Abstract
Elevated urinary albumin excretion in patients with type 1 diabetes reverts to normoalbuminuria in a majority of patients but advances toward proteinuria in some. In order to gain valuable insights into the early pathophysiology of diabetic nephropathy we evaluated the association of kidney tubular injury biomarkers with changes in albuminuria in patients with type 1 diabetes mellitus. Urine levels of kidney injury molecule-1 (KIM-1), N-acetyl-β-D-glucosaminidase (NAG), and some inflammatory markers were determined in 38 healthy individuals and 659 patients with type 1 diabetes mellitus having varying degrees of albuminuria. Urinary interleukin-6, CXCL10/IP-10, NAG, and KIM-1 levels were very low in healthy individuals, increased in type 1 patients with normoalbuminuria, and were highest in diabetic patients that had microalbuminuria. Low baseline concentrations of urinary KIM-1 and NAG both individually and collectively were significantly associated with the regression of microalbuminuria over the subsequent 2 years; an effect independent of clinical characteristics. Progression and regression of microalbuminuria were unrelated to urinary levels of interleukins 6 and 8, CXCL10/IP-10, and monocyte chemoattractant protein-1. Thus our results show that lower urinary KIM-1 and NAG levels were associated with the regression of microalbuminuria in type 1 diabetes mellitus. Hence, tubular dysfunction is a critical component of the early course of diabetic nephropathy.
Keywords: chronic kidney disease, diabetic nephropathy, renal proximal tubule cell, tubular epithelium
Diabetic nephropathy (DN) is the most common cause of chronic kidney failure and end-stage kidney disease in the world. An estimated 20.8 million people (7% of the population) in the United States have diabetes, and nearly 180,000 people are living with kidney failure as a result of diabetes.1
The earliest known noninvasive biomarker for diagnosis of DN is microalbuminuria (MA).2 MA in type 1 diabetic patients is often considered the first inexorable step toward progression to macroalbuminuria, overt proteinuria, and end-stage renal failure.2–4 Recent prospective studies, however, have shown that the elevated urinary albumin excretion in patients with type 1 diabetes regresses to normoalbuminuria in a majority and advances toward proteinuria in only a minority of patients.5 Identifying sensitive biomarkers that can predict the course of MA will facilitate early diagnosis and prognosis and guide interventional strategies. Furthermore, biomarkers that are informative may provide valuable insights into the early pathophysiology of DN.
Progressive glomerular dysfunction is traditionally thought to be the primary mechanism for increase in urine protein excretion; however, tubulointerstitial disease may have an important role in pathogenesis and progression of DN.6–9 Although it has been proposed that renal proximal tubule injury and dysfunction could be important in the early increases in urine albumin excretion, this issue has not been adequately investigated due to lack of sensitive tests of proximal tubule injury in humans.10,11 It is also important to recognize that albuminuria is a marker of tubular injury in addition to increases in glomerular permeability. In fact, the US Food and Drug Administration and the European Medicines Agency have qualified urinary albumin as a biomarker of tubular injury with nephrotoxins.12
Because tubular injury markers can provide important insight into the status of the proximal tubule in humans and because it is known that an inflammatory component is involved in the DN in type 1 diabetes mellitus (DM),9,13,14 the primary objective of this study was to evaluate the behavior of sensitive biomarkers of tubular injury and inflammation relative to albumin excretion early in the course of type 1 diabetes.
Kidney injury molecule-1 (KIM-1) is a type 1 cell membrane glycoprotein, which contains, in its extracellular portion, immunoglobulin- and mucin-like domains, with N-and O-glycosylation sites. KIM-1 expression is undetectable in normal kidneys but the mRNA and protein are markedly upregulated with acute kidney injury.15–18 KIM-1 is expressed on the apical membrane of proximal tubule cells and its ectodomain is cleaved and released into the lumen of the tubule ultimately appearing in the urine where it is stable. KIM-1 is a highly sensitive and specific biomarker for proximal tubule injury because of a wide variety of pathophysiological states and toxins in animals and humans.19–25 N-acetyl-β-D-glucosaminidase (NAG) is a 140 kDa proximal tubular brush border lysosomal enzyme, which is released into the urine after renal proximal tubule injury.23,26
Specifically the goals of this study were to compare and evaluate an association between abnormalities in urinary albumin excretion and urinary concentrations of tubular injury markers, KIM-1 and NAG, and tubular inflammatory markers interleukin-6 (IL-6), interleukin-8 (IL-8), CXCL10 (formerly referred to as IP-10), and monocyte chemoattractant protein-1 (MCP-1) using data on longitudinal changes in urinary albumin excretion that were obtained in the large Second Joslin Study on Natural History of Microalbuminuria in type 1 diabetes.
RESULTS
Cross-sectional study
The study groups consisted of 363 patients with type 1 diabetes and normoalbuminuria (DM-NA group), 296 patients with type 1 diabetes and microalbuminuria (DM-MA group), and 38 healthy nondiabetic individuals (controls). Clinical characteristics, baseline concentration of urinary chemokines, and markers of tubular injury in the study groups are shown in Table 1. Patients in the DM-MA group were more likely to be male, slightly older, with longer diabetes duration, higher serum hemoglobin A1c (HbA1c) and, by design, higher albumin-to-creatinine ratio (ACR) compared with patients in the DM-NA group. Individuals in the control group were slightly older, and by design had low HbA1c and low ACR in comparison with the DM-NA group.
Table 1.
Clinical characteristics and concentrations at baseline of urinary chemokines and urinary markers of tubular injury according to the study group
| Clinical characteristics | Controls (n=38) | Patients with type 1 diabetes |
P-value, controls vs DM-NA | P-value, DM-MA vs DM-NA | |
|---|---|---|---|---|---|
| Normoalbuminuria (DM-NA) (n=363) | Microalbuminuria (DM-MA) (n=296) | ||||
| Age (years) | 43±10 | 39±12 | 41±12 | 0.025 | 0.009 |
| Gender (% male) | 50 | 44 | 61 | NS | <0.0001 |
| Duration (years) | NA | 20+9 | 23+10 | NA | <0.0001 |
| HbA1c (g%) | 5.3±0.4 | 8.3±1.2 | 8.6±1.5 | <0.0001 | 0.006 |
| AER (μg/min) | 10 (7–15) | 14 (10–19) | 67 (41–124) | <0.0001 | Different by design |
| Urinary chemokines | |||||
| IL-6 (ng/g cr) | 0.20 (0.00–0.55) | 0.48 (0.21–1.45) | 0.63 (0.27–1.69) | 0.0002 | 0.007 |
| IL-8 (ng/g cr) | 0.3 (0.0–7.1) | 1.8 (0.3–8.5) | 2.1 (0.5–12.5) | 0.014 | 0.19 |
| IP-10 (ng/g cr) | 2.3 (1.3–9.3) | 7 (2–29) | 21 (8–55) | 0.010 | <0.0001 |
| MCP-1 (ng/g cr) | 38 (10–71) | 42 (19–75) | 59 (33–92) | 0.24 | <0.0001 |
| Urinary markers of tubular injury | |||||
| NAG (U/g cr) | 0.6 (0.1–1.2) | 2.0 (1.2–3.5) | 3.4 (2.0–5.7) | <0.0001 | <0.0001 |
| KIM-1 (ng/g cr) | 21 (2–46) | 29 (13–73) | 60 (29–133) | 0.008 | <0.0001 |
Abbreviations: AER, albumin excretion rate; DM-MA, type 1 diabetes and microalbuminuria; DM-NA, type 1 diabetes and normoalbuminuria; KIM-1, kidney injury molecule-1; MCP-1, monocyte chemoattractant protein-1; NAG, N-acetyl-β-D-glucosaminidase; NS, not significant.
Clinical characteristics are presented as mean±standard deviation except for AER: median (25–75th percentile). Urinary markers with creatinine adjustment presented as median (IQR). Urinary markers and AER log-transformed for the purpose of the statistical analysis. t-Test and χ2-test with continuity correction were used for the comparison between appropriate groups.
Urinary concentrations of the chemokines, IL-6 and IP-10, and markers of tubular injury, NAG and KIM-1, were significantly different among the three groups. Concentrations were the lowest in the control group, increased in the DM-NA group, and were highest in the DM-MA group. The differences between the controls and DM-NA group and between the DM-NA and DM-MA groups were highly significant for both KIM-1 and NAG. Among urinary chemokines, the differences reached the level of statistical significance only for IL-6, IP-10, and MCP-1 when the DM-MA group was compared with the DM-NA group.
Follow-up study of patients with MA
A total of 296 patients in the DM-MA group were followed for a 2-year interval. MA regressed in 71 patients (24%), remained stable in 188 patients (63.5%), and progressed in 37 individuals (12.5%). The median values of albumin excretion rate (AER) at the end of the 2-year follow-up were as follows: 22 (15–35) μg/min for the MA regression group, 72 (44–130) μg/min for MA stable group, and 324 (195–612) μg/min for the MA progression group.
The characteristics of the patients according to changes in AER during the follow-up are presented in Table 2. Subjects did not differ in regards to age, diabetes duration, glomerular filtration rate, and blood pressure (BP) measures. Patients with MA regression in comparison with MA progression group had lower AER and HbA1c values and were slightly more frequently treated with ACE inhibitors, but did not differ by the frequency of the other antihypertensive treatment.
Table 2.
Baseline clinical characteristics according to changes in microalbuminuria during 2 years of follow-up
| Clinical characteristics at baseline | Changes in microalbuminuria during 2-year follow-up |
P-value |
||||
|---|---|---|---|---|---|---|
| MA Regression (n=71) | MA Stable (n=188) | MA Progression (n=37) | Regression vs stable | Progression vs stable | Progression vs regression | |
| Age (years) | 40+12 | 42+11 | 38+14 | NS | 0.07 | NS |
| Gender (% male) | 37 (52) | 126 (67) | 17 (46) | 0.03 | 0.03 | NS |
| Duration (years) | 24+9 | 23+10 | 21+9 | NS | NS | NS |
| AER (μg/min) | 65 (46–108) | 66 (44–134) | 108 (49–150) | NS | 0.06 | 0.024 |
| CcGFR (ml/min per 1.73m2) | 101+24 | 98+27 | 96+32 | NS | NS | NS |
| Systolic BP (mm Hg) | 124+13 | 125+14 | 123+13 | NS | NS | NS |
| Diastolic BP (mm Hg) | 74+8 | 73+8 | 73+7 | NS | NS | NS |
| HbA1c (g%) | 8.3+1.5 | 8.6+1.4 | 9.0+1.9 | NS | NS | 0.03 |
| Cholesterol (mg/dl) | 194+29 | 188+30 | 200+33 | NS | 0.03 | NS |
| ACEi Tx, n (%) | 70 | 62 | 43 | NS | 0.05 | 0.011 |
| Anti-Hyp Tx, n (%) | 30 | 20 | 22 | NS | NS | NS |
| Current smokers, n (%) | 14 | 21 | 14 | NS | NS | NS |
Abbreviations: AER, albumin excretion rate; BP, blood pressure; GFR, glomerular filtration rate; MA, microalbuminuria; NS, not significant.
MA regression, defined as 50% decrease in AER (averaged value from 2-year follow-up interval) in reference to 2-year interval preceding baseline measurements.
MA progression, defined as 100% increase in AER.
Continuous variables reported as mean±s.d. except for AER where median (IQR) was used.
AER, median albumin excretion rate of all AER determination carried out during the 2-year interval preceding the baseline examination.
CcGFR, estimated GFR based on serum cystatin C concentration according to Macisaac et al.39 formula.
HbA1c, mean value of multiple measurements of HbA1c carried out during the 2-year interval preceding the baseline examination.
AER was log10 transformed for the purpose of the statistical analysis.
P-value represents significance of analysis of variance for unbalanced design for all variables except for categorical covariates where χ2-test with continuity correction was applied.
Urinary markers and regression and progression of MA
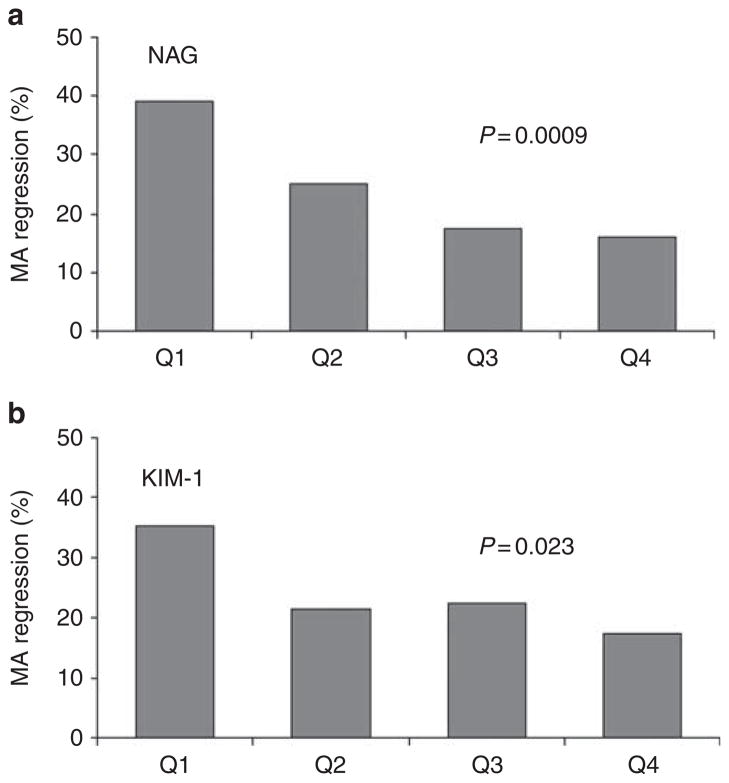
Table 3 shows the median (25th, 75th percentiles) concentrations of baseline and follow-up urinary markers normalized to urinary creatinine concentration as they relate to AER changes over the 2-year follow-up in the DM-MA group. Patients in each of the groups: MA regression, MA stable and MA progression, did not differ with regard to baseline urinary concentrations of IL-6, MCP-1, or IP10. IL-8 concentrations differ among the groups, but the effect did not parallel the increase in AER in a linear pattern. Patients in the MA regression group had significantly lower urinary concentrations of tubular markers, NAG and KIM-1, than subjects in the MA stable group, and also NAG had a tendency to lower values in comparison of MA regression vs progression. Figure 1a and b shows the percent of subjects in which MA regressed during 2-year follow-up in the DM-MA study group according to quartiles of urinary concentrations of NAG and KIM-1 at baseline. MA regression was frequent in patients with low levels of NAG or KIM-1 at baseline. For the MA regression, the effect of NAG was proportional across quartiles whereas effect of KIM-1 was the most pronounced for Q1. The frequency of MA regression declined with increased baseline urinary levels of NAG (test for trend P =0.0009). A total of 39% of patients with urinary NAG at baseline in quartile 1 (NAG Q1 <2.0 U/g cr) experienced MA regression, whereas albuminuria decreased in only 16% of patients if baseline urinary NAG levels were in quartile 4 (NAG Q4>5.71 U/g cr). Similarly, 36% subjects with low urinary KIM-1 (Q1 <29 ng/g cr) had regression of MA, in comparison with only 18% of subjects with high KIM-1 (Q4>132 ng/g cr) in which MA regressed. The odds of MA regression were 4.8 (95% CI 1.9–12.7) and 2.6 (95% CI 1.1–6.1) for NAG Q1 or KIM-1Q1 in comparison with high levels (Q4) of the markers (Table 4). The effect of NAG and KIM-1 on regression of MA was independent from other clinical characteristics, as the odds ratio hardly changed after adjustment for such characteristics as age, gender, AER, hemoglobin A1c, systolic BP, renoprotective treatment, and serum cholesterol levels (Table 4). Crude and adjusted analysis of the associations of NAG and KIM-1 with MA course, performed without creatinine adjustment, yielded comparable results.
Table 3.
Concentration of urinary markers at baseline according to changes in microalbuminuria during 2 years of follow-up
| Biomarkers | Changes in microalbuminuria during 2-year follow-up |
P-value |
||||
|---|---|---|---|---|---|---|
| MA Regression (n=71) | MA Stable (n=188) | MA Progression (n=37) | Regression vs stable | Progression vs stable | Progression vs regression | |
| Urinary chemokines | ||||||
| IL-6 (ng/g cr) | 0.7 (0.2–1.7) | 0.6 (0.3–1.6) | 0.6 (0.3–1.9) | NS | NS | NS |
| IL-8 (ng/g cr) | 2.2 (0.9–19.2) | 1.9 (0.5–7.2) | 6.5 (0.9–63.4) | 0.04 | 0.007 | NS |
| IP10 (ng/g cr) | 18 (7–45) | 23 (8–52) | 33 (14–65) | NS | NS | NS |
| MCP-1 (ng/g cr) | 60 (30–88) | 58 (33–95) | 62 (47–92) | NS | NS | NS |
| Urinary markers of tubular injury | ||||||
| NAG (U/g cr) | 2.8 (1.7–4.1) | 3.8 (2.2–6.1) | 3.4 (2.2–5.7) | 0.005 | NS | 0.06 |
| KIM-1 (ng/g cr) | 44 (18–109) | 61 (34–136) | 77 (23–186) | 0.026 | NS | NS |
Abbreviations: KIM-1, kidney injury molecule-1; MA, microalbuminuria; MCP-1, monocyte chemoattractant protein-1; NAG, N-acetyl-β-D-glucosaminidase; NS, not significant. Median (25th, 75th percentile) levels of urinary markers adjusted for creatinine are presented in the table.
They were log10 transformed for the purpose of the analysis. P value represents analysis of variance for unbalanced design.
Figure 1. Frequency of MA regression during the 2-year follow-up as a function of creatinine-normalized urinary concentrations of markers of tubular injury.
In (a and b) the proportion of subjects who regressed is plotted as a function of baseline quartiles of NAG and KIM-1. MA: microalbuminuria; Q1, Q2, Q3, Q4: quartiles 1–4, respectively. Quartile ranges: NAG, Q1 <2.0, Q2 2.0–3.4, Q3 3.4–5.6, Q4>5.7 (U/g cr); KIM-1, Q1 <29, Q2 29–60, Q3 60–132, Q4>132 (ng/g cr). P-values for a test of trend are presented.
Table 4.
Effect of the salutary (low) levels of NAG and KIM-1 at the baseline on MA regression
| Biomarkers | Crude model |
Adjusted model |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| NAG | ||||
| Q1 vs Q4 | 3.4 (1.5–7.6) | 0.003 | 4.8 (1.9–12.7) | 0.0013 |
| Q2 vs Q4 | 1.8 (0.7–4.1) | 0.20 | 2.3 (0.9–6.3) | 0.089 |
| Q3 vs Q4 | 1.1 (0.5–2.7) | 0.81 | 1.5 (0.5–4.2) | 0.45 |
| Q4 REF | 1.0 REF | 1.0 REF | ||
| KIM-1 | ||||
| Q1 vs Q4 | 2.6 (1.2–5.8) | 0.019 | 2.6 (1.1–6.1) | 0.03 |
| Q2 vs Q4 | 1.3 (0.6–3.0) | 0.55 | 1.0 (0.4–2.6) | 0.95 |
| Q3 vs Q4 | 1.4 (0.6–3.2) | 0.47 | 1.3 (0.5–3.4) | 0.52 |
| Q4 REF | 1.0 REF | 1.0 REF | ||
Quartiles (Q1, Q2, Q3, Q4) based on the creatinine-adjusted values. Quartile ranges: NAG, Q1<2.0, Q2 2.0–3.4, Q3 3.4–5.6, Q4>5.7 (U/g cr); KIM-1, Q1 <29, Q2 29–60, Q3 60–132, Q4>132 (ng/g cr). P-value for the crude and adjusted logistic regression models. Adjusted model controlled for age, gender, AER, hemoglobin A1c, systolic BP, renoprotective treatment, and serum cholesterol levels.
NAG and KIM-1 concentrations were markedly correlated (Spearman’s rank coefficient r=0.55, P<0.0001); nevertheless the effect of NAG Q1 and of KIM-1 Q1 remained significant when they were both entered in the adjusted model, resulting with OR: NAG Q1 vs Q2–Q4 2.8 (1.4–5.6) and KIM-1 Q1 vs Q2–Q4 2.1 (1.1–4.0), respectively.
DISCUSSION
Although MA has been proposed to be an early predictive biomarker of DN, it is clear that in the majority of patients MA can regress to normoalbuminuria and in a minority it progresses to proteinuria.5 Although MA has generally been attributed to glomerular injury, nephrotoxicity studies in animals reveal that albuminuria is a sensitive marker of early tubular toxicity.12 It is therefore possible that the early MA observed in many patients with type 1 diabetes may be partially due to tubular injury resulting from hyperglycemia and other metabolic factors. The degree of tubular injury may be associated with more favorable albuminuria outcome. We evaluated biomarkers that specifically measure tubular cell injury with the hypothesis that this injury may have a role in the development and progression/regression of MA in type 1 diabetes.
Using the clinical and albuminuria data from the Second Joslin Study, we found, in a cross-sectional comparison, that urinary levels of tubular injury biomarkers KIM-1 and NAG were significantly elevated in patients with type 1 diabetes and MA in comparison with diabetics with normoalbuminuria and nondiabetic healthy controls. Low urinary KIM-1 and NAG at baseline were strongly associated with regression of MA during a 2-year follow-up and the effect was independent of clinical characteristics.
To the best of our knowledge this is the first prospective study showing the association between urinary excretion of very specific tubular injury biomarkers and time-dependent changes in MA in patients with type 1 diabetes. Our findings provide strong evidence that tubular injury is an important component of the natural history of MA in type 1 diabetes. Less injury to the proximal tubule, as reflected by lower levels of urinary KIM-1 and NAG, is associated with regression of MA independently from glycemic control, or BP, or treatment with ACE inhibitors. There has been a great deal of focus on the role of proteinuria and progression of tubulointerstitial damage,27 and it is well appreciated that tubulointerstitial scarring is the best predictor of renal outcome in diabetic and nondiabetic renal disease. Tubulointerstitial disease has been proposed to be secondary to both enhanced protein uptake by proximal tubule cells with lysosomal rupture resulting in direct tubule toxicity as well as cytokines and chemokines generated by the proximal tubule after albumin uptake, which enhance the inflammatory response and activate fibrotic processes in the interstitial compartment.28 It is possible, however, that proximal tubule injury is primary rather than secondary in the development of MA. In an attempt to identify early urinary biomarkers of nephrotoxicity the Predictive Safety Testing Consortium tested many proximal tubule toxins in rats and found that MA was a excellent biomarker for tubular injury.12 The fact that cultured proximal29 and interstitial30 cells respond directly to high levels of glucose with production of profibrotic mediators also supports the concept that tubulointerstitial disease may be primary rather than secondary in DM. Although there is some controversy over how much protein is filtered in a normal kidney,31 it is generally agreed upon that there is some normally filtered albumin that is reabsorbed by the proximal tubule. If this reabsorption is impaired, we would expect to see MA; therefore, the earliest kidney lesion in type 1 DM may be tubular injury, not glomerular injury.
We propose an important role for urinary NAG and KIM-1 for the diagnosis and monitoring of the course of renal disease in DM. As a lysosomal enzyme, urinary NAG elevation would be expected to be enhanced with proximal tubule injury although it may also be increased because of enhanced lysosomal activity without injury, per se.23 NAG has been extensively studied in both the adult and pediatric populations and has proven to be a sensitive, persistent, and robust indicator of proximal tubule injury.23 Many studies have established that the KIM-1 ectodomain serves as a very reliable biomarker of kidney injury both in rodents and in humans in which the data suggest it is not only a sensitive indicator of injury but can be a predictive biomarker of outcome.6,18,23,32 We have reported recently an algorithm using four biomarkers, including KIM-1 and NAG, to identify optimally acute kidney injury in hospitalized patients.23
IL-6, IL-8, IP-10, and MCP-1 have been previously implicated in the development of DN, renal function decline, and chronic inflammation.9,33 Although elevated levels of markers in urine indicative of low-level inflammation were specific for early progressive renal function decline, none of them was associated with MA in a previous study.9 Consistent with these prior results the present study shows that patients with MA had significantly higher levels of IL-6, IP-10, and MCP-1 levels when compared with patients with normoalbuminuria. In no case, however, were changes in one of these urinary cytokine levels associated with progression or regression of MA.
One of the limitations of this study is the small number of patients who had MA progression, thereby preventing adequate evaluation of biomarkers associated with MA progression. In addition, the follow-up period was only 2 years and hence too short to adequately determine the potential of urinary KIM-1 and NAG to predict permanent regression or progression of MA. Because this study was focused on the relationship between proximal tubule injury and MA early in type 1 DM, it does not establish the relationship of urinary tubular injury biomarkers with changes in glomerular filtration rate over time. It is possible that the findings in patients with advanced proteinuria may be different, and findings in type 1 DM may not be generalized to patients with type 2 DM and MA. Nevertheless this study provides important new insight into the importance of proximal tubule injury with associated MA early in the course of type 1 DM and identifies proximal tubule injury biomarkers that, when at low levels are associated with reduction in albuminuria over a 2-year follow-up period. It is possible, for example, that MA in the absence of significant tubule injury may inflict a functional state that is more readily reversible. This may relate to reversible changes in glomerular hemodynamics, for example.
In summary, the results of our study show that low urinary levels of KIM-1 and NAG are associated with regression of MA in type 1 diabetes and suggest that tubular dysfunction is a critical component of the early course of DN.
METHODS
Study populations
Nondiabetic subjects
For comparison of diabetic patients with nondiabetic subjects, we recruited a control group that consisted of 38 healthy individuals without any history of diabetes, kidney disease, hypertension, or other serious comorbidities and comparable in age to the type 1 DM group. These individuals were recruited and examined during a family study on the genetics of type 2 diabetes carried out at the Joslin Clinic, Boston, MA.
Type 1 diabetic subjects
The study groups with diabetes consisted of patients without overt proteinuria who were enrolled into the Second Joslin Study on the Natural History of Microalbuminuria (Second Joslin Kidney Study). Detailed descriptions of the Joslin Clinic population and the recruitment protocol for Second Joslin Kidney Study have been published previously.34 Briefly, between 1 January 2003 and 31 December 2004 patients with type 1 DM cared for at the Joslin Clinic were recruited into the Second Joslin Kidney Study. Eligibility criteria included residence in New England, diabetes diagnosed before age 40 years, treatment with insulin, current age 18–64 years, diabetes duration 3–39 years, and at least two measurements of HbA1c and urinary ACR during a baseline 2-year interval. Exclusion criteria included advanced DN (proteinuria or end-stage renal disease), or other serious comorbidities including nondiabetic kidney disease, HIV, or HCV infection, or extreme obesity (body mass index >40 kg/m2).
Enrollment and examination
Trained recruiters administered a structured interview and brief examination to eligible patients at a routine visit to the clinic. This is termed ‘the entry examination.’ The interview solicited the history of diabetes and its treatment, other health problems, use of medications, and self-reports of height and weight. Current and past use of medications (particularly ACE inhibitors, angiotensin II receptor blockers, and other antihypertensive drugs) was recorded. The recruiter measured seated BP twice (in the 5-min interval) using an automatic monitor (Omron Healthcare, Vernon Hills, IL), and obtained samples of blood and urine. Details of the assays used were described previously.35,36 For characterizing patients’ baseline exposures, repeated measures in the 2 years up to and including entry examination were summarized by their mean values (HbA1c, cholesterol).
Assessment of urinary albumin excretion
Albumin-to-creatinine ratio was measured in multiple random urine samples in the ‘baseline’ 2 years up to and including entry examination (at least three measurements). Subsequently, to determine baseline albuminuria status, ACR measurements were converted to AER according to a formula published previously.37 Individuals with the median of all available AER measurements in the range 30–300 μg/min were classified as DM-MA group and those with albumin excretion lower than 30 μg/min were regarded as the DM-NA (normoalbuminuria) group. Patients with AER ≥300 μg/min were considered as having proteinuria and were excluded from the study.
AER follow-up study
Individuals with type 1 diabetes and MA (DM-MA group) at baseline (n =296) were followed for the next 2 consecutive follow-up years. Albumin excretion was determined within this follow-up interval based on a median of 3 (range 2–6) measurements of ACR and converted to AER using the same formula as for baseline measurements. Regression of MA (MA regression group) was defined as at least a 50% reduction in albumin excretion from baseline. Progression of MA (MA progression group) was defined as increase in AER by 100% or more over baseline. MA was regarded to be stable over time (stable MA group) if values of AER decreased by <50% or increased <100%. These definitions have previously been implemented.5
Urine collection for biomarkers
Urine samples were collected at the baseline examination at a single time point. All urine specimens for the measurements of the markers were handled in the same way. Patient specimens were collected into sterile cups (Vacutainer Urine Collection Cup; Cardinal Health, Dublin, OH) during the daytime and then aliquotted into 1.5 ml volumes into sterile, nontoxic, nonpyrogenic cryogenic tubes (CryoTubes CryoLine System; NUNC Serving Life Science, Rochester, NY), and frozen at −80 °C until further analysis.
Measurement of urinary biomarkers
Urine samples were thawed, vortexed and centrifuged, and biomarker measurements were performed on the supernatant. Urinary NAG was measured spectrophotometrically according to the manufacturers’ protocols (Roche Diagnostics). Urinary KIM-1, IL-8, IP-10, and MCP-1 measurements were performed using microsphere-based Luminex xMAP technology as described previously.9,23 This is a multiplex particle-enhanced, sandwich type, liquid-phase immunoassay with laser-based detection system based on flow cytometry.38 The assay to measure KIM-1 was developed and evaluated in our laboratory23 whereas Beadlyte Human Multi-Cytokine Detection (48-011) (Upstate-Millipore, Billerica, MA) with protocol B was used to measure IL-8, IP-10, and MCP-1. Urine samples (25 μl for IL-8, IP-10, and MCP-1 or 30 μl for KIM-1) were analyzed in duplicate and respective analytes were quantitated using 8-point (or 13-point for KIM-1 assay) five parametric logarithmic standard curves. The inter- and intraassay variability was <20% for all assays. Urinary IL-6 was measured by enzyme-linked immunosorbent assay (high sensitive immunoassay: HS600B; R&D, Minneapolis, MN) according to the manufacturer’s protocol.9 The urinary levels of the respective antigens were expressed in absolute terms and also normalized to the urinary creatinine concentration. Investigators who were unaware of the patients’ clinical characteristics performed all of the urinary analyte measurements. All the urine specimens for the measurements of the markers had the same number of freeze–thaw cycles (two freeze–thaw cycles).
Statistical analysis
Continuous clinical characteristics were presented as mean± standard deviation, and the categorical ones as proportion. Urinary markers normalized to creatinine were presented as median (25th, 75th percentiles). Statistical analysis was performed on creatinine-normalized and the log-transformed urinary markers. t-Test and χ2-test were used for comparisons between the study groups. In the follow-up analysis, we categorized the investigated markers into quartiles to evaluate relationships between levels of the markers and the odds of regression and progression using logistic regression. In the adjusted model, adjustment was made for the clinical characteristics that were significant in the crude analyses or clinically relevant based on the formerly published reports.5 Clinical characteristics were considered in two ways: as quartiles and as strata by formerly assigned clinical cutoffs regarded associated with MA course (considered cutoff points: HbA1c, 8 g/l; systolic BP, 115 mm Hg; serum cholesterol level, 198 mg/dl; median baseline ACR, 68 mg/g). All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC). The Committee on Human Subjects of the Joslin Diabetes Center approved the protocol and informed consent procedures for this study.
Acknowledgments
This work was supported by NIH grants ES016723 (to VSV), DK74099 and DK72381 (to JVB), DK41526 and DK67638 (to ASK), and by ADA Grant 7-03-MN-28 (to MAN).
Footnotes
DISCLOSURE
JVB is co-inventor on patents for KIM-1, which have been assigned to Partners Health Care and licensed to Johnson and Johnson, Genzyme, and Biogen Idec.
References
- 1.Collins AJ, Kasiske B, Herzog C, et al. Excerpts from the United States Renal Data System 2006 Annual Data Report. Am J Kidney Dis. 2007;49:A6–A7. S1–296. doi: 10.1053/j.ajkd.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Viberti GC, Hill RD, Jarrett RJ, et al. Microalbuminuria as a predictor of clinical nephropathy in insulin-dependent diabetes mellitus. Lancet. 1982;1:1430–1432. doi: 10.1016/s0140-6736(82)92450-3. [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE. Microalbuminuria as a predictor of clinical diabetic nephropathy. Kidney Int. 1987;31:673–689. doi: 10.1038/ki.1987.50. [DOI] [PubMed] [Google Scholar]
- 4.Mogensen CE, Christensen CK. Predicting diabetic nephropathy in insulin-dependent patients. N Engl J Med. 1984;311:89–93. doi: 10.1056/NEJM198407123110204. [DOI] [PubMed] [Google Scholar]
- 5.Perkins BA, Ficociello LH, Silva KH, et al. Regression of microalbuminuria in type 1 diabetes. N Engl J Med. 2003;348:2285–2293. doi: 10.1056/NEJMoa021835. [DOI] [PubMed] [Google Scholar]
- 6.Bangstad HJ, Seljeflot I, Berg TJ, et al. Renal tubulointerstitial expansion is associated with endothelial dysfunction and inflammation in type 1 diabetes. Scand J Clin Lab Invest. 2009;69:138–144. doi: 10.1080/00365510802444080. [DOI] [PubMed] [Google Scholar]
- 7.Mauer SM, Steffes MW, Ellis EN, et al. Structural–functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips AO, Steadman R. Diabetic nephropathy: the central role of renal proximal tubular cells in tubulointerstitial injury. Histol Histopathol. 2002;17:247–252. doi: 10.14670/HH-17.247. [DOI] [PubMed] [Google Scholar]
- 9.Wolkow PP, Niewczas MA, Perkins B, et al. Association of urinary inflammatory markers and renal decline in microalbuminuric type 1 diabetics. J Am Soc Nephrol. 2008;19:789–797. doi: 10.1681/ASN.2007050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abrass CK. Diabetic proteinuria. Glomerular or tubular in origin? Am J Nephrol. 1984;4:337–346. doi: 10.1159/000166849. [DOI] [PubMed] [Google Scholar]
- 11.Thomas MC, Burns WC, Cooper ME. Tubular changes in early diabetic nephropathy. Adv Chronic Kidney Dis. 2005;12:177–186. doi: 10.1053/j.ackd.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Yu Y, Jin H, Holder D, et al. Biomarkers of kidney tubule injury: urinary trefoil factor 3 and albumin. Nat Biotechnol. 2010;28:470–477. doi: 10.1038/nbt.1624. [DOI] [PubMed] [Google Scholar]
- 13.Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- 14.Bonventre JV, Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 15.Bailly V, Zhang Z, Meier W, et al. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- 16.Bonventre JV. Kidney injury molecule-1 (KIM-1): a urinary biomarker and much more. Nephrol Dial Transplant. 2009;24:3265–3268. doi: 10.1093/ndt/gfp010. [DOI] [PubMed] [Google Scholar]
- 17.Han WK, Bailly V, Abichandani R, et al. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62:237–244. doi: 10.1046/j.1523-1755.2002.00433.x. [DOI] [PubMed] [Google Scholar]
- 18.Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008;48:463–493. doi: 10.1146/annurev.pharmtox.48.113006.094615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273:4135–4142. doi: 10.1074/jbc.273.7.4135. [DOI] [PubMed] [Google Scholar]
- 20.Prozialeck WC, Vaidya VS, Liu J, et al. Kidney injury molecule-1 is an early biomarker of cadmium nephrotoxicity. Kidney Int. 2007;72:985–993. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaidya VS, Ford GM, Waikar SS, et al. A rapid urine test for early detection of kidney injury. Kidney Int. 2009;76:108–114. doi: 10.1038/ki.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaidya VS, Ramirez V, Ichimura T, et al. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- 23.Vaidya VS, Waikar SS, Ferguson MA, et al. Urinary biomarkers for sensitive and specific detection of acute kidney injury in humans. Clin Transl Sci. 2008;1:200–208. doi: 10.1111/j.1752-8062.2008.00053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Timmeren MM, Vaidya VS, van Ree RM, et al. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation. 2007;84:1625–1630. doi: 10.1097/01.tp.0000295982.78039.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Curr Opin Nephrol Hypertens. 2007;16:557–564. doi: 10.1097/MNH.0b013e3282f08745. [DOI] [PubMed] [Google Scholar]
- 26.Emeigh Hart SG. Assessment of renal injury in vivo. J Pharmacol Toxicol Methods. 2005;52:30–45. doi: 10.1016/j.vascn.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 27.Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- 28.Strutz FM. EMT and proteinuria as progression factors. Kidney Int. 2009;75:475–481. doi: 10.1038/ki.2008.425. [DOI] [PubMed] [Google Scholar]
- 29.Ziyadeh FN, Snipes ER, Watanabe M, et al. High glucose induces cell hypertrophy and stimulates collagen gene transcription in proximal tubule. Am J Physiol. 1990;259:F704–F714. doi: 10.1152/ajprenal.1990.259.4.F704. [DOI] [PubMed] [Google Scholar]
- 30.Polhill TS, Saad S, Poronnik P, et al. Short-term peaks in glucose promote renal fibrogenesis independently of total glucose exposure. Am J Physiol. 2004;287:F268–F273. doi: 10.1152/ajprenal.00084.2004. [DOI] [PubMed] [Google Scholar]
- 31.Russo LM, Sandoval RM, McKee M, et al. The normal kidney filters nephrotic levels of albumin retrieved by proximal tubule cells: retrieval is disrupted in nephrotic states. Kidney Int. 2007;71:504–513. doi: 10.1038/sj.ki.5002041. [DOI] [PubMed] [Google Scholar]
- 32.Vaidya VS, Ozer JS, Dieterle F, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28:478–485. doi: 10.1038/nbt.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruster C, Wolf G. The role of chemokines and chemokine receptors in diabetic nephropathy. Front Biosci. 2008;13:944–955. doi: 10.2741/2734. [DOI] [PubMed] [Google Scholar]
- 34.Rosolowsky ET, Ficociello LH, Maselli NJ, et al. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol. 2008;3:706–713. doi: 10.2215/CJN.04271007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ficociello LH, Perkins BA, Silva KH, et al. Determinants of progression from microalbuminuria to proteinuria in patients who have type 1 diabetes and are treated with angiotensin-converting enzyme inhibitors. Clin J Am Soc Nephrol. 2007;2:461–469. doi: 10.2215/CJN.03691106. [DOI] [PubMed] [Google Scholar]
- 36.Krolewski AS, Laffel LM, Krolewski M, et al. Glycosylated hemoglobin and the risk of microalbuminuria in patients with insulin-dependent diabetes mellitus. N Engl J Med. 1995;332:1251–1255. doi: 10.1056/NEJM199505113321902. [DOI] [PubMed] [Google Scholar]
- 37.Warram JH, Gearin G, Laffel L, et al. Effect of duration of type I diabetes on the prevalence of stages of diabetic nephropathy defined by urinary albumin/creatinine ratio. J Am Soc Nephrol. 1996;7:930–937. doi: 10.1681/ASN.V76930. [DOI] [PubMed] [Google Scholar]
- 38.Vignali DA. Multiplexed particle-based flow cytometric assays. J Immunol Methods. 2000;243:243–255. doi: 10.1016/s0022-1759(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 39.Macisaac RJ, Tsalamandris C, Thomas MC, et al. The accuracy of cystatin C and commonly used creatinine-based methods for detecting moderate and mild chronic kidney disease in diabetes. Diabet Med. 2007;4:443–448. doi: 10.1111/j.1464-5491.2007.02112.x. [DOI] [PubMed] [Google Scholar]