G protein receptor kinases or β arrestins increase the efficiency of morphine to induce μOR internalization in cell lines 27, 48, but whether this also occurs with native receptors in highly differentiated cells like neurons is unknown. Our study is the first to show that morphine induces rapid and pronounced μOR internalization in enteric neurons in a condition of tolerance, which is comparable to the level of internalization induced by other opiates and endogenous opioids 24. In order to better understand the mechanism responsible for the switch of morphine from an inefficient to an efficient internalizing ligand, we analyzed whether intracellular proteins regulating receptor trafficking were altered by chronic morphine treatment. Our findings of increased expression and translocation of dynamin from the cytosol to the cell surface suggest that this cellular adaptation to chronic treatment mediates the translocation of morphine-activated μORs from the cell surface to the cytoplasm. The inhibition of morphine-induced receptor internalization by dynasore, a dynamin inhibitor that blocks coated vesicle formation 35 further supports this hypothesis. Dynamin upregulation and translocation observed in the ileum are in agreement with previous findings in the central nervous system and cell lines following chronic opiate treatment 29. By contrast, β arrestins do not appear to have a significant role in increasing morphine ability to induce receptor internalization in enteric neurons. Thus, it is possible that the distinct internalizing potencies of μOR ligands depend upon differences in ligand capacity to interact with intracellular proteins regulating receptor trafficking.
This study confirms the high internalizing efficiency of DAMGO in enteric neurons and shows that DAMGO-induced μOR internalization persists at a similar degree in enteric neurons from tolerant animals suggesting that μORs remain functional in a condition of tolerance. These findings are consistent with previous observations in the spinal cord, where the magnitude of DAMGO-induced internalization was comparable in naïve neurons and neurons that developed tolerance to morphine-induced analgesia 49. Thus, prolonged activation of μOR does not appear to impair receptor trafficking in the enteric nervous system as in the central nervous system. Indeed, the μOR internalization time course in enteric neurons from tolerant animals is similar to what we previously reported in normal neurons24, with receptor remaining inside of the cell for over 4 hours and returning at the cell surface at 6 hours, suggesting receptor recycling (data not shown), though lysosomal degradation might also occur, since endocytosed GPCRs are sorted into both recycling and lysosomal pathways50. DAMGO-induced μOR internalization in enteric neurons is dynamin-dependent as supported by the inhibition with the dynamin inhibitor dynasore. However, the increase in dynamin occurring in condition of tolerance does not appear to enhance DAMGO efficiency to induce internalization in tolerant neurons. We can speculate that the increased levels and translocation of dynamin affect morphine-activated receptors that are at the cell surface, without influencing DAMGO-activated receptors that are rapidly internalizing.
It is worthy pointing out some differences in different systems and species. Morphine has been reported to induce rapid μOR internalization in embryonic striatal neurons in culture 51 which was β arrestin dependent. Since the levels of β arrestins in these cell cultures were not elevated, the authors speculate that morphine-induced μOR internalization might be due to cell-type specific differences in β arrestins or other types of intracellular proteins like G protein receptor kinases. Furthermore, it is of interest that morphine has been associated with μOR endocytosis in dendrites but not in cell bodies in the nucleus accumbens and in the locus ceruleus 52, 53. However, in both studies, morphine was injected in vivo, therefore it cannot be excluded that μOR internalization was due to release of endogenous opioids in response to morphine, and not to a direct interaction of morphine with μOR, since morphine induces opioid release in the central nervous system 54. By contrast, in our system, we can conclude that morphine-induced μOR endocytosis in enteric neurons from tolerant animals was a primary effect of morphine on μOR since suppression of endogenous transmitter released by TTX did not prevent receptor translocation. All together, these findings suggest that ligand-receptor interaction and endocytosis might vary according to the cell type (e.g. embryonic vs. adult) and stimulation condition (e.g. acute vs. chronic).
In summary, our findings that morphine induces substantial μOR internalization in enteric neurons rendered tolerant with chronic morphine treatment provide evidence for a modified regulation of μOR endocytosis following prolonged receptor activation. It is reasonable to propose that different mechanisms at the receptor levels and perhaps downstream of the receptor contribute to the regulation of opiate drug action in the enteric nervous system depending upon the stimulation conditions. These findings are clinically relevant, since μORs are expressed by functionally distinct enteric neurons, including motor neurons and interneurons of both the ascending and descending pathways, which form the neuronal circuits controlling gastrointestinal functions including motility, which is severely impaired by chronic opiate treatment.
Acknowledgement
This research was supported by the National Institutes of Health Grants DK54155 (C.S.), DK41301 (Morphology/Imaging subsection, C.S.), and a Pilot Grant from the NIH-NIDA “Center for Study of Opioid Receptors and Drugs of Abuse” P50 DA05010. We are grateful to Dr. Lefkowitz for providing the β-arrestin, Dr. George Sachs for providing the facilities for the subcellular fractionation studies, and Dr. Nicholas Brecha for critical reading of the manuscript.
Supported by NIH grants DK 54155, DK41301 and DA05010.
Abbreviations
- CTOP
d-Phe-Cys-Tyr-d-Trp-Orn-Yhr-NH2
- DAMGO
[D-Ala2, MePhe4,Gly-ol5] enkephalin
- LMMP
longitudinal muscle-myenteric plexus preparation
- μOR
μ opioid receptor
- PB
phosphate buffer
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflict of interest to disclose for all authors.
Patierno was involved with acquisition, analysis and interpretation of data, statistical analysis and drafting of the manuscript. Anselmi was involved with acquisition, analysis and interpretation of the data and statistical analysis. Jaramillo, Scott and Garcia were involved with data acquisition and analysis, and technical support. Sternini was involved with study concept and design, interpretation of data, critical revision of the manuscript for important intellectual content, and overall supervision of the study; she obtained funding for these studies.
References
- 1.Pasternak GW. Pharmacological mechanisms of opioid analgesics. Clin. Neuropharmacol. 1993;16:1–18. doi: 10.1097/00002826-199302000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Minami M, Satoh M. Molecular biology of the opioid receptors: structures, functions and distributions. Neurosci. Res. 1995;23:121–145. doi: 10.1016/0168-0102(95)00933-k. [DOI] [PubMed] [Google Scholar]
- 3.Nelson CJ, Schneider GM, Lysle DT. Involvement of central mu- but not delta- or kappa-opioid receptors in immunomodulation. Brain Behav Immun. 2000;14:170–184. doi: 10.1006/brbi.1999.0575. [DOI] [PubMed] [Google Scholar]
- 4.Stefano GB, Scharrer B, Smith EM, et al. Opioid and opiate immunoregulatory processes. Crit Rev Immunol. 1996;16:109–144. doi: 10.1615/critrevimmunol.v16.i2.10. [DOI] [PubMed] [Google Scholar]
- 5.Sternini C. Receptors and transmission in the brain-gut axis: potential for novel therapies. III. μ opioid receptors in the enteric nervous system. Am. J. Physiol. 2001;281:G8–G15. doi: 10.1152/ajpgi.2001.281.1.G8. [DOI] [PubMed] [Google Scholar]
- 6.Evans CJ. Agonist selective mu-opioid receptor trafficking in rat central nervous system. Mol Psychiatry. 2000;5:121. doi: 10.1038/sj.mp.4000734. [DOI] [PubMed] [Google Scholar]
- 7.Kieffer BL. Opioids: first lessons from knockout mice. Trends Pharmacol Sci. 1999;20:19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- 8.Bagnol D, Mansour A, Akil H, et al. Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience. 1997;81:579–591. doi: 10.1016/s0306-4522(97)00227-3. [DOI] [PubMed] [Google Scholar]
- 9.Burks TF. Opioid peptides in gastrointestinal functions. In: Tseng LF, editor. The Pharmacology of Opioid Peptides. Harwood Academic Publishers; 1995. pp. 397–409. [Google Scholar]
- 10.Ho A, Lievore A, Patierno S, et al. Neurochemically distinct classes of myenteric neurons express the μ-opioid receptor in the guinea pig ileum. J Comp Neurol. 2003;458:404–411. doi: 10.1002/cne.10606. [DOI] [PubMed] [Google Scholar]
- 11.Sternini C, Patierno S, Selmer IS, et al. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16 Suppl 2:3–16. doi: 10.1111/j.1743-3150.2004.00553.x. [DOI] [PubMed] [Google Scholar]
- 12.Panchal SJ, Muller-Schwefe P, Wurzelmann JI. Opioid-induced bowel dysfunction: prevalence, pathophysiology and burden. Int J Clin Pract. 2007 doi: 10.1111/j.1742-1241.2007.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pappagallo M. Incidence, prevalence, and management of opioid bowel dysfunction. Am J Surg. 2001;182:11S–18S. doi: 10.1016/s0002-9610(01)00782-6. [DOI] [PubMed] [Google Scholar]
- 14.Schug SA, Zech D, Grond S. Adverse effects of systemic opioid analgesics. Drug Saf. 1992;7:200–213. doi: 10.2165/00002018-199207030-00005. [DOI] [PubMed] [Google Scholar]
- 15.Philippe D, Dubuquoy L, Groux H, et al. Anti-inflammatory properties of the mu opioid receptor support its use in the treatment of colon inflammation. J Clin Invest. 2003;111:1329–1338. doi: 10.1172/JCI16750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohm S, Grady EF, Bunnett NW. Mechanisms attenuating signaling by G protein-coupled receptors. Biochem J. 1997;322:1–18. doi: 10.1042/bj3220001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Zastrow M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003;74:217–224. doi: 10.1016/j.lfs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 18.von Zastrow M, Svingos A, Haberstock-Debic H, et al. Regulated endocytosis of opioid receptors: cellular mechanisms and proposed roles in physiological adaptation to opiate drugs. Curr Opin Neurobiol. 2003;13:348–353. doi: 10.1016/s0959-4388(03)00069-2. [DOI] [PubMed] [Google Scholar]
- 19.Qiu Y, Law PY, Loh HH. Mu-opioid receptor desensitization: role of receptor phosphorylation, internalization, and representation. J Biol Chem. 2003;278:36733–36739. doi: 10.1074/jbc.M305857200. [DOI] [PubMed] [Google Scholar]
- 20.von Zastrow M. Role of endocytosis in signalling and regulation of G-protein-coupled receptors. Biochem Soc Trans. 2001;29:500–504. doi: 10.1042/bst0290500. [DOI] [PubMed] [Google Scholar]
- 21.Keith DE, Anton B, Murray SR, et al. μ-opioid receptor internalization: opiate drugs have differential effects on a conserved endocytic mechanism in vitro and in mammalian brain. Mol. Pharmacol. 1998;53:377–384. [PubMed] [Google Scholar]
- 22.Keith DE, Murray SR, Zaki PA, et al. Morphine activates opioid receptors without causing their rapid internalization. J. Biol. Chem. 1996;271:19021–19024. doi: 10.1074/jbc.271.32.19021. [DOI] [PubMed] [Google Scholar]
- 23.McConalogue K, Grady EF, Minnis J, et al. Activation and internalization of the μ opioid receptor by the newly described endogenous agonists, endomorphin-1 and endomorphin-2. Neuroscience. 1999;90:1051–1059. doi: 10.1016/s0306-4522(98)00514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minnis J, Patierno S, Kohlmeier SE, et al. Ligand-induced μ opioid receptor endocytosis and recycling in enteric neurons. Neuroscience. 2003;119 doi: 10.1016/s0306-4522(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 25.Sternini C, Spann M, Anton B, et al. Agonist-selective endocytosis of μ opioid receptor by neurons in vivo. Proc Natl Acad Sci USA. 1996;93:9241–9246. doi: 10.1073/pnas.93.17.9241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whistler JL, Chuang H-H, Chu P, et al. Functional dissociation of μ opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 27.Zhang J, Ferguson SSG, Barak LS, et al. Role of G protein-coupled receptor kinase in agonist-specific regulation of μ-opioid receptor responsiveness. Proc. Natl. Acad. Sci. USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duttaroy A, Yoburn BC. The effect of intrinsic efficacy on opioid tolerance. Anesthesiology. 1995;82:1226–1236. doi: 10.1097/00000542-199505000-00018. [DOI] [PubMed] [Google Scholar]
- 29.Noble F, Szuc M, Kieffer B, et al. Overexpression of dynamin is induced by chronic stimulation of μ- but not δ-opioid receptors: relationships with μ-related morphine dependence. Mol. Pharmacol. 2000;58:159–166. doi: 10.1124/mol.58.1.159. [DOI] [PubMed] [Google Scholar]
- 30.Yoburn BC, Purohit V, Patel K, et al. Opioid agonist and antagonist treatment differentially regulates immunoreactive mu-opioid receptors and dynamin-2 in vivo. Eur J Pharmacol. 2004;498:87–96. doi: 10.1016/j.ejphar.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Q, Purohit V, Yoburn BC. Continuous opioid agonist treatment dose-dependently regulates mu-opioid receptors and dynamin-2 in mouse spinal cord. Synapse. 2005;56:123–128. doi: 10.1002/syn.20137. [DOI] [PubMed] [Google Scholar]
- 32.Patierno S, Raybould HE, Sternini C. Abdominal surgery induces μ opioid receptor endocytosis in enteric neurons of the guinea pig ileum. Neuroscience. 2004;123:101–109. doi: 10.1016/j.neuroscience.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 33.Sternini C, Brecha NC, Minnis J, et al. Role of agonist-dependent receptor internalization in the regulation of μ opioid receptors. Neuroscience. 2000;98:233–241. doi: 10.1016/s0306-4522(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 34.Kromer W. Endogenous and exogenous opioids in the control of gastrointestinal motility and secretion. Pharmacol Rev. 1988;40:121–162. [PubMed] [Google Scholar]
- 35.Macia E, Ehrlich M, Massol R, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 36.Patierno S, Zellalem W, Ho A, et al. N-Methyl-d-Aspartate Receptors Mediate Endogenous Opioid Release in Enteric Neurons After Abdominal Surgery. Gastroenterology. 2005;128:2009–2019. doi: 10.1053/j.gastro.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 37.Harada T, Moore BA, Yang R, et al. Ethyl pyruvate ameliorates ileus induced by bowel manipulation in mice. Surgery. 2005;138:530–537. doi: 10.1016/j.surg.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 38.Miller MS, Galligan JJ, Burks TF. Accurate measurement of intestinal transit in the rat. J Pharmacol Methods. 1981;6:211–217. doi: 10.1016/0160-5402(81)90110-8. [DOI] [PubMed] [Google Scholar]
- 39.Chavkin C, Goldstein A. Opioid receptor reserve in normal and morphine-tolerant guinea pig ileum myenteric plexus. Proc. Natl. Acad. Sci. USA. 1984;81:7253–7257. doi: 10.1073/pnas.81.22.7253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alvarez VA, Arttamangkul S, Dang V, et al. mu-Opioid receptors: Ligand-dependent activation of potassium conductance, desensitization, and internalization. J Neurosci. 2002;22:5769–5776. doi: 10.1523/JNEUROSCI.22-13-05769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovoor A, Celver JP, Wu A, et al. Agonist induced homologous desensitization of mu-opioid receptors mediated by G protein-coupled receptor kinases is dependent on agonist efficacy. Mol Pharmacol. 1998;54:704–711. [PubMed] [Google Scholar]
- 42.Schulz S, Mayer D, Pfeiffer M, et al. Morphine induces terminal micro-opioid receptor desensitization by sustained phosphorylation of serine-375. Embo J. 2004;23:3282–3289. doi: 10.1038/sj.emboj.7600334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu Y, Zhang L, Yin X, et al. Mu opioid receptor phosphorylation, desensitization, and ligand efficacy. J Biol Chem. 1997;272:28869–28874. doi: 10.1074/jbc.272.46.28869. [DOI] [PubMed] [Google Scholar]
- 44.Borgland SL, Connor M, Osborne PB, et al. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. doi: 10.1074/jbc.M300525200. [DOI] [PubMed] [Google Scholar]
- 45.Burford NT, Tolbert LM, Sadee W. Specific G protein activation and mu-opioid receptor internalization caused by morphine, DAMGO and endomorphin I. Eur J Pharmacol. 1998;342:123–126. doi: 10.1016/s0014-2999(97)01556-2. [DOI] [PubMed] [Google Scholar]
- 46.Arttamangkul S, Quillinan N, Low MJ, et al. Differential activation and trafficking of micro-opioid receptors in brain slices. Mol Pharmacol. 2008;74:972–979. doi: 10.1124/mol.108.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Ferguson SSG, Barak LS, et al. Dynamin and beta-arrestin reveal distinct mechanisms for G protein-coupled receptor internalization. J. Biol. Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 48.Whistler JL, von Zastrow M. Morphine-activated opioid receptors elude desensitization by β-arrestin. Proc. Natl. Acad. Sci. USA. 1998;95:9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trafton JA, Basbaum AI. [d-Ala2,N-MePhe4,Gly-ol5]enkephalin-induced internalization of the micro opioid receptor in the spinal cord of morphine tolerant rats. Neuroscience. 2004;125:541–543. doi: 10.1016/j.neuroscience.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 50.Hanyaloglu AC, von Zastrow M. Regulation of GPCRs by Membrane Trafficking and Its Potential Implications. Annu Rev Pharmacol Toxicol. 2007 doi: 10.1146/annurev.pharmtox.48.113006.094830. [DOI] [PubMed] [Google Scholar]
- 51.Haberstock-Debic H, Kim KA, Yu YJ, et al. Morphine promotes rapid, arrestin-dependent endocytosis of mu-opioid receptors in striatal neurons. J Neurosci. 2005;25:7847–7857. doi: 10.1523/JNEUROSCI.5045-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haberstock-Debic H, Wein M, Barrot M, et al. Morphine acutely regulates opioid receptor trafficking selectively in dendrites of nucleus accumbens neurons. J Neurosci. 2003;23:4324–4332. doi: 10.1523/JNEUROSCI.23-10-04324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scavone JL, Van Bockstaele EJ. Mu-opioid receptor redistribution in the locus coeruleus upon precipitation of withdrawal in opiate-dependent rats. Anat Rec (Hoboken) 2009;292:401–411. doi: 10.1002/ar.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olive MF, Bertolucci M, Evans CJ, et al. Microdialysis reveals a morphine-induced increase in pallidal opioid peptide release. Neuroreport. 1995;6:1093–1096. doi: 10.1097/00001756-199505300-00005. [DOI] [PubMed] [Google Scholar]