Abstract
Pesticides such as chlorpyrifos (CPF) and metals such as copper can impair swimming behavior in fish. However, the impact to swimming behavior from exposure to mixtures of neurotoxicants has received little attention. In the current study, we analyzed spontaneous swimming rates of adult zebrafish (Danio rerio) to investigate in vivo mixture interactions involving two chemical classes. Zebrafish were exposed to the neurotoxicants copper chloride (CuCl, 0.1 μM, 0.25 μM, 0.6 μM, or 6.3, 16, 40 ppb), chlorpyrifos (CPF, 0.1 μM, 0.25 μM, 0.6 μM, or 35, 88, 220 ppb) and binary mixtures for 24 hr to better understand the effects of Cu on CPF neurotoxicity. Exposure to CPF increased the number of animals undergoing freeze responses (an anti-predator behavior) and, at the highest CPF dose (0.6 μM), elicited a decrease in zebrafish swimming rates. Interestingly, the addition of Cu caused a reduction in the number of zebrafish in the CPF-exposure groups undergoing freeze responses. There was no evidence of additive or synergistic toxicity between Cu and CPF. Although muscle AChE activity was significantly reduced by CPF, there was a relatively poor relationship among muscle AChE concentrations and swimming behavior, suggesting non-muscle AChE mechanisms in the loss of swimming behavior. In summary, we have observed a modulating effect of Cu on CPF swimming impairment that appears to involve both AChE and non-AChE mechanisms. Our study supports the utility of zebrafish in understanding chemical mixture interactions and neurobehavioral injury.
Keywords: mixtures, metals, organophosphates, behavior, acetylcholinesterase
Introduction
Surface waters within many watersheds receive run-off and discharges from urban, agricultural, and household sources which can lead to the accumulation of contaminant mixtures containing neurotoxic compounds (Gilliom 2007). The biological significance of these mixtures represents an important ecological issue, as neurobehavioral swimming impairment from metals and pesticides occurs in a number of fish, including threatened and endangered salmonids (Brewer et al. 2001; Sandahl et al. 2007; Tierney et al. 2008). However, understanding mixture interactions on behavioral function can be difficult to address. For these reasons, it is of utility to develop rapid diagnostic assays of neurobehavioral impacts on fish. Such an approach necessitates that the model species of choice exhibits a relevant behavioral phenotype. In this regard, zebrafish (Danio rerio) are an established neurobehavioral laboratory fish model with a well characterized genome allowing for the application of sophisticated molecular approaches to investigate mechanisms of toxicity (Nikonov et al. 2001; Levin et al. 2004; Swain et al. 2004). Furthermore, the extensive number of potential environmental mixture combinations favors the use of small laboratory fish species such as zebrafish as surrogates for ecological species.
Of the established environmental neurotoxicants, organophosphate pesticides (OPs) such as chlorpyrifos are potent inhibitors of acetylcholinesterase (AChE), a key enzyme pathway responsible for terminating transmission of many neuronal cell types across synapses (Szabo et al. 1992; Behra et al. 2002; Yang et al. 2008). Accordingly, chemical inhibition of AChE results in overwhelming of post-synaptic acetylcholine receptors and hyper-stimulation, which leads to physiologic aberrations ranging from behavioral impairment to death. The measurement of AChE inhibition is a widely used biomarker of exposure to OPs and carbamate insecticides in wild fish species (Tierney et al. 2008). However, non AChE-associated mechanisms of CPF neurotoxicity and behavioral injury have also been described (Garcia et al. 2002; Howard et al. 2005; Eaton et al. 2008). Similar to the organophosphates, metals such as copper may cause behavioral abnormalities which can be manifested by a number of swimming impairments (Sandahl et al. 2004; De Boeck et al. 2006; McIntyre et al. 2008). As with chlorpyrifos, the mechanisms of copper mediated neurobehavioral injury are complex, and can involve disruption of olfactory sensor cells and olfactory transduction signaling (De Boeck et al. 2006; Linbo et al. 2006; Tilton et al. 2008).
In the current project, we used a swimming behavior assay in adult zebrafish exposed to relevant neurobehavioral toxicants to further our understanding of neurobehavioral impacts from environmentally relevant mixtures. Our experimental approach was to expose zebrafish to copper, chlorpyrifos, or a mixture of the two neurotoxicants in various molar ratios, and to assess the consequences of exposures on lateral swimming movement and muscle AChE inhibition. Our results indicate in vivo interactions at a behavioral level of two different and relevant chemical classes and support the use of zebrafish to study interactive effects among environmental neurotoxicants on AChE and non AChE mechanisms of behavioral injury.
Materials and Methods
Animals and Exposures
All zebrafish studies were conducted in accordance with University of Washington Institutional Animal Care and Use Committee regulations. One year old adult AB zebrafish (Danio rerio, Cyprinidae) were maintained in 38 L aquaria with re-circulating filtration at 4 animals/L. Fish were fed twice daily and water quality was recorded daily. The water source was Seattle municipal water passed through a treatment system containing 0.2 μm filtration, activated carbon, ionic and mixed bed filters. The resulting water pH was 5.5 and devoid of conductivity and chlorine. Water for fish was reconstituted freshwater using Instant Ocean® salts at 1000±100 μS, pH adjusted to 7.0 using Na2HCO3 and heated to 27 °C in a holding reservoir. Tanks received a 20% (v/v) daily water change or more as necessary. The zebrafish were exposed in groups of five to each experimental concentration of CuCl2, CPF or the CuCl2/CPF mixtures and this was replicated three times. Vehicle controls with one group of five fish were carried out as closely time-matched as practically possible for each type of the three toxicant exposures (i.e. Cu, CPF or CPF+Cu), resulting in a total of three groups of vehicle controls. A stock solution of CPF (Chem Service Inc, West Chester, PA, USA) was made up in dimethylsulfoxide (DMSO) and the final DMSO concentration in all exposure jars was 0.001%. Nominal chlorpyrifos concentrations employed throughout this study were 0.1 μM, 0.25 μM, 0.6 μM, or 35, 88, 220 μg/L. The CuCl stock was prepared in distilled water. All copper treatments were spiked with 0.001% DMSO to equalize any carrier solvent effects across treatment groups. Nominal copper concentrations were 0.1 μM, 0.25 μM, 0.6 μM, or 6.3, 16, 40 μg/L or ppb. For the binomial mixtures, the copper concentration was maintained at 0.25 μM (16 ppb) in combination with each of the three concentrations of CPF. This experimental design resulted in three experimental conditions in which the binomial concentrations were equivalent (1:1 molar ratio), or in which Cu or CPF predominated the mixtures (2.5:1 molar ratio). Exposures were conducted in 3L of water held in aerated sealed 3.5L glass jars maintained at 28 °C using a water bath. The exposure jars containing CPF were pretreated with the respective concentrations for 24 h prior to use and were reused (but with fresh CPF solution) for each of the treatments of CPF to minimize possible adsorption to the glass surfaces. Exposures occurred over 24 h prior to post-exposure behavioral testing and tissue was collected within one hour of the final behavioral trial. The three experimental treatment groups included copper chloride (CuCl2), chlorpyrifos (CPF), and binomial mixtures of the two agents. Exposures were conducted independently. The three replications of each treatment group were rotated such that no replicate was initiated (or terminated) at the same time of day as the other experimental replicates. Each replicate pool (exposure jar) contained two males and three females (or vice versa) that were alternated so that the ratios across replicates approximated 1:1 (males: females). After 24 h of exposure, water quality parameters (pH, dissolved oxygen, and temperature) were recorded. Water quality parameters did not appreciably change over the course of the experiment. Water samples were analyzed at 24 h for CPF and CPF-oxon (CPO) concentrations (Pacific Agricultural Laboratory, Portland, OR, USA) and for total copper analysis (Frontier GeoSciences Inc., Seattle, WA, USA).
Muscle cholinesterase measurements
Cholinesterase measurements were conducted on zebrafish tissues following removal of the head (above the operculum) and the internal organs. Cholinesterase (EC 3.1.1.7/8) enzymatic activities were determined at 25 °C using an Optimax plate reader (Molecular Devices) with absorbance monitored at 412 nm (Sandahl et al. 2002). Briefly, the group of 5 fish per replicate were pooled and homogenized prior to centrifugation at 16,000 g for 10 min at 4 °C. Supernatant was collected and stored at −80 °C until analysis. After thawing, the tissue extracts were incubated in 10 mM PBS containing 0.7 mM DTNB (Sigma-Aldrich Chemical Co., St. Louis, MO, USA), pH 7.4 for 15 min. at 25 °C. Acetylthiocholine iodide (3 mM) was added to the samples prior to vortexing and transferred to a 96 well microtiter plate. Each sample was assayed three times in separate wells and averaged to provide a single value, and each 96 well plate contained tissue and substrate blanks. Enzymatic activities were corrected for spontaneous hydrolysis of acetylthiocholine iodide and for nonspecific reduction of DTNB in the presence of tissue extracts. An AChE inhibitor, 1,5-bis(4-allyldimethyl-ammoniumphenyl)pentane-2-one dibromide (BW284c51, Sigma) was used to distinguish between the different cholinesterases that might be present in zebrafish muscle tissue. The inhibitor was co-applied with DTNB during the 15 min incubation that preceded the addition of substrate.
Swimming Behavior Assay and Behavioral Trials
A 19 L aquarium with one inch squares drawn across the outside bottom of the tank with distinguishing marks to form ten 7.6 × 8.9 cm grids was used as the behavioral testing chamber. Prior to testing each experimental group, the behavioral chamber was filled with 5 L of water (~7.5 cm deep) to provide each fish with ample room to swim horizontally while limiting its vertical movement. The testing chamber was wrapped in white bench paper leaving only the top open for viewing. A Nikon A95 digital camera was mounted above the center of the tank to record video of the swimming trials and a clip lamp using a 15W incandescent light bulb was placed approximately 1 meter above the camera to provide uniform lighting throughout the tank.
Preliminary studies established an average number of times a fish crossed over one of the 7.6 × 8.9 cm grid lines in a one min trial (75 ± 35 crossings over one min). For each 1 min behavioral trial, individual adult fish were placed in the center of the testing chamber and allowed to swim for 1.25 min to equilibrate (as determined to be a sufficient amount of time for these animals to resume normal swimming rates and behavior). Animals that showed little or no movement (i.e. by crossing < 35 grids/min) were not used in the experiments. Each exposure group of five animals was randomly selected from the group tanks. Gender was phenotypically determined and no more than three animals of one gender were used per replicate. Every attempt was made to maintain equal ratios of males and females across the three treatment replicates. After the equilibration period, the video recording was initiated and the timer was allowed to count down 15 s before the one minute trial. A handheld counter was used to record the number of times the eyes (e.g. head) of the animal crossed a 7.6 cm line. This was accomplished for 5 consecutive 1 min trials per animal with 15 s intervals between trials, resulting in 25 data points per treatment group. Swimming behavior was measured in identical fashion for each individual animal pre-and post-exposure to the test agents. A random blind sub-sample of the video recordings was later evaluated to confirm the real-time data collection. Before and after behavioral testing, the fish were held in the appropriate exposure water until tissue samples were collected.
Statistical analysis
A linear mixed effects model was used to calculate the statistical significance of the data displayed in Table 2 and Figures 1, 2, 3 and 4. More specifically, the R/Bioconductor limma package was used (http://www.R-project.org). This approach was employed to determine a) whether the swimming behavior of the various pre- and post-exposure controls differed significantly from each other (data presented in Table 2); b) whether Cu, CPF or CPF+Cu exposures resulted in statistically significant inhibition of AChE activity relative to the control (data presented in Figure 4), and c) whether the difference in swimming behavior between the pre and post-exposure control groups was statistically significantly different from the difference between the corresponding pre and post-toxicant exposure groups (data presented in Figures 1,2 and 3). Statistical significance of the freeze response in toxicant exposed versus control animals (data presented in Table 3) was assessed using a Fisher’s exact test from the R statistical software package’s function fisher.test (http://www.R-project.org). The R statistical software package’s function lm (“lm” stands for linear model) was used to fit a linear model to determine the statistically significant relationship between “OD min mg protein” and “mean swim behavior data for series” (data presented in Figure 5) (http://www.R-project.org).
Table 2.
Statistical analysis of swimming behavior of pre and post-exposure controls
| Specific pre and post-exposure control comparisons | p-value |
|---|---|
| a control Cu pre-exposure versus control Cu post-exposure | 0.194 |
| a control CPF pre-exposure versus control CPF post-exposure | 0.878 |
| a control Cu+CPF pre-exposure versus control Cu+CPF post-exposure | 0.513 |
| a control Cu post-exposure versus control CPF post-exposure | 0.326 |
| a control Cu post-exposure versus control Cu+CPF post-exposure | 0.112 |
| a control CPF post-exposure versus control Cu+CPF post-exposure | 0.576 |
five fish were used for each of the pre-exposure and the post-exposure control groups.
Figure 1.
Effect of exposure to CuCl2 on lateral line swimming behavior in adult zebrafish. The bars % post-exposure swimming rates relative to the corresponding pre-exposure rates. Data represent mean (SD) of 5 consecutive 1 min trials per animal with 15 s intervals between trials. 5 fish each were used for the pre- and post-exposure control groups resulting in 25 data points per group. 15 fish (3 replicate trials with 5 animals each) were used for each of the pre- and post-exposure Cu groups for each Cu concentration resulting in a total of 75 data points for each of these groups. The difference in swimming behavior between the pre- and post-exposure controls was not statistically significant when compared to the difference between the pre and post-Cu exposure groups at any Cu concentration examined. A p-value of <0.01 was considered significant.
Figure 2.
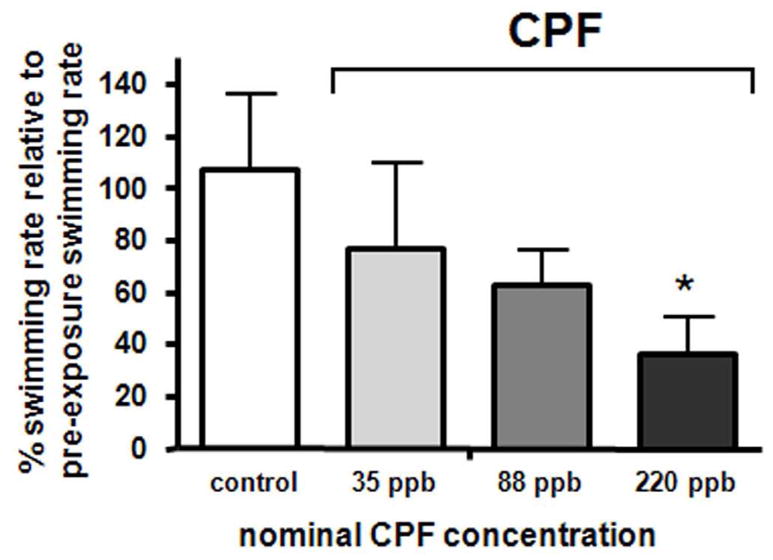
Effect of exposure to CPF on lateral line swimming behavior in adult zebrafish. The bars show % post-exposure swimming rates relative to the corresponding pre-exposure swimming rates. Data represent mean (SD) of 5 consecutive 1 min trials per animal with 15 s intervals between trials. Five fish each were used for the pre- and post-exposure control groups resulting in 25 data points per group. 15 fish (3 replicate trials with 5 animals each) were used for each of the pre- and post-exposure chlorpyrifos (CPF) groups for each CPF concentration resulting in a total of 75 data points for each of these groups. The difference in swimming behavior between the pre- and post-exposure controls was statistically significant when compared to the difference between the pre- and post-220 CPF exposure group only. A p-value of <0.001 was considered significant. * indicates p<0.01.
Figure 3.
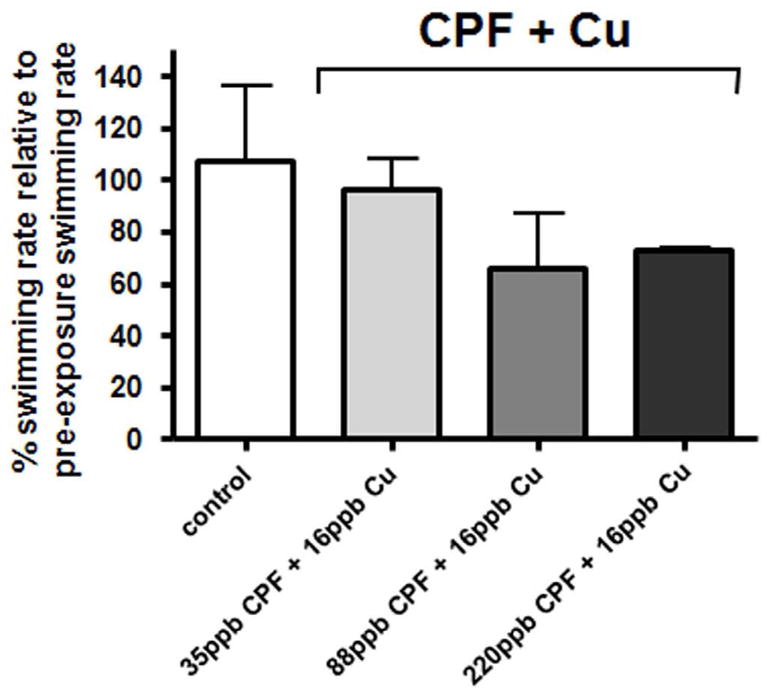
Effect of exposure to a binomial CPF+Cu mixture on lateral line swimming behavior in adult zebrafish. The bars show % post-exposure swimming rates relative to the corresponding pre-exposure swimming rates. Data represent mean (SD) of 5 consecutive 1 min trials per animal with 15 s intervals between trials. 5 fish each were used for the pre- and post-exposure control groups resulting in 25 data points per group. 15 fish (3 replicate trials with 5 animals each) were used for each of the pre- and post-exposure CPF+Cu groups for each of the three CPF+Cu concentration combinations resulting in a total of 75 data points for each of these groups. The difference in swimming behavior between the pre- and post-exposure controls was not statistically significant when compared to the difference between the pre and post- CPF+Cu exposure groups at any of the CPF+Cu concentration combinations used. A p-value of <0.01 was considered significant.
Figure 4.
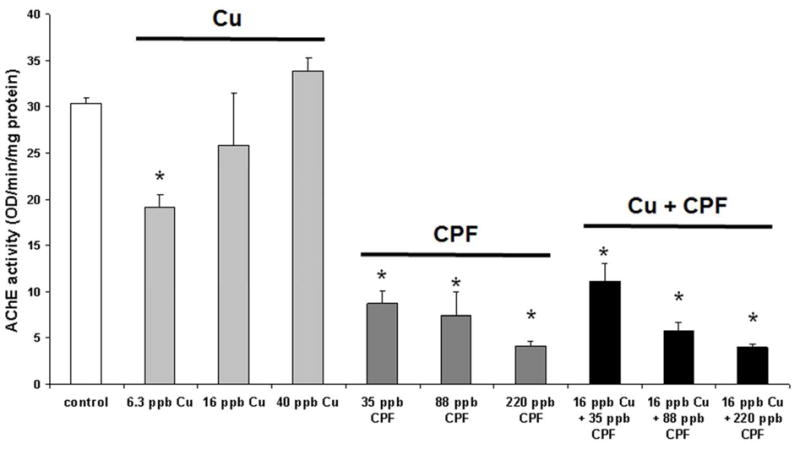
Muscle AChE activity in adult zebrafish exposed to CuCl2, CPF or a mixture of the two chemicals. Data represents mean (SD) of 3 pools of 5 fish each resulting in 3 data points per condition. AChE activity is shown as optical density measurement per min per mg of protein (OD/min/mg protein). * indicates p< 0.01 relative to the control values.
Table 3.
Post-exposure freeze responses in zebrafish
| Treatment controls | Number of animals with post-exposure freeze response (%; p-valuec) | Number of one min intervals with fish exhibiting post-exposure freeze responses (%;p-valuec) |
|---|---|---|
| 0/15 (0%; N/Aa) | 0/75 (0%; N/Aa) | |
| 6.3 ppb Cu | 1/15 (7%; 1.0000) | 2/75 (2.6%; 0.497) |
| 16 ppb Cu | 2/15 (13%; 0.486) | 2/75 (2.6%; 0.497) |
| 40 ppb Cu | 3/14 (21%; 0.229) | 11/70 (15.7%; 0.0007) |
| 35 ppb CPF | 4/15 (27%; 0.113) | 6/75 (8%; 0.029) |
| 88 ppb CPFb | 6/15 (40%; 0.030b) | 17/75 (22.6%; 0.00003b) |
| 220 ppb CPFb | 4/15 (27%; 0.113) | 14/75 (18.6%; 0.0001b) |
| 35 ppb CPF + 16 ppb Cu | 3/15 (20%; 0.233) | 4/75 (5.3%; 0.121) |
| 88 ppb CPF + 16 ppb Cub | 6/15 (40%; 0.030b) | 7/75 (9.3%; 0.014b) |
| 220 ppb CPF + 16 ppb Cu | 2/15 (13%; 0.486) | 2/75 (2.6%; 0.497) |
not applicable
p<0.05 in bold print
p-values assesses statistical significance relative to the controls
Figure 5.

Scatter plots showing the relationship among swimming rates (number of gridlines crossed per minute) and AChE activity in adult zebrafish exposed to CuCl2, CPF, or CPF+Cu binomial mixtures. Linear regression analysis indicated a negative correlation between Cu exposure and swimming behavior (R2=0.076, p=0.440), whereas fish exposed to CPF alone (R2=368, p=0.063) or a mixture of CPF+Cu (R2=0.212, p=0.180), exhibited a positive correlation. However, none of these correlations were statistically significant.
Results
Nominal and measured copper and CPF concentrations
Presented in table 1 are the nominal and measured aqueous chemical concentrations of the two neurotoxicants. The CPF concentrations measured at 24 hours were in reasonable agreement with the nominal concentrations in the jars that did not contain fish. More specifically, the measured CPF concentrations were 137% (48 ppb), 125% (110 ppb) and 109% (240 ppb) of the 35, 88 and 220 ppb corresponding nominal concentrations respectively. There was little loss of CPF over 24 hours in exposure jars devoid of fish (Table 1). By 24 hours, however, CPF concentrations in exposure jars containing fish had decreased by more than 80% for each concentration, indicating efficient uptake of CPF. A small amount of chlorpyrifos oxon (CPO) was detected at the different CPF concentrations (mean of 0.8 ppb, Table 1). The copper concentrations measured were 170% (10.7 ppb), 116% (18.5 ppb), and 99% (39.7 ppb), of the 6.3, 16 and 40 ppb corresponding nominal concentrations respectively Table 1). Thus, with exception of the low concentrations, the measured and nominal concentrations were in good agreement. As anticipated, there was a minor background level of 1.4 ppb Cu in the reconstituted zebrafish control water which could not be removed by filtration.
Table 1.
Nominal and actual waterborne copper and chlorpyrifos concentrations1
| Nominal CPF (ppb) | Mean measured CPF at 24 hr without fisha (ppb) | Mean measured CPF at 24 hr with fishb (ppb) | Mean measured CPO without fish at 24 hra (ppb) | Measured CPO with fish at 24 hrb (ppb) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 35 | 48 | 6.2 (1.4c) | 0.5 | 0.7 (0.4c) |
| 88 | 110 | 10.2 (5.8c) | 1.1 | 0.6 (0.2c) |
| 220 | 240 | 12.9 (11.4c) | 1.6 | 1.3 (1.1c) |
| Nominal Cu | Mean measured Cu at 24 hr without fishb | Mean measured Cu at 24 hr with fish | ||
| 0 | 1.4 (0.5c) | NDd | ||
| 6.3 | 10.7 (3.2c) | ND | ||
| 16 | 18.5 (0.9c) | ND | ||
| 40 | 39.7 (2.3c) | ND |
Analytical analyses for Cu were conducted using EPA Method 1669 by inductively coupled plasma mass spectrometry (detection limit of 0.04 μg/L), and for CPF and CPO using EPA method 8141B (Gas chromatography-flame photometric detection.
One grab sample from independent sample containers, N=1
One grab sample from each exposure, N=3
Standard Deviation
ND: not determined
Zebrafish swimming performance of pre- and post-exposure control groups
Behavioral trials were conducted in pre- and post-exposure control animals. Pre-exposure controls were not exposed to the vehicle DMSO, whereas the post-exposure controls were exposed to 0.001% DMSO for the same duration as the actual exposures to a toxicant. Each pre and post-exposure control group (n=5 fish) had a corresponding pre- and post-toxicant exposure group (n=5 fish) assigned and measurements in these groups were as closely time matched as practically possible. This design was chosen to take into account the effect any non-toxicant related parameters (e.g. time of day and its effect on circadian rhythm, slight temperature differences) may have on swimming behavior. There was no statistically significant difference between any of the pre- and their corresponding post exposure controls (e.g. pre-exposure control Cu versus post-exposure control Cu) that were as closely time matched as practically possible with the actual Cu, CPF and Cu+CPF exposure scenarios (Table 2). Similarly, the differences between any of the possible post-exposure control comparisons (e.g. post-exposure control Cu versus post-exposure control CPF, etc.) were not statistically significant (Table 2).
Zebrafish swimming performance following Cu exposure
There was extensive individual variation in swimming responses in the high dose Cu group (40 ppb Cu), with 3 of the 15 animals accounting for the variability. Specifically, 2 animals in the first replication and 1 animal in the third replication froze upon being placed in the behavior chamber, accumulating a score in only 3 of the 15 one minute trials that were carried out for these three fish. The number of fish and the number of trials in which this freeze response was observed for all exposure groups are presented in Table 3. Essentially, freeze response indicates that these animals simply stopped their swimming behavior and dropped to the bottom of the tank. This is a common predator avoidance response observed in other fish exposed to environmental chemicals (Treberg et al. 2002).
In both the 16 ppb and 40 ppb Cu behavioral trials, there was a noticeable change in zebrafish swimming behavior as reflected by periods of little to no swimming, followed by high rates of swimming. In contrast to 40 ppb Cu exposure, there were no significant differences in swimming behavior between the 16 ppb pre and post-exposure groups and the corresponding pre and post-exposure control groups (Figure 1). Some of the animals exposed to the medium (16 ppb) and high doses (40 ppb) of copper jumped out of the water of the behavioral chamber after spending time facing the corners and swimming up and down in the water column. This aforementioned behavior was not observed at the lowest dose of 6.3 ppb Cu, which did not differ significantly from its corresponding controls (Table 1).
Zebrafish swimming performance following CPF exposure
Exposure to 35, 88 and 220 ppb CPF reduced swimming rates in a dose dependent manner (Figure 2). However, only the highest exposure resulted in a statistically significant effect (p<0.01). Out of the 15 fish each exposed to 35, 88 or 220 ppb CPF, 4, 6 and 4 showed freeze responses respectively (table 3). These animals would typically stay immediately adjacent to the testing chamber wall with both pectoral fins rigid and extended. It is note worthy that 2 fish exposed to 220 ppb CPF exhibited a freeze response the entire time swimming behavior was measured and, thus, resulted in 0 scores. The same scenario was observed for 1 fish exposed to 88 ppb CPF, but none of the 35 ppb CPF exposed animals.
Three animals in the highest CPF treatment group showed clear toxic responses with obvious muscle twitches as they swam. One of these fish had significant buoyancy issues which resulted in sporadic movement to maintain position, resulting in an obvious reduced swimming score. There were no other obvious behavioral impairments in the CPF treated animals.
Zebrafish swimming performance following exposure to a mixture containing CPF and Cu
The three treatment groups from the binomial exposures were designed to investigate the possible interactions of Cu and CPF. One group was exposed to equimolar concentrations of Cu and CPF. In the other groups, Cu or CPF predominated by a molar ratio of 2.5:1. Accordingly, the measured data are described primarily on a molar basis for clarity. In the first treatment group Mix A, consisting of 0.1 μM CPF (35 ppb) + 0.25 μM Cu (16 ppb), Cu was in 2.5-fold molar excess of CPF. Animals exposed to Mix A showed a slightly reduced swimming rate, but this reduction was not significant (Figure 3). In the second treatment group Mix B, 0.25 μM CPF (88 ppb) + 0.25 μM Cu (16 ppb), the toxicants were added in equal molar concentrations. The swimming rate in this group was also reduced relative to the control group. However, this decrease was not statistically significant. Finally, in the third treatment group Mix C, 0.6 μM CPF (220 ppb) + 0.25 μM Cu (16 ppb), CPF was in 2.5 fold molar excess. A trend in decreased swimming rate was observed for animals exposed to Mix C, but it did not reach statistical significance (Figure 3).
Muscle AChE activity and correlation analysis of AChE activity and swimming behavior
Increasing Cu concentrations were inversely correlated with AChE activity with the lowest Cu exposure (6.3 ppb) resulting in a statistically significant (p=0.007) reduction (63% of control) in AChE activity, whereas 16 and 40 ppb Cu had no significant effects (Figure 4). Exposure to 35, 88 and 220 ppb CPF resulted in significant decreases of AChE activity to 29% (p=0.001), 25% (p=0.010) and 14% (p=4.53 × 10−6) of control respectively. Similarly, Mix A (35ppb CPF + 16ppb Cu), Mix B (88ppb CPF + 16ppb Cu), and Mix C (220ppb CPF + 16ppb Cu), lowered AChE activity in a dose response like fashion to 37% (p=0.007), 19% (p=5.15 × 10−5) and 13% (p=5.82 × 10−6) of control and were statistically significant.
In order to investigate whether AChE activity was correlated with swimming behavior, we assessed the correlation of these two types of measurements. Interestingly, linear regression analysis indicated a weak negative correlation between AChE activity and swimming behavior (R2=0.076, p=0.440) in the Cu exposed animals. However, this correlation was not statistically significant (Figure 5). In contrast, fish exposed to CPF displayed a positive correlation (R2=0.368; p=0.063) that approached, but was not statistically significant. Fish exposed to the CPF+Cu binomial mixtures also exhibited a positive correlation that was not statistically significant (R2=0.212, p=0.180; (Figure 5).
Discussion
A number of sensitive neurobehavioral measures have been used to assess the effects of pesticide exposures on behaviors critical to survival in ecologically sensitive fish species. One of the more sensitive techniques, electro-olfactogram (EOG) recordings, is an in vivo measure of peripheral olfactory function and sublethal neurotoxicity in fish involving analysis of odorant stimulated negative potentials generated from the olfactory epithelium (Baldwin et al. 2005). However, such approaches, while quantitative and linked to system-specific behavioral injury (i.e. impairment of olfaction originating from the olfactory rosette receptor neurons) are logistically difficult in large experiments assessing the effects of complex mixtures. By contrast, using assessment of lateral swimming behavior, although not olfactory-specific or pinpointing the target organ origin of the behavioral impairment, integrates physiological impacts that lead to modulation of behavior. The potential myriad of combinations of binomial interactions that can occur under natural conditions between metals and organophosphates were reduced in the present study to three environmentally relevant exposure scenarios. While the 88 and 220 ppb CPF doses used in this study were higher than those reported in threshold studies elsewhere for wild fish (Sandahl et al. 2004; Scholz et al. 2006), the lowest dose (35 ppb approached more realistic environmental levels. The fact that the CPF doses employed resulted in a dose-dependent reduction in swimming behavior suggests that zebrafish may be somewhat more resistant to the neurobehavioral toxicity of this compound relative to wild fish. As discussed, the doses used in our study allowed us to investigate hypothetical environmental exposure situations in which CPF or Cu predominated, or that were roughly similar on a molar basis.
The fact that the addition of Cu to the moderate dose of CPF partially attenuated the number of animals undergoing freeze responses suggests that copper may somehow block biochemical neurological impacts of CPF exposures, or modify its uptake or action at sensitive neuronal sights. However, the fact that swimming behavior, as reflected by the number of grid lines crossed per minute was still impaired for each CPF exposure group in which CPF predominated, underscores the complexity of neurobehavioral injury by CPF. Neurobehavioral impairment in fish exposed to CPF has been shown to involve AChE and non-AChE mechanisms, the latter of which can include disruption of cAMP-related cell signaling, apoptosis, oxidative stress, excitotoxicity, development of neurotransmitter synthesis, serotonin norepinephrine and dopamine receptor modulation (Slotkin et al. 2007; Geter et al. 2008; Saulsbury et al. 2009; Slotkin et al. 2009). Using microarray analysis, we have reported that exposure of zebrafish to CPF preferentially impacts olfactory gene expression pathways associated with the maintenance of cellular morphogenesis, growth and development, and odorant binding (Tilton et al., 2010). Some of these aforementioned mechanisms are likely not unique to CPF and organophosphates, as alterations in swimming behavior of delta smelt exposed to the pyrethroid pesticide esfenvalerate is associated with alterations in the expression of genes associated with immune responses, along with apoptosis, redox status, osmotic stress, detoxification, and growth and development (Connon et al. 2009).
One particularly interesting aspect of the responses observed on exposure to Cu was that the behavioral effects predominately consisted of freeze responses, an anti-predator behavior, as opposed to modulation of lateral swimming movement. Such responses have been observed on exposure to copper in other aquatic species (Sandahl et al. 2007). While the molecular basis for this behavior is not well established, we have shown that copper modulates signal transduction pathways in the zebrafish olfactory system with a likely involvement of oxidative stress (Tilton et al. 2008). Inhibition of olfaction by metals such as copper can underlie neurobehavioral injury (Sandahl et al. 2007). Copper is also known to have neuronal excitability potential (Aedo et al. 2007) and it was interesting that we observed excitatory responses (e.g. jumping behaviors and rapid bursts of swimming) in a number of fish exposed to 40 ppb Cu. This observation may be indicative of a neurostimulatory potential associated with copper exposure
In general, the responses observed in adult zebrafish exposed to either Cu or CPF on AChE and swimming behavior in our study are consistent with those reported for other species of fish. For example, brief CPF exposure increases startle responses in adult zebrafish (Eddins et al. 2010). It is also interesting that despite the fact that copper and chlorpyrifos are both behavioral toxicants, most of the neurobehavioral toxicity observed in the present study (including both swimming behavior and on AChE levels) were associated with the CPF treatments and not Cu. Although we did not anticipate a modulating effect of Cu on muscle AChE, the reduction in muscle AChE on exposure to a low dose copper is consistent with other studies reporting metal induced alterations of AChE in several vertebrate and invertebrates (Frasco et al. 2005; Frasco et al. 2007; Pari et al. 2007; Frasco et al. 2008; Petraglio et al. 2008). Potentially, the behavioral effects observed following Cu exposure may have been due to a transient effect in muscle AChE. Clearly, the relatively small sample size used in the present study, which was necessitated by the logistics of conducting mixture exposures, suggests that the observed modulating effect of Cu on muscle AChE requires a follow-up study with larger sample sizes. It is also important to note that brain AChE plays a crtical role in mediating CPF effects on locomotion. The pronounced impact on both AChE and swimming impairment of CPF relative to copper suggests that muscle AChE is informative with regards to assessing the contribution of AChE impairment to changes in swimming behavior.
Of consideration is the dose-response characteristics of the agents used in the present study relative to those of others. Copper effects on fish neurotoxicity have been most closely studied in salmonids. By contrast, several others have observed quantitative behavioral impairment of larval zebrafish on exposure to chlorpyrifos at concentrations 10 to 100 μg/L, which were within the concentrations used in this present study (Levin et al. 2004). Similarly, exposure to chlorpyrifos at concentrations from 180–750 ppb CPF concentrations increases locomotor activity in zebrafish embryos (Kienle et al. 2009, Selderslaghs, #344). Developmental differences in sensitivity of copper behavioral injury susceptibilities have not been well established in zebrafish, and thus it is important to discriminate results of those studies conducted in adults from those involving earlier life stages. Studies in adult mosquito fish (Gambusia affinis) exposed to chlorpyrifos for 20 days at 60 μg/L revealed decreases in both brain AChE and swimming behavior (Rao et al. 2005). When viewed collectively, our study and those of others suggest that zebrafish are an attractive in vivo model to address behavioral outcomes from multiple compounds of different chemical classes that are commonly encountered in surface waters. Studies investigating the molecular and biochemical basis by which environmental neurotoxicants and their mixtures are acting are continuing in our laboratory.
Acknowledgments
We thank Drs. Richard Beyer and Kathleen Kerr for statistical analysis expertise. This project was supported by the University of Washington NIEHS Superfund program project P42-ES04696.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aedo F, Delgado R, Wolff D, Vergara C. Copper and zinc as modulators of neuronal excitability in a physiologically significant concentration range. Neurochem Int. 2007;50:591–600. doi: 10.1016/j.neuint.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Baldwin DH, Scholz NL. The electro-olfactogram: An in vivo meaure of peripheral olfactory function and sublethal neurotoxicity in fish. In: Ostrander GK, editor. Techniques in aquatic toxicology. Vol. 2. CRC Press, Inc; Boca Raton, Fl: 2005. pp. 257–276. [Google Scholar]
- Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, Strahle U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat Neurosci. 2002;5:111–118. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- Brewer SK, Little EE, DeLonay AJ, Beauvais SL, Jones SB, Ellersieck MR. Behavioral dysfunctions correlate to altered physiology in rainbow trout (Oncorynchus mykiss) exposed to cholinesterase-inhibiting chemicals. Arch Environ Contam Toxicol. 2001;40:70–76. doi: 10.1007/s002440010149. [DOI] [PubMed] [Google Scholar]
- Connon RE, Geist J, Pfeiff J, Loguinov AV, D’Abronzo LS, Wintz H, Vulpe CD, Werner I. Linking mechanistic and behavioral responses to sublethal esfenvalerate exposure in the endangered delta smelt; Hypomesus transpacificus (Fam. Osmeridae) BMC Genomics. 2009;10:608. doi: 10.1186/1471-2164-10-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Boeck G, van der Ven K, Hattink J, Blust R. Swimming performance and energy metabolism of rainbow trout, common carp and gibel carp respond differently to sublethal copper exposure. Aquat Toxicol. 2006;80:92–100. doi: 10.1016/j.aquatox.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Eaton DL, Daroff RB, Autrup H, Bridges J, Buffler P, Costa LG, Coyle J, McKhann G, Mobley WC, Nadel L, Neubert D, Schulte-Hermann R, Spencer PS. Review of the toxicology of chlorpyrifos with an emphasis on human exposure and neurodevelopment. Crit Rev Toxicol. 2008;38(Suppl 2):1–125. doi: 10.1080/10408440802272158. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicol Teratol. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasco MF, Colletier JP, Weik M, Carvalho F, Guilhermino L, Stojan J, Fournier D. Mechanisms of cholinesterase inhibition by inorganic mercury. FEBS J. 2007;274:1849–1861. doi: 10.1111/j.1742-4658.2007.05732.x. [DOI] [PubMed] [Google Scholar]
- Frasco MF, Fournier D, Carvalho F, Guilhermino L. Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers. 2005;10:360–375. doi: 10.1080/13547500500264660. [DOI] [PubMed] [Google Scholar]
- Frasco MF, Fournier D, Carvalho F, Guilhermino L. Does mercury interact with the inhibitory effect of dichlorvos on Palaemon serratus (Crustacea: Decapoda) cholinesterase? Sci Total Environ. 2008 doi: 10.1016/j.scitotenv.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos targets developing glia: effects on glial fibrillary acidic protein. Brain Res Dev Brain Res. 2002;133:151–161. doi: 10.1016/s0165-3806(02)00283-3. [DOI] [PubMed] [Google Scholar]
- Geter DR, Kan HL, Lowe ER, Rick DL, Charles GD, Gollapudi BB, Mattsson JL. Investigations of oxidative stress, antioxidant response, and protein binding in chlorpyrifos exposed rat neuronal PC12 cells. Toxicol Mech Methods. 2008;18:17–23. doi: 10.1080/15376510701389530. [DOI] [PubMed] [Google Scholar]
- Gilliom RJ. Pesticides in U.S. streams and groundwater. Environ Sci Technol. 2007;41:3408–3414. doi: 10.1021/es072531u. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicol Appl Pharmacol. 2005;207:112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Kienle C, Kohler HR, Gerhardt A. Behavioural and developmental toxicity of chlorpyrifos and nickel chloride to zebrafish (Danio rerio) embryos and larvae. Ecotoxicol Environ Saf. 2009;72:1740–1747. doi: 10.1016/j.ecoenv.2009.04.014. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26:719–723. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Linbo TL, Stehr CM, Incardona JP, Scholz NL. Dissolved copper triggers cell death in the peripheral mechanosensory system of larval fish. Environ Toxicol Chem. 2006;25:597–603. doi: 10.1897/05-241r.1. [DOI] [PubMed] [Google Scholar]
- McIntyre JK, Baldwin DH, Meador JP, Scholz NL. Chemosensory deprivation in juvenile Coho salmon exposed to dissolved copper under varying water chemistry conditions. Environ Sci Technol. 2008;42:1352–1358. doi: 10.1021/es071603e. [DOI] [PubMed] [Google Scholar]
- Nikonov AA, Caprio J. Electrophysiological evidence for a chemotopy of biologically relevant odors in the olfactory bulb of the channel catfish. J Neurophysiol. 2001;86:1869–1876. doi: 10.1152/jn.2001.86.4.1869. [DOI] [PubMed] [Google Scholar]
- Pari L, Murugavel P. Diallyl tetrasulfide improves cadmium induced alterations of acetylcholinesterase, ATPases and oxidative stress in brain of rats. Toxicology. 2007;234:44–50. doi: 10.1016/j.tox.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Petraglio G, Bartolini M, Branduardi D, Andrisano V, Recanatini M, Gervasio FL, Cavalli A, Parrinello M. The role of Li+, Na+, and K+ in the ligand binding inside the human acetylcholinesterase gorge. Proteins. 2008;70:779–785. doi: 10.1002/prot.21560. [DOI] [PubMed] [Google Scholar]
- Rao JV, Begum G, Pallela R, Usman PK, Rao RN. Changes in behavior and brain acetylcholinesterase activity in mosquito fish, Gambusia affinis in response to the sub-lethal exposure to chlorpyrifos. Int J Environ Res Public Health. 2005;2:478–483. doi: 10.3390/ijerph2005030013. [DOI] [PubMed] [Google Scholar]
- Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL. Odor-evoked field potentials as indicators of sublethal neurotoxicity in juvenile coho salmon exposed to copper, chlorpyrifos, or esfenvalerate. Can J Fish Aquat Sci. 2004;61:404–413. [Google Scholar]
- Sandahl JF, Baldwin DH, Jenkins JJ, Scholz NL. A sensory system at the interface between urban stormwater runoff and salmon survival. Environ Sci Technol. 2007;41:2998–3004. doi: 10.1021/es062287r. [DOI] [PubMed] [Google Scholar]
- Sandahl JF, Jenkins JJ. Pacific steelhead (Oncorhynchus mykiss) exposed to chlorpyrifos: benchmark concentration estimates for acetylcholinesterase inhibition. Environ Toxicol Chem. 2002;21:2452–2458. [PubMed] [Google Scholar]
- Saulsbury MD, Heyliger SO, Wang K, Johnson DJ. Chlorpyrifos induces oxidative stress in oligodendrocyte progenitor cells. Toxicology. 2009;259:1–9. doi: 10.1016/j.tox.2008.12.026. [DOI] [PubMed] [Google Scholar]
- Scholz NL, Truelove NK, Labenia JS, Baldwin DH, Collier TK. Dose-additive inhibition of chinook salmon acetylcholinesterase activity by mixtures of organophosphate and carbamate insecticides. Environ Toxicol Chem. 2006;25:1200–1207. doi: 10.1897/05-030r1.1. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Oxidative and excitatory mechanisms of developmental neurotoxicity: transcriptional profiles for chlorpyrifos, diazinon, dieldrin, and divalent nickel in PC12 cells. Environ Health Perspect. 2009;117:587–596. doi: 10.1289/ehp.0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain HA, Sigstad C, Scalzo FM. Effects of dizocilpine (MK-801) on circling behavior, swimming activity, and place preference in zebrafish (Danio rerio) Neurotoxicol Teratol. 2004;26:725–729. doi: 10.1016/j.ntt.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Szabo A, Nemcsok J, Asztalos B, Rakonczay Z, Kasa P, Hieu LH. The effect of pesticides on carp (Cyprinus carpio L). Acetylcholinesterase and its biochemical characterization. Ecotoxicol Environ Saf. 1992;23:39–45. doi: 10.1016/0147-6513(92)90020-4. [DOI] [PubMed] [Google Scholar]
- Tierney KB, Sampson JL, Ross PS, Sekela MA, Kennedy CJ. Salmon olfaction is impaired by an environmentally realistic pesticide mixture. Environ Sci Technol. 2008;42:4996–5001. doi: 10.1021/es800240u. [DOI] [PubMed] [Google Scholar]
- Tilton F, Tilton S, Bammler T, Beyer R, Farin F, Stapleton PL, Gallagher EP. Transcriptional biomarkers and mechanisms of copper-induced olfactory injury in zebrafish. Environ Sci Technol. 2008;42:9404–9411. doi: 10.1021/es801636v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treberg JR, Wilson CE, Richards RC, Ewart KV, Driedzic WR. The freeze-avoidance response of smelt Osmerus mordax: initiation and subsequent suppression of glycerol, trimethylamine oxide and urea accumulation. J Exp Biol. 2002;205:1419–1427. doi: 10.1242/jeb.205.10.1419. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]



