Abstract
Objective:
Multimodal CT, including noncontrast CT (NCCT), CT with contrast, CT angiography (CTA), and perfusion CT (CTP), is increasingly used in acute stroke patients to identify candidates for endovascular therapy. Our goal is to explore the cost-effectiveness of multimodal CT as a diagnostic test.
Methods:
A Markov model compared multimodal CT to NCCT in a hypothetical cohort of nonhemorrhagic stroke patients presenting within 3 hours of symptom onset who were potential IV tPA candidates. Patients who failed to improve after IV tPA or in whom IV tPA was contraindicated were candidates for endovascular therapy. Direct costs (2008 USD), outcomes, and probabilities were obtained from the literature.
Results:
For the 3-month time horizon, multimodal CT had lower costs (−$1,716), had greater quality-adjusted life-years (QALYs, 0.004), and was the cost-effective choice 100% of the time for a willingness-to-pay of $100,000/QALY (probabilistic sensitivity analysis). The number needed to screen with multimodal CT to avoid 1 diagnostic angiogram was 2. Over a lifetime, multimodal CT had lower costs (−$2,058), had greater QALYs (0.008), and was cost-effective, with a 90.1% likelihood, for a willingness-to-pay of $100,000/QALY.
Conclusions:
Multimodal CT appears to be a cost-saving screening tool over the short term. However, additional data regarding clinical outcomes following multimodal CT-guided intra-arterial treatment are needed before the long-term cost-effectiveness can be suitably addressed. This analysis can be incorporated into future discussions of multimodal CT as a diagnostic test for unselected patients, within and beyond the 3-hour IV tPA time window.
GLOSSARY
- CTA
= CT angiography;
- CTP
= perfusion CT;
- IA
= intra-arterial;
- ICER
= incremental cost-effectiveness ratio;
- mRS
= modified Rankin Scale;
- NCCT
= noncontrast CT;
- NE
= northeast;
- NW
= northwest;
- QALY
= quality-adjusted life-year;
- SE
= southeast;
- SW
= southwest;
- tPA
= tissue plasminogen activator;
- WTA
= willingness to accept;
- WTP
= willingness to pay.
Recommendations for treatment of acute ischemic stroke emphasize timely IV tissue plasminogen activator (tPA) administration. The minimum requirement is imaging excluding hemorrhage while allowing for other MR or CT-based imaging so long as IV tPA is not delayed.1 At some institutions, multimodal CT imaging is performed prior to intra-arterial (IA) procedures. CT-based imaging is typically utilized because it requires less time to complete than MR-based imaging, thus minimizing the time to IA procedures in the setting of acute stroke. Multimodal CT imaging including CT with and without contrast, CT angiography (CTA), and perfusion CT (CTP) rapidly identifies the presence or absence of clot suitable for extraction and salvageable ischemic tissue.2,3
At centers providing endovascular therapies for stroke, multimodal CT rapidly identifies candidates for these therapies in lieu of conventional angiography. However, multimodal CT is a costly screening tool where a proportion of subjects will be screened without a subsequent change in clinical management. Thus, the objective of this study was to compare cost-effectiveness of 2 strategies in patients who would be considered for IV tPA and subsequently for IA procedures if IV tPA failed or could not be given: 1) noncontrast CT (NCCT) followed by conventional cerebral angiography to screen candidates for IA procedures vs 2) multimodal CT followed by IA procedures in those with identified intraluminal thrombus. IA procedures include any emergent endovascular thrombolysis, thrombectomy, or combination of treatments.
METHODS
Model description.
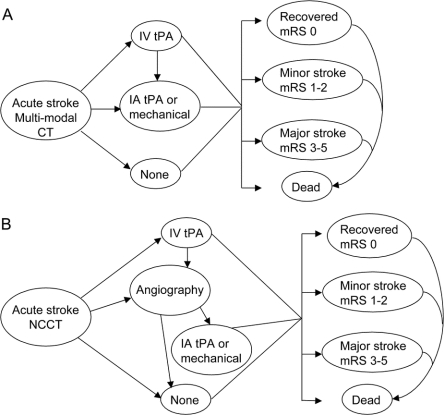
We developed a Markov model to calculate future costs and quality of life over the lifetime of the cohort. Permanent health states, based on the modified Rankin Scale (mRS), were categorized as complete recovery (mRS 0), minor stroke (mRS 1-2), major stroke (mRS 3-5), and death (mRS 6). IA data were primarily grouped as indicated and this stratification simplified analysis.
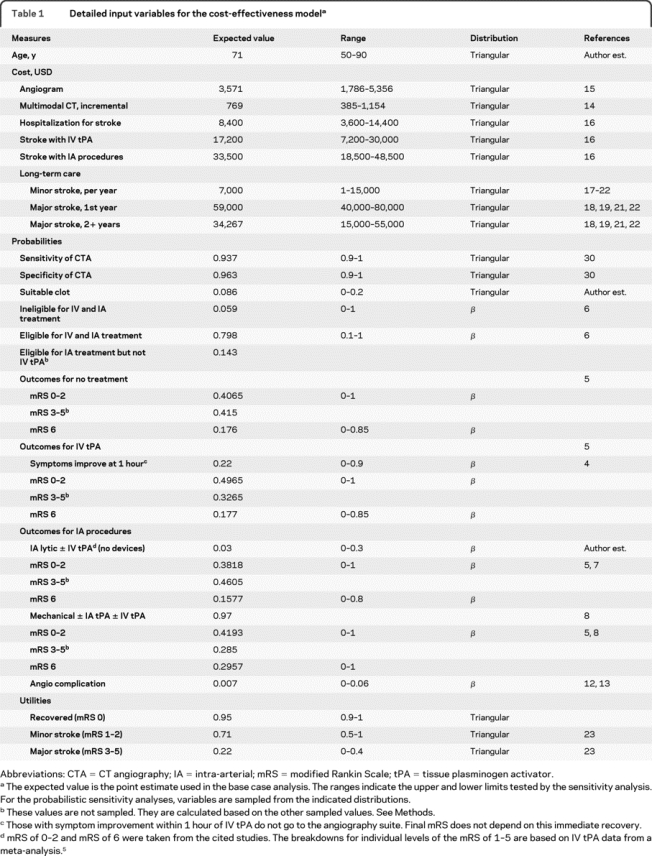
The hypothetical cohort started either with multimodal CT or with NCCT alone. Subjects in either group who received IV tPA and showed appreciable improvement after 1 hour would not undergo IA procedures.4 Outcomes for those showing symptom improvement within the first hour were assumed to follow the established outcomes after IV tPA.5 Patients ineligible for IV tPA or who failed to improve in the first hour after IV tPA could undergo IA procedures if there were no clinical contraindications and intraluminal thrombus was found on multimodal CT scans (multimodal CT branch) or by conventional diagnostic angiography (NCCT branch) (figure 1).4 The costs, probabilities, and utilities of the model are listed in table 1.
Figure 1 Diagram of the procedures and health states used in the Markov model following an initial imaging strategy of multimodal CT (A) or NCCT (B)
IA = intra-arterial; mRS = modified Rankin Scale; NCCT = noncontrast CT; tPA = tissue plasminogen activator.
Table 1 Detailed input variables for the cost-effectiveness model
Target population.
The target population was a hypothetical cohort of stroke patients presenting within 3 hours of symptom onset who would be considered for IV tPA and for IA procedures, when needed. The 3-hour time window was chosen because meta-analysis data for IV tPA outcomes and trials of IA procedures reflect the 3-hour time IV tPA window.
Perspective and time horizons.
The payer perspective (including direct medical costs) was used (2008 USD). No indirect costs were included. Two time horizons were considered. First, a 3-month time horizon was evaluated since outcome data for IV tPA administration and stroke outcomes are well-established at 3 months follow-up. The second time horizon was the lifetime of the cohort; the analysis was continued until the cohort had accumulated in the Markov state for death.
Probability data.
Eligibility for IV tPA and IA procedures were taken from a cohort presenting within 3 hours of symptom onset.6 Six percent of the hypothetical cohort were ineligible for both IV tPA and IA procedures. Almost 80% had no contraindication to either IV tPA or IA procedures. The rest were ineligible for IV tPA but remained eligible for IA procedures. The outcomes after IV tPA and after no intervention were taken from a meta-analysis.5 This meta-analysis also provided the proportion of subjects in each mRS category.
Outcomes after IA thrombolysis alone were assumed to be the same as outcomes after IV tPA plus IA thrombolysis.7 Outcome probabilities after mechanical thrombectomy were calculated from Penumbra POST and Multi-MERCI mechanical thrombectomy with IA thrombolysis data.8,9 Outcomes after mechanical thrombectomy alone were also assumed to be the same as outcomes after mechanical thrombectomy plus IV tPA.
The recent trials involving IA procedures reported outcomes in the following categories: mRS of 0-2 and mRS of 6.7,8,10,11 The remaining population was assumed to represent the likelihood of a mRS of 3-5. The distribution of mRS scores of 0, 1, and 2, as individual scores, were assumed to be distributed as the IV tPA branch for mRS 0-2 from a meta-analysis.5 Finally, the prevalence of thrombus was a weighted estimate from the IMS, IMS2, MERCI, and Multi-MERCI trials, where the range of subjects with thrombus divided by the number screened was 2%-16%.7,8,10,11
Minor stroke and major stroke due to cerebral angiography were also accounted for as adverse events.12,13 Half of the complications from angiography were considered minor strokes, the other half were major strokes.12 Adverse events for contrast reaction, renal failure, and radiation exposure were not included in the model because they would be present in both arms to varying levels.
Sensitivity and specificity of CTA to detect thrombus were included with conventional angiography as the gold standard. A priori, CTP scan data relating to penumbra were not included in the main model. Improvements in functional outcomes due to CTP data were tested in a deterministic (one-way) sensitivity analysis by increasing the relative risk of the probability of a mRS 0-2 after IA procedures.
Cost and utilization data.
All costs were adjusted to 2008 USD using the Consumer Price Index for medical care. As our purpose was to explore potential benefits of multimodal CT, only direct medical costs that would differ between multimodal CT and NCCT were included. For example, the cost of a NCCT was not included because it was initially incurred by both branches. The incremental cost of multimodal CT over NCCT and the cost of angiography plus intervention were estimated from the literature.14,15 Costs for the hospitalization were based on nationwide US estimates of Medicare costs.16 Long-term care costs were estimated from the literature.17–22
Utility data.
Health state preferences were obtained from the literature and represented utilities that describe the mRS scores used in our study.23 Perfect health was anchored at 1 and death was 0. Quality-adjusted life-years (QALYs) were the utility of a health state multiplied by the duration of the health state.
Analysis.
For the analysis over the lifetime of the cohort, the Markov cycle length was 3 months. Three months was chosen as it was a common time frame for reporting outcome measures in recent acute stroke trials. Multimodal CT was considered the intervention, because it was the newer technique. NCCT was the comparator. The incremental cost-effectiveness ratio (ICER) was calculated as the difference in costs divided by the difference in QALYs [($Multimodal CT − $NCCT)/(QALYsMultimodal CT − QALYsNCCT)]. Future costs and utilities were discounted at 3%.24 For the base case analysis, each variable is set to its expected value. Assumptions about each variable were tested individually over prespecified ranges using a deterministic (one-way) sensitivity analysis. Since variables are unlikely to change in isolation, a probabilistic sensitivity analysis simultaneously sampled from distributions of cost, probability, and utility values (n = 1,000 trials).
Cost-effectiveness plane.
The costs and health gains of NCCT were placed at the origin of a cost-effectiveness plane. The incremental costs and incremental QALYs for multimodal CT were plotted relative to NCCT. The quadrants (northeast [NE], southeast [SE], northwest [NW], southwest [SW]) were interpreted as follows: (NE) willingness to pay (WTP) more money for better health, (SE) multimodal CT is the dominant, favored, imaging strategy because of increased QALYs at lower costs, (NW) NCCT was dominant because of reduced costs for increased QALYs, and (SW) willingness to accept (WTA) QALY losses for lower costs.25
RESULTS
Base case analysis: 3-month time horizon.
The model predicted 0.171 QALYs for multimodal CT and 0.167 QALYs for NCCT over a 3-month time horizon. The costs of multimodal CT were $20,165, while the costs of NCCT were $21,881 (table 2). Because multimodal CT had a lower incremental cost (−$1,716) with greater QALYs, it was the dominant imaging strategy (figure 2).
Table 2 Base case analysis for the 3-month and lifetime horizon
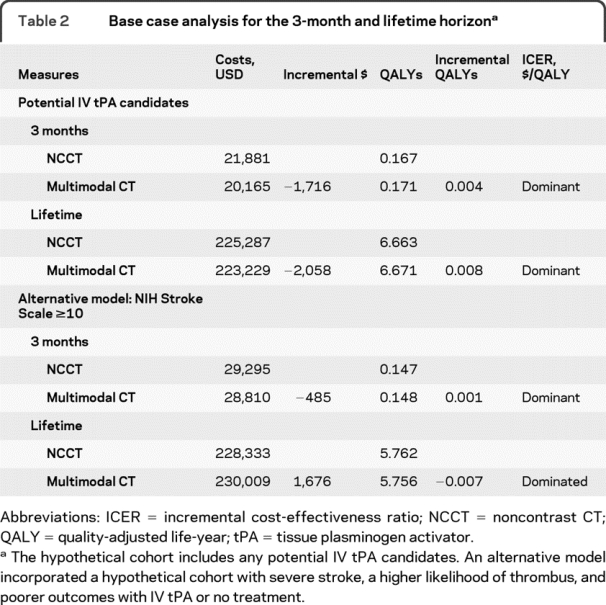
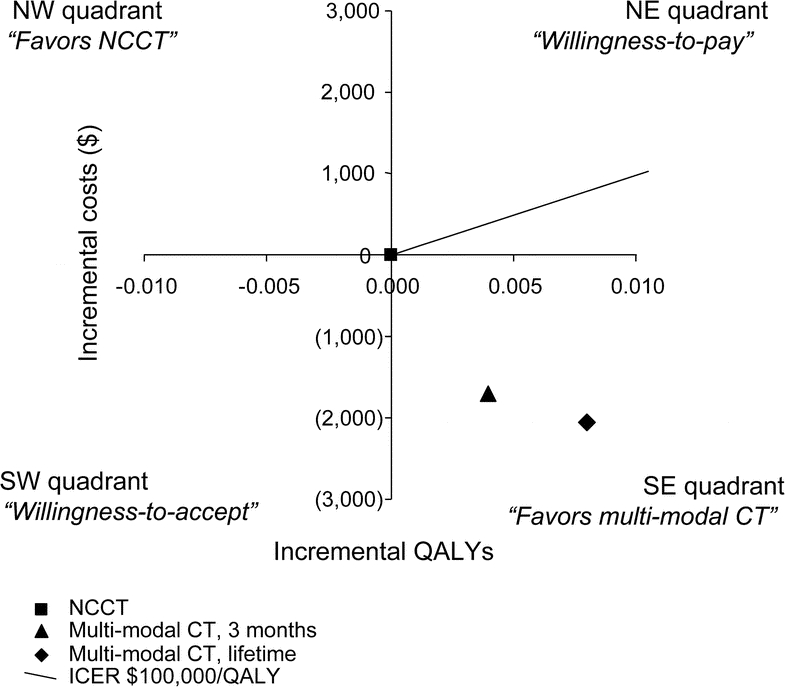
Figure 2 Base case analysis for the 3-month and lifetime horizons
A cost-effectiveness plane is illustrated with quality-adjusted life-years (QALYs) on the x-axis and costs on the y-axis. Incremental costs and QALYS for multimodal CT are graphed with respect to noncontrast CT (NCCT), which is placed at the origin. The cost-effectiveness estimate for multimodal CT is in the southeast (SE) quadrant with lower costs and greater QALYs at 3 months (diamond) and over a lifetime (square). The line representing an incremental cost-effectiveness ratio (ICER) of $100,000/QALY is graphed as a reference. NE = northeast; NW = northwest; SW = southwest.
The number needed to screen with multimodal CT to avoid 1 unnecessary diagnostic cerebral angiogram was 2.
Sensitivity analyses: 3-month time horizon.
A series of deterministic sensitivity analyses were conducted for each variable. None of the individual assumptions about the variables influenced the cost-effectiveness estimate of multimodal CT. It remained the dominant strategy with lower costs and greater QALYs.
We tested the assumption that information from CTP scans could select patients who may have more favorable outcomes. Increasing the relative likelihood of mRS of 0-2 caused minimal QALY gains and cost savings over a 3-month time horizon as multimodal CT remained the dominant imaging strategy.
A probabilistic sensitivity analysis, sampling all variables simultaneously, showed which choice, multimodal CT or NCCT, was beneficial at different economic values for 1 year of optimal health ($/QALY, net monetary benefits). Multimodal CT was the cost-effective option with 99.7% probability for a WTP of $0/QALY and with 100% probability for WTP of $100,000/QALY.
Base case analysis: Lifetime.
Over the lifetime of the cohort, multimodal CT was associated with 6.671 QALYs at a cost of $223,229. NCCT produced 6.663 QALYs at a cost of $225,287 (table 2). The incremental costs were −$2,058 while the incremental QALYs were 0.008. With a longer time horizon, multimodal CT remained the dominant imaging strategy (figure 2).
Sensitivity analyses: Lifetime.
Each variable was tested over a range of values for the deterministic sensitivity analyses. In contrast to the 3-month time horizon, several variables affected the cost-effectiveness estimate over the lifetime of the cohort. Assuming the sensitivity and specificity of CTA for finding intraluminal thrombus was less than conventional angiography, on average, more patients in the NCCT branch with conventional angiography will undergo IA procedures. Thus, increasing favorable outcomes after IA procedures (≥61% likelihood of a mRS 0-2) led to a net gain in QALYs for NCCT. Costs for multimodal CT remained below the costs of NCCT, thereby shifting the cost-effectiveness estimate into the SW quadrant (figure e-1 on the Neurology® Web site at www.neurology.org). Similarly, decreasing the likelihood of mRS 0-2 after IV tPA would lead to fewer QALYs in both branches, though decreasing QALYs in the NCCT branch could be offset by the QALY gain from more IA procedures. When the likelihood of mRS 0-2 after IV tPA was ≤38%, the cost-effectiveness estimate again moved into the SW quadrant (figure e-1). Finally, if the probability of symptom improvement 1 hour after IV tPA was ≥91%, the costs of multimodal CT exceeded the costs of NCCT, moving the point estimate into the NE quadrant (figure e-2).
Assumptions about the quality of life after a stroke influenced the model. When the utility for the recovered state was <0.53, the cost-effectiveness estimate moved into the SW quadrant. However, the ICER for a WTA did not decrease below $3.5 million/QALY. Changing the sensitivity or specificity of CTA did not influence the model. To incorporate the putative benefits of CTP acquired during multimodal imaging in identifying salvageable tissue, a relative risk term increased the likelihood of a mRS of 0-2 following IA procedures. Better outcomes led to additional QALY gains and increased cost savings (figure 3A). Specifically, an increase of 10% in relative risk by incorporating presumed benefits of CTP generated 0.034 incremental QALYs and −$2,562, making multimodal CT an even more favorable choice.
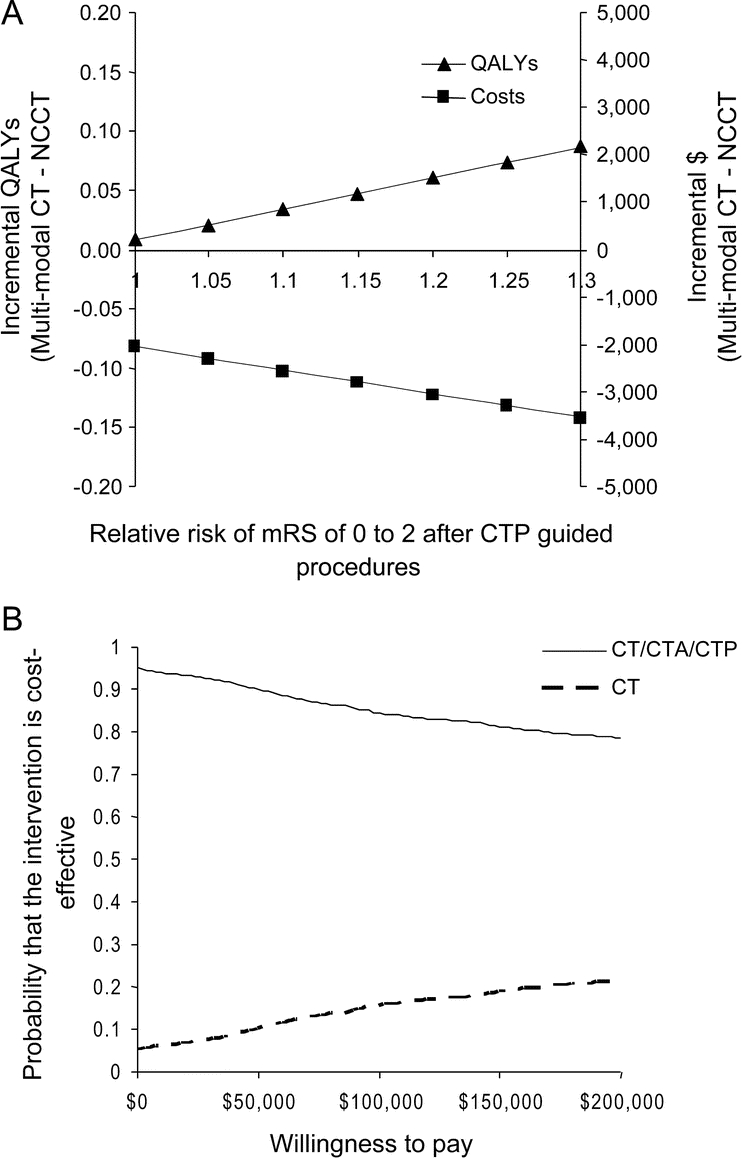
Figure 3 Sensitivity analyses
(A) A deterministic, 1-way sensitivity analysis varied the likelihood of a modified Rankin Scale (mRS) of 0-2 by using perfusion CT (CTP) data in addition to CT angiography (CTA) detection of clot. Incorporating information about the ischemic penumbra to improve outcomes leads to increased incremental quality-adjusted life-years (QALYs) for multimodal CT and increased cost savings. The time horizon is the life of the cohort. (B) A probabilistic sensitivity analysis sampled model variables simultaneously over the lifetime of the cohort. The probability that multimodal CT is the optimal imaging pathway (solid line) or that noncontrast CT (NCCT) is the optimal pathway (dashed line) varies with the willingness to pay ($/QALY, x-axis). Note that the probability of multimodal CT being the optimal choice never falls below 79% out to $200,000/QALY.
The probabilistic sensitivity analysis showed that for a WTP of $0/QALY multimodal CT was the cost-effective option with 95% probability. For a WTP of $50,000/QALY, multimodal CT was the cost-effective option with 90.1% probability. Finally, for a WTP of $100,000/QALY, multimodal CT was the cost-effective option with 84% probability (figure 3B).
An alternative model: Presenting with a severe stroke.
In an alternative model, subjects had NIH Stroke Scale scores ≥10, thus increasing the likelihood of clot (expected value: 78%).7 The outcomes for IV tPA and no treatment were modified to reflect severe stroke.7 For the 3-month time horizon, multimodal CT had reduced costs, −$485, and slightly greater QALYs, 0.001. Over a lifetime, NCCT was the dominant strategy with lower incremental costs, $1,676, and an incremental QALY gain of 0.007. Increasing the likelihood of mRS of 0-2 with CTP data also influenced this cost-effectiveness estimate. An increase of 1% in the probability of favorable outcomes was enough to create an ICER for multimodal CT of $76,005/QALY. Once the likelihood of mRS of 0-2 with CTP data were ≥4.0%, multimodal CT was the dominant strategy (figure e-3).
DISCUSSION
With the increasing availability of multimodal CT and IA procedures, we examined the cost-effectiveness of multimodal CT compared to NCCT with the option for conventional angiography. Multimodal CT for subjects presenting with symptoms of an acute stroke severe enough to warrant consideration of IV tPA had lower costs and greater QALYs than NCCT, making multimodal CT cost-saving over the 3-month poststroke phase and over the lifetime of the cohort.
An alternative model evaluated a cohort with more severe symptoms and a higher prevalence of thrombus. In this alternative model, multimodal CT was cost-effective at 3 months, but NCCT became the dominant strategy over the lifetime of the cohort. With the increased prevalence of thrombus, the detection of thrombus by multimodal CT became an extra screening test in those who ultimately needed conventional angiography combined with IA procedures. Outcomes after IA procedures were based on recent studies which did not incorporate CTP-guided treatment, thus our base case analysis did not include the sensitivity and specificity of perfusion maps to identify viable parenchyma. If CTP maps can improve patient selection for IA procedures and subsequent outcomes, multimodal imaging would be more effective.
We tested the ability of perfusion-guided IA procedures to improve outcomes using a relative risk term in a 1-way sensitivity analysis. Assuming that an improvement in the percentage of mRS of 0-2 reflects greater discriminating power of CTP, then only a small change (4%) in favorable outcomes would make multimodal CT dominant for a cohort with a high prevalence of thrombus. Two small cohort studies reported that 16 of 34 patients (47%) and 12 of 27 patients (44%) had favorable outcomes following CTP-guided procedures.26,27 The probabilities of 44-47% for mRS of 0-2 following CTP-guided procedures compared to 41% from the sample weighted data suggest that a relative improvement of 7%, let alone 4%, is possible.
Our model assumed immediate access to multimodal imaging, that physical capabilities for multimodal CT were already in place, and that no cost was incurred for setup. We excluded sequential NCCT, with or without IV tPA, followed by multimodal CT prior to IA procedures as an alternative imaging strategy. First, it is convenient to proceed with the entire multimodal CT protocol once the person is in the CT scanner rather than arranging a return trip to the CT suite. Second, the angiographic suite can be prepared based on the multimodal CT data while IV tPA is administered. Finally, sequential NCCT with multimodal CT prior to angiography would only reduce costs compared to the multimodal CT branch. QALYs would be equivalent between sequential NCCT and multimodal CT.
Price per QALY boundaries in the NE and SW quadrants may not be the same. That is, the 2008 USD saved for a QALY loss in the SW quadrant may not have the same relative value as 2008 USD spent for QALY gains in the NE quadrant.25,28 Though we presented a threshold of $100,000/QALY as a point of reference, we also presented data over a wide range of ICERs because there is no accepted US economic value for a QALY.24 This presentation style also allows for evaluation of other prices for a QALY (e.g., $40,000/QALY), which vary by payer or by country.
A previous study examined the cost-effectiveness of mechanical thrombectomy.29 Mechanical thrombectomy itself was cost-effective compared to no other IA therapy. Imaging modalities to qualify someone for IA treatment were not clearly outlined nor was a probabilistic sensitivity analysis conducted. Our study differs by also addressing imaging modalities, a necessary component prior to thrombectomy, and by simultaneously sampling multiple variables.
Our model has several limitations. Only 1 acute event was allowed, such that after the initial acute ischemic stroke, no future events, other than death, were allowed. This assumption allowed us to address the cost-effectiveness of multimodal CT as a diagnostic tool for IA procedures in a group of subjects with an immediate life-threatening condition and immediate payoffs. Risks of exacerbating renal disease or a contrast reaction were not included in our model (although both multimodal CT and catheter angiography involve ionizing radiation and contrast administration). The prevalence of clot was a weighted estimate (8.6%) from recent trials (range 1.5%-16.1%).7,8,10,11 This expected value was reasonable because it did not exceed the clot burden reported in Multi-MERCI and was near the midpoint of the prevalence range. Randomized data were not available for outcomes after IA procedures or for multimodal imaging. Our sample weighted outcome estimates after IA procedures may be subject to unknown confounding factors.
Multimodal CT is a cost-effective screening tool for individuals presenting with an acute stroke who would be considered for IV tPA or IA procedures. This assessment of imaging screening strategies with the potential for acute thrombectomy or thrombolysis suggested that multimodal CT would be the imaging modality of choice 90.1% of the time for a WTP of $100,000/QALY over a lifetime. For a cohort with a high prevalence of clot, improving outcomes following multimodal CT guided IA procedures by 4% (e.g., by incorporating CTP data in patient selection) would enhance the cost-effectiveness of multimodal CT. While the hypothesis of improvements with CTP-guided IA procedures cannot be directly tested yet, increasing evidence points to the utility of CTP in determining salvageable penumbra in stroke patients, and such patients may fare better following revascularization.2,3,26,27 Future models should incorporate new data with longer follow-up from such cohorts.
AUTHOR CONTRIBUTIONS
Markov analysis was conducted by Dr. Kate Young.
DISCLOSURE
Dr. Young reports no disclosures. Dr. Benesch has served as a consultant for and received funding for travel from ZZ Biotech; and receives/has received research support from AGA Medical Corporation, CoAxia, Inc., and the NIH (NHLBI 5R01HL080107 [PI], U01NS044876-01A2 [subinvestigator], R01NS38384 [subinvestigator], and R01NS38539-04A1 [Site PI]). Dr. Jahromi has received funding for travel from Cordis Corporation (Johnson & Johnson).
Supplementary Material
Address correspondence and reprint requests to Dr. Kate C. Young, Department of Neurology, University of Rochester, 601 Elmwood Ave., Box 681, Rochester, NY 14642 kate_young@urmc.rochester.edu
Supplemental data at www.neurology.org
e-Pub ahead of print on October 6, 2010, at www.neurology.org.
Study funding: Supported by the University of Rochester Departments of Neurology and Neurosurgery and the NIH (RO1HL080107 to C.G.B.).
Disclosure: Author disclosures are provided at the end of the article.
Received March 17, 2010. Accepted in final form July 19, 2010.
REFERENCES
- 1.Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for imaging of acute ischemic stroke: a scientific statement from the American Heart Association. Stroke 2009;40:3646–3678. [DOI] [PubMed] [Google Scholar]
- 2.Murphy BD, Fox AJ, Lee DH, et al. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke 2006;37:1771–1777. [DOI] [PubMed] [Google Scholar]
- 3.Parsons MW, Pepper EM, Bateman GA, Wang Y, Levi CR. Identification of the penumbra and infarct core on hyperacute noncontrast and perfusion CT. Neurology 2007;68:730–736. [DOI] [PubMed] [Google Scholar]
- 4.Felberg RA, Okon NJ, El-Mitwalli A, Burgin WS, Grotta JC, Alexandrov AV. Early dramatic recovery during intravenous tissue plasminogen activator infusion: clinical pattern and outcome in acute middle cerebral artery stroke. Stroke 2002;33:1301–1307. [DOI] [PubMed] [Google Scholar]
- 5.Hacke W, Donnan G, Fieschi C, et al. Association of outcome with early stroke treatment: pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet 2004;363:768–774. [DOI] [PubMed] [Google Scholar]
- 6.Barber PA, Zhang J, Demchuk AM, Hill MD, Buchan AM. Why are stroke patients excluded from TPA therapy? An analysis of patient eligibility. Neurology 2001;56:1015–1020. [DOI] [PubMed] [Google Scholar]
- 7.The Interventional Management of Stroke (IMS) II Study. Stroke 2007;38:2127–2135. [DOI] [PubMed] [Google Scholar]
- 8.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–1212. [DOI] [PubMed] [Google Scholar]
- 9.Penumbra POST. Presented at the 2009 International Stroke Conference, February 20, 2009, San Diego.
- 10.Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004;35:904–911. [DOI] [PubMed] [Google Scholar]
- 11.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke 2005;36:1432–1438. [DOI] [PubMed] [Google Scholar]
- 12.Heiserman JE, Dean BL, Hodak JA, et al. Neurologic complications of cerebral angiography. AJNR Am J Neuroradiol 1994;15:1401–1407. [PMC free article] [PubMed] [Google Scholar]
- 13.Dawkins AA, Evans AL, Wattam J, et al. Complications of cerebral angiography: a prospective analysis of 2,924 consecutive procedures. Neuroradiol 2007;49:753–759. [DOI] [PubMed] [Google Scholar]
- 14.Gleason S, Furie KL, Lev MH, et al. Potential influence of acute CT on inpatient costs in patients with ischemic stroke. Acad Radiol 2001;8:955–964. [DOI] [PubMed] [Google Scholar]
- 15.Brown DL, Hoffman SN, Jacobs TL, Gruis KL, Johnson SL, Chernew ME. CT angiography is cost-effective for confirmation of internal carotid artery occlusions. J Neuroimaging 2008;18:355–359. [DOI] [PubMed] [Google Scholar]
- 16.HCUP Nationwide inpatient sample (NIS). Healthcare Cost and Utilization Project (HCUP). In: Agency for Healthcare Research and Quality, 2006-2007. Available at: http://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed January 9, 2010. [PubMed]
- 17.Oster G, Huse DM, Lacey MJ, Epstein AM. Cost-effectiveness of ticlopidine in preventing stroke in high-risk patients. Stroke 1994;25:1149–1156. [DOI] [PubMed] [Google Scholar]
- 18.Kuntz KM, Kent KC. Is carotid endarterectomy cost-effective? An analysis of symptomatic and asymptomatic patients. Circulation 1996;94:II194–II198. [PubMed] [Google Scholar]
- 19.Cronenwett JL, Birkmeyer JD, Nackman GB, et al. Cost-effectiveness of carotid endarterectomy in asymptomatic patients. J Vasc Surg 1997;25:298–309. [DOI] [PubMed] [Google Scholar]
- 20.Yin D, Carpenter JP. Cost-effectiveness of screening for asymptomatic carotid stenosis. J Vasc Surg 1998;27:245–255. [DOI] [PubMed] [Google Scholar]
- 21.Post PN, Kievit J, van Baalen JM, van den Hout WB, van Bockel JH. Routine duplex surveillance does not improve the outcome after carotid endarterectomy: a decision and cost utility analysis. Stroke 2002;33:749–755. [DOI] [PubMed] [Google Scholar]
- 22.Kilaru S, Korn P, Kasirajan K, et al. Is carotid angioplasty and stenting more cost effective than carotid endarterectomy? J Vasc Surg 2003;37:331–339. [DOI] [PubMed] [Google Scholar]
- 23.Post PN, Stiggelbout AM, Wakker PP. The utility of health states after stroke: a systematic review of the literature. Stroke 2001;32:1425–1429. [DOI] [PubMed] [Google Scholar]
- 24.Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 25.Klok RM, Postma MJ. Four quadrants of the cost-effectiveness plane: some considerations on the south-west quadrant. Expert Rev Pharmacoecon Outcomes Res 2004;4:599–601. [DOI] [PubMed] [Google Scholar]
- 26.Knoepfli AS, Sekoranja L, Bonvin C, et al. Evaluation of perfusion CT and TIBI grade in acute stroke for predicting thrombolysis benefit and clinical outcome. J Neuroradiol 2009;36:131–137. [DOI] [PubMed] [Google Scholar]
- 27.Gasparotti R, Grassi M, Mardighian D, et al. Perfusion CT in patients with acute ischemic stroke treated with intra-arterial thrombolysis: predictive value of infarct core size on clinical outcome. AJNR Am J Neuroradiol 2009;30:722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Brien BJ, Gertsen K, Willan AR, Faulkner LA. Is there a kink in consumers' threshold value for cost-effectiveness in health care? Health Econ 2002;11:175–180. [DOI] [PubMed] [Google Scholar]
- 29.Patil CG, Long EF, Lansberg MG. Cost-effectiveness analysis of mechanical thrombectomy in acute ischemic stroke. J Neurosurg 2009;110:508–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lev MH, Farkas J, Rodriguez VR, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr 2001;25:520–528. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.