Abstract
Background
Hemorrhagic transformation (HT) after fibrinolytic therapy may be less common in patients with acute cerebral ischemia confined to single penetrator artery (SPA) territories than in patients with large artery ischemia. However, prior investigations of HT diagnosed small vessel ischemia based on lacunar clinical syndromes, an approach known to yield misdiagnosis in one-third to one-half of cases.
Methods
Consecutive intravenous t-PA treated patients in a prospectively maintained hospital registry were analyzed. Patients were classified as having SPA ischemia if they had imaging evidence of: 1) deep location, 2) diameter ≤1.5 cm, and 3) distribution in a single penetrator territory, regardless of presenting clinical syndrome. Lacunar clinical syndrome was defined according to the Oxfordshire Community Stroke Project classification.
Results
Among 93 intravenous t-PA-treated patients, mean age was 71.5, 62.4% were female, and median pretreatment NIHSS score was 14. Single penetrator artery ischemia was imaged in 13 (14.0%) and large artery ischemia in 75 (80.6%), with no visualized ischemic injury in 5 (5.4%). Lacunar clinical syndromes were present in 23 (24.7%), including 10 with SPA ischemia and 9 with large artery ischemia. No patient with imaging-confirmed SPA infarcts experienced any hemorrhagic transformation, while any radiologic HT occurred in 29.3% of large artery infarcts, p = 0.03. Symptomatic ICH occurred in 0% of SPA infarcts vs. 4.0% of large artery infarcts.
Conclusion
HT after lytic therapy in imaging-confirmed SPA infarcts is uncommon. Imaging demonstration of ischemia confined to a SPA territory better identifies this population at low risk of hemorrhagic complications than clinical lacunar syndromes.
Keywords: intracranial hemorrhage, fibrinolysis, tissue plasminogen activator, lacunar infarction
The risk of hemorrhagic transformation after fibrinolytic therapy for acute cerebral ischemia is related to the volume of ischemic tissue,1, 2 so hemorrhagic transformation may be less common in patients with ischema confined to a single penetrator artery territory than in patients with infarcts involving large artery or multiple artery territories. This hypothesis has not been previously reliably investigated, as prior studies of hemorrhagic transformation have diagnosed small vessel infarct only on the basis of lacunar clinical syndromes, an approach known to yield misdiagnosis in one-third to one-half of cases. With the spread of multimodal MRI and CT, modern lytic treatment decision s are increasingly based on direct imaging delineation, rather than clinical inference, regarding site and size of arterial occlusion.
The purpose of this study was to determine the rate of hemorrhagic transformation after intravenous fibrinolysis in patients with imaging-confirmed single penetrator artery (SPA) ischemia.
Methods
In a prospectively maintained hospital registry, consecutive patients were identified who were treated with intravenous t-PA within 3 hours after the onset of symptoms at the University of California, Los Angeles Medical Center from 1999 to 2008. Patients subsequently treated with combined intra-arterial fibrinolysis, angioplasty with stent, or mechanical clot disruption, were excluded in this study.
Patients were then divided into two subgroups, SPA infarcts versus large or multiple artery territories (non-SPA) infarcts. This classification was based on the visulaized topography of ischemia as delineated on all available sequences, including diffusion and perfusion MRI and noncontrast and perfusion CT. Patients were classified as having SPA infarcts if they had imaging evidence of: 1) deep location, 2) diameter ≤ 1.5 cm and 3) distribution in a single penetrator territory, regardless of the clinical syndrome, the presence of steno-occlusion in responsible arteries, and the presence of cardioembolic, hypercoagulable, or other etiologic mechanisms.
Patients were also classified as exhibiting lacunar or nonlacunar clinical syndromes. Patients were considered to have a lacunar clinical syndromes if they presented with pure motor hemiparesis, pure sensory stroke, sensorimotor stroke, dyarthria clumsy hand, or ataxic-hemiparesis, using the classification algorithm of the Oxfordshire Community Stroke Project (OCSP).3
All patients underwent control CT or MR images approximately 24 hours after t-PA therapy, and earlier imaging if clinical deterioration occurred. All under 36 hour scans were reviewed for the presence of hemorrhagic transformation. Post-lytic radiologic hemorrhagic transformation within the ischemic field was classified as hemorrhagic infarct (HI)-1, HI-2, parenchymal hematoma (PH)-1 and PH-2 using standard criteria.4 Symptomatic ICH was defined, using the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SIST-MOST) criteria, as the occurrence of an increase in the National Institutes of Health Stroke Scale (NIHSS) score of 4 or more points in the setting of local or remote PH2.5, 6
Image interpretation determining the presence of SPA and non-SPA infarcts was done by an experienced stroke neurologist (S.J.L), blinded to clinical data except for the side of clinically suspected ischemia. Lesion diameter ≤ or > 1.5 cm was estimated by DWI when MR images were available. The presence of HT on follow-up CT and MRI scans was rated by a stroke neurologist and a neuroradiologist. Interobserver agreement about the presence of HT was assessed using the κ statistic. Disagreements were resolved by a consensus discussion. For statistical analyses, we compared the mean between groups using the 2-tailed t test for normally distributed variables and Mann-Whitney U test for non-normally distributed variables. Normality was evaluated with the Kolmogorov-Smirnov test. Patient groups were compared by contingency tables for categorical variables with use of a χ2 test and Fisher’s exact test if the numbers of expected frequencies in each cell of the contingency table were more than five. P values <0.05 were considered statistically significant. As at least one candidate predictor variable had zero values, stepwise multivariate logistic analysis was performed using Monte Carlo simulation and then exact permutational methods to estimate model parameters (LogXact program, version 8, Cytel Inc, Cambridge Mass, 2008), with variable retention for p<0.15.
Results
Among 134 patients treated with intravenous t-PA during the study period, 93 met study entry criteria. The other 41 patients were excluded because they were treated with endovascular recanalization therapies following intravenous t-PA, including clot retrieval (n=26), intra-arterial fibrinolysis (n=12) and angioplasty with stent (n=3). Among the 93 patients treated with intravenous t-PA alone, mean±SD age was 71.5±15.8 years (range, 23 – 91) and 62.4% were female. The median NIHSS score before administration of intravenous t-PA was 14.0 [interquartile range, 7 – 21]. The median last known well to treatment time was 150 minutes (range, 50 – 180). Demographic data, risk factors profiles, and initial laboratory data for these patients are shown in supplemental Table available online at http://stroke.ahajournals.org.
Imaging performed prior to intravenous t-PA infusion included: noncontrast CT only in 58 patients, CT perfused blood volume imaging in 3, diffusion and perfusion MRI without CT in 22, and both noncontrast CT and diffusion and perfusion MR sequences in 10. Post-lytic follow-up imaging included CT only in 17 patients, MR only in 8, and both CT and MR in 68.
Of the 93 patients, 88 had acute ischemic changes visualized on imaging, while 5 showed no responsible acute lesion on initial and follow-up CT and/or MR images. Among these 5 patients, 4 showed complete clinical resolution of the symptoms (averted stroke) and the other had marked improvement with mild residual aphasia after thrombolyic therapy. Pretreatment diffusion and perfusion MRI was obtained in 32 patients, permitting assignment to the categories of SPA or non-SPA infarct on the basis of pretreatment imaging alone in all instances. In the remaining 56 cases (60.2%), only noncontrast CTs were performed pretreatment and SPA categorization was made based on post-treatment imaging, generally multimodal MRI performed within the first 72 hours after presentation. Acute ischemic changes confined to a SPA territory were visualized in 13 patients (14.0%). Of the 13 patients, 8 had a SPA infarct in internal capsule, 2 in corona radiata, 2 in thalamus, and 1 in pons. The remaining patients had acute ischemic changes in large artery territories (75, 80.6%) or no visualized ischemic injury (5, 5.4%). In 8 cases, SPA territory infarcts were visualized on pretreatment diffusion/perfusion MRI or perfusion CT. Post-treatment imaging did not alter SPA vs non-SPA categorization in any case undergoing pretreatment multimodal MR or CT.
Lacunar clinical syndromes were present in 23 patients (24.7%), including 10 with sensorimotor stroke, 8 patients with pure motor hemiparesis, 4 with dysarthria clumsy hand syndrome, and 1 with ataxic hemiparesis. Among patients with SPA infarcts, 10 had a clinical lacunar syndrome and 3 nonlacunar. Among non-SPA infarct patients, 66 had a nonlacunar syndrome and 9 a lacunar syndrome. The predictive value of a classical lacunar syndrome for identifying an underlying SPA infarct was sensitivity 76.9% (95% CI 46.2% – 94.7%), specificity 85.3% (95% CI 75.3% – 92.4%), positive predictive value 47.6% (95% CI 25.7% – 70.2%), negative predictive value 95.5% (95% CI 87.5% – 99.1%), and overall accuracy 84.1% (95% CI 76.5% – 91.7%).
Post-lytic radiologic hemorrhagic transformation, ranging from small petechiae to parenchymal hematoma (HI or PH), was found in 22 patients (23.7%; Table 1). Inter-rater agreement for the presence of hemorrhagic transformation was high, κ = 0.94. Symptomatic ICH occurred in 3 patients (3.2%). No patient with imaging-confirmed SPA infarcts experienced any hemorrhagic transformation, including no symptomatic ICH, no HI and no PH; hemorrhagic transformation was observed only in patients with infarcts involving large or multiple artery territories. This difference reached statistical significance for the occurrence of any radiologic hemorrhagic transformation, SPA infarcts 0% (95% CI 0% – 14.5%) versus large artery infarcts 29.2% (95% CI 19.0% –39.6%), p=0.03. In the subgroup of 9 patients who presented with clinical lacunar syndromes but were actually experiencing underlying large artery infarct, any radiologic hemorrhagic transformation occurred in 3 patients (1 HT1 and 2 PH1; Table 2). Remote parenchymal hemorrhages (PHr) outside of the initially ischemic area occurred in 3 patients with non-SPA ischemia, symptomatic n 1 and asymptomatic in 2, all in patients age 90 or older (average age of PHr vs. non-PHr patients, 91.7 vs. 70.9, p = 0.006).
Table 1.
Profiles of radiologic hemorrhagic transformations
| Hemorrhagic Transformations | N (%) | |
|---|---|---|
| Hemorrhagic Infarction (HI) | 10 (10.7%) | HI1: 4, HI2: 6 |
| Parenchymal Hematoma (PH) | 10 (10.7%)* | PH1: 8, PH2: 2 |
| Remote Parenchymal Hematoma (PHr) | 3 (3.2%)* | PHr1: 2, PHr2: 1 |
| SICH | 3 (3.2%) | PH2: 2, PHr2:1 |
SICH (Symptomatic intracerebral hemorrhage): NIHSS clinical deterioration of 4 points or more in the setting of local or remote PH2.
One patient with both PH1 and PHr1 is counted in both rows.
Table 2.
Classification of stroke subtypes and development of hemorrhagic transformations
| HT | LCS | non-LCS | SPA infarct | non-SPA infarct |
|---|---|---|---|---|
| n (%) | n=23 | n=70 | n=13 | n=75 |
| Any HT | 3 (13.0) | 19 (27.1) | *0 | *22 (29.3) |
| HI | 1 (4.3) | 9 (12.9) | 0 | 10 (13.3) |
| PH | 2 (8.7) | 10 (14.3) | 0 | 12 (16.0) |
| SICH | 0 | 3 (4.8) | 0 | 3 (4.0) |
HT, hemorrhagic transformation; HI, hemorrhagic infarct; PH, parenchymal hematoma; SICH, symptomatic intracerebral hemorrhage; LCS, lacunar clinical syndrome; SPA, single penetrator artery. Values are number of patients (%) if not indicated
Among 19 candidate predictor variables, in univariate analysis, SPA infarct and NIHSS score were the variables most strongly associated with reduced risk of hemorrhagic transformation (supplemental Table available online at http://stroke.ahajournals.org). On multivariate analysis, four variables were selected for inclusion in the predictive model for the occurrence of any HT, with higher NIHSS score (OR 1.07, 95% CI 0.99–1.15) and higher SBP (OR 1.03, 95% CI 1.00–1.06) increasing HT risk and presence of SPA infarct (OR 0.17, 95% CI upper limit 1.17) and prior stroke history (OR 0.19, 95% CI 0.12–1.18) decreasing HT risk.
Clinical outcome at discharge varied among patients with different stroke subtypes, as shown in Figure.
Figure.
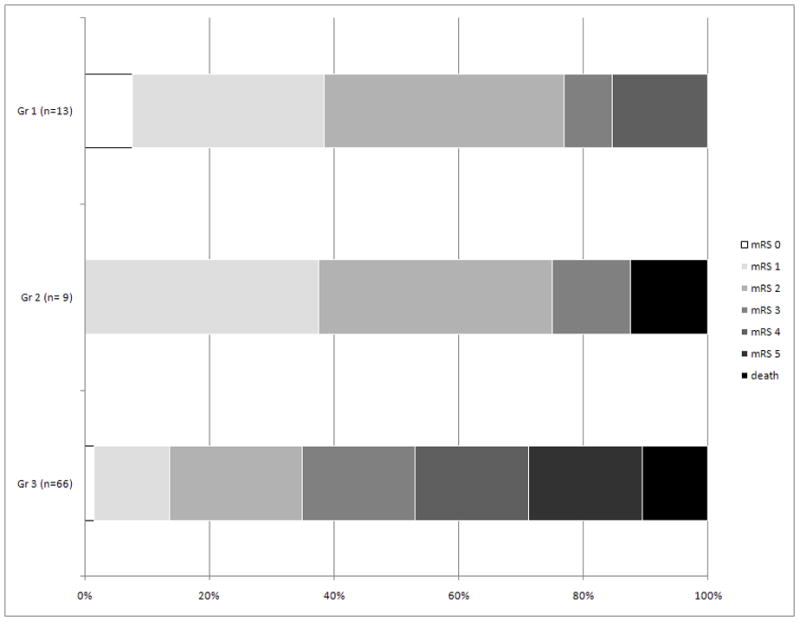
Modified Rankin Scale at discharge among 3 patient groups: Group 1) SPA infarcts, Group 2) non-SPA infarcts with LCS, and 3) non-SPA infarcts with non-LCS
SPA, single penetrator artery; LCS, lacunar clinical syndrome
Discussion
This study confirms that patients with imaging-verified single penetrator artery ischemia are at low risk of hemorrhagic transformation after intravenous thrombolysis. No instances of even minor petechial hemorrhage were observed among the SPA infarct patients in this series. Multivariate analysis suggested that imaging-confirmed SPA ischemia was an independent predictor of reduced risk of hemorrhagic transformation.
No previous investigation has investigated the subset of patients with imaging-confirmed lacunar infarcts. Prior studies have analyzed the frequency of hemorrhagic transformation in patients with clinical lacunar syndromes, but a substantial proportion of these patients actually harbor underlying large artery infarcts. In our series, the false positive rate for clinical lacunar syndromes in predicting underlying SPA ischemia was 52%. This frequency accords with prior studies. In the multicenter TOAST trial and the population-based NOMASS study, false positive rates of 34% and 25% were noted.7, 8 The false positive rate may be higher among patients undergoing lytic therapy, both because of the brevity of the clinical evaluation before treatment and the requirement that patients have a potentially disabling deficit, resulting in a high frequency of semsorimotor and pure motor syndromes and reduced frequency of pure sensory and dysarthria-clumsy hand syndromes. The first ECASS trial showed that clinical presentation as lacunar syndrome within a few hours of stroke onset corresponded to lacunar infarcts in less than one-third of patients.9 In another study among patients with pure motor hemiparesis and sensorimotor syndromes evaluated within 12 to 24 hours from stroke onset, only half had lacunar infarcts.10
Our study findings accord with those of prior studies that investigated hemorrhagic transformation rates after intravenous thrombolysis for patients with lacunar clinical syndromes. The rate of any radiologic hemorrhagic transformation in patients with lacunar clinical syndrome in prior studies has ranged from 6.9%11 to 9.1%12, comparable to the rate of 13.0% in the current series. Our findings suggest that the hemorrhages in these patients likely cluster in the subset of patients with lacunar clinical syndrome but actual underlying large artery ischemia. In our study, any radiologic HT occurred in 3 of 10 of such patients versus none of 13 with genuine single penetrating artery territory ischemia.
Several studies have identified volume of ischemically compromised tissue as a critical risk determinant for HT after intravenous thrombolysis. Early ischemic changes on CT were independently associated with an increased risk of symptomatic hemorrhagic transformation in the two NINDS-tPA trials.13 In a large series, the volume of early CT change, indexed by lower Alberta Stroke Program Early CT Score (ASPECTS), was associated with increased risk of symptomatic ICH in patients treated with intravenous t-PA.14 In an MRI study, the rate of symptomatic ICH increased with increasing DWI lesion volume, from 2% with small lesions (< 10 ml) to 12 to 16% with lesions exceeding 100 ml.1 Imaging-confirmed SPA infarcts have small lesion volumes, no more than 3.8 cc (1.53=3.75). However, we don’t know whether a lacunar stroke was an independent variable in the reduced hemorrhagic risk after thrombolysis or whether this finding is purely secondary to the small volume of lacunar strokes. Of 13 patients with SPA infarct 9 patients had small vessel occlusion (lacunar stroke) based on TOAST classification. The number of included patients is too small to demonstrate the question.
As the risk of bleeding into an ischemic field varies directly with volume of injured tissue, ischemic lesions confined to a single penetrating artery are at a low risk of hemorrhagic transformation. The risk of bleeding outside the ischemic field is low, but nonzero, and may be higher in the oldest old (age 85 and higher), who may have a greater burden of subclinical amyloid angiopathy.
Increasingly in academic and advanced community practice, thrombolytic treatment decisions are made based on using multimodal CT and MRI imaging, permitting direct imaging delineation of the site and size of the ischemic lesion and the presence or absence of a large artery occlusion. Our results suggest that imaging-based recognition of patients harboring SPA infarcts is a better guide than classical lacunar clinical syndromes in identifying patients at very low risk of hemorrhagic transformation after intravenous lytic therapy.
This study has limitations. The sample size was modest. Confirmation of our findings in larger datasets is desirable. We excluded from analysis patients who underwent endovascular interventions after intravenous thrombolysis. This exclusion was required to avoid attributing to intravenous t-PA hemorrhagic transformations actually related to the endovascular interventions. This exclusion could lead to an underestimation of the rate of HT in non-SPA patients. However, since patients with SPA infarcts are not candidates for endovascular therapy, this exclusion does not affect the SPA patient analysis. We used infarct topography -“distribution in a single penetrator territory” - as part of the operational definition of single penetrator infarcts. This approach allows the exclusion of lesions that are small and deep but not confined to a standard penetrator field, such as small pontine lesions that cross the midline or juxtaposed thalamic and internal capsule infarcts. However, even with typical penetrator territory infarcts, we cannot rule out that some cases occurred in the distribution of two adjacent, very small penetrators, rather than one penetrator. The requirement that infarct diameter not exceed 1.5 cm would be expected to make such occurrences uncommon.
A potential source of bias in adjudicating the presence of HT was that the same post-treatment scans used to ascertain HT were also one source of information regarding classifying cases as SPA vs non-SPA. Several factors worked to mitigate this bias, though not completely remove it, including that different sequences on MR scans are the primary source of information on HT vs infarct topography, a different reader rated the scans independently for SPA vs non-SPA classification than the readers rating for the presence of HT, and that in patients with pretreatment MRIs, pretreatment scan assessments of SPA topography assignment concurred with those obtained on post-treatment scans. A related source of potential bias is that, if TPA worked to reduce the size of final infarcts, it could have led to some cases of non-SPA ischemia being classified as SPA-ischemia. This concern is attenuated by the fact that no such instance was observed in the cases with pretreatment diffusion MR of CT perfusion imaging. However, confirmation in a larger series with multimodal pretreatment imaging is needed. A final limitation is that we could not tease out whether small ischemic lesion volume fully accounts for the lower HT risk of SPA infarcts, of if other factors contribute, as pretreatment diffusion MRI or perfusion CT assessments of ischemia lesion size were not obtained in all cases.
In conclusion, hemorrhagic transformation after lytic therapy in imaging-confirmed single penetrator artery infarcts is uncommon. Imaging demonstration of ischemia confined to single penetrator artery territory better identifies this population at low risk of the hemorrhagic complications than classical clinical lacunar syndromes.
Supplementary Material
Acknowledgments
Funding
This work was supported by a grant (A060171) of the Korea Health 21 R&D project, Ministry of Health, Welfare and Family Affairs, Republic of Korea (S.J. Lee) and NIH-NINDS Award P50 NS044378 (J.L. Saver).
We thank Jeffrey Gornbein, PHD, for expert statistical consultation.
Footnotes
Statement of substantial contributions to the intellectual content of the paper
Study concept and design (SJL, JLS, and SS); acquisition of data (JLS, DSL, LA, BO, DK, PV, SS, MF, and MT); analysis and interpretation of data (SJL, JLS, and SS); statistical analysis (SJL and JLS);drafting of the manuscript (SJL); critical revision of the manuscript for important intellectual content (JLS, DSL, LA, BO, DK, MF, MT, PV, and SS).
Conflicts of interest/Disclosures
JLS is an employee of the University of California, which holds a patent on retriever devices for stroke; is a scientific consultant regarding trial design and conduct to CoAxia, Concentric Medical, Talecris, Ferrer, AGA Medical, BrainsGate, PhotoThera, and Cygnis (all modest); has received lecture honoraria from Ferrer and Boehringer Ingelheim (modest); received devices for use in an NIH multicenter clinical trial from Concentric Medical (modest); has declined consulting/honoraria monies from Genentech since 2002; has declined serving as a medicolegal expert in TPA litigation since 2002; is a site investigator in multicenter trials sponsored by AGA Medical, Vernalis, Paion, Lundbeck, and Neurobiological Technologies for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is a site investigator in the NIH IRIS, CLEAR, and IMS 3 multicenter clinical trials for which the UC Regents receive payments based on the clinical trial contracts for the number of subjects enrolled; administers stroke thrombolytic therapy in his practice (<5% of effort); and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364.
SS is an employee of the University of California, which holds a patent on retriever devices for stroke; has been a site investigator in multicenter trials sponsored by Vernalis, Paion, Lundbeck, and Neurobiological Technologies for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is a site investigator in a multicenter registry run by Concentric for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is a site investigator in the NIH CLEAR and IMS 3 multicenter clinical trials for which the UC Regents receive payments based on the clinical trial contracts for the number of subjects enrolled; administers stroke thrombolytic therapy in his practice (<5% of effort); has served as a medicolegal expert in acute stroke litigation; and is funded by NIH-NINDS Awards P50 NS044378 and U01 NS 44364.
DSL is an employee of the University of California, which holds a patent on retriever devices for stroke; is a scientific consultant regarding trial design and conduct to CoAxia(modest) and Concentric Medical (modest).
LA, BO, DK, PV, MF, MT, and JG are employees of the University of California, which holds a patent on retriever devices for stroke.
References
- 1.Singer OC, Humpich MC, Fiehler J, Albers GW, Lansberg MG, Kastrup A, Rovira A, Liebeskind DS, Gass A, Rosso C, Derex L, Kim JS, Neumann-Haefelin T. Risk for symptomatic intracerebral hemorrhage after thrombolysis assessed by diffusion-weighted magnetic resonance imaging. Ann Neurol. 2008;63:52–60. doi: 10.1002/ana.21222. [DOI] [PubMed] [Google Scholar]
- 2.Singer OC, Kurre W, Humpich MC, Lorenz MW, Kastrup A, Liebeskind DS, Thomalla G, Fiehler J, Berkefeld J, Neumann-Haefelin T. Risk assessment of symptomatic intracerebral hemorrhage after thrombolysis using DWI-ASPECTS. Stroke. 2009;40:2743–2748. doi: 10.1161/STROKEAHA.109.550111. [DOI] [PubMed] [Google Scholar]
- 3.Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-o. [DOI] [PubMed] [Google Scholar]
- 4.Larrue V, von Kummer R, Muller A, Bluhmki E. Risk factors for severe hemorrhagic transformation in ischemic stroke patients treated with recombinant tissue plasminogen activator: a secondary analysis of the European-Australasian Acute Stroke Study (ECASS II) Stroke. 2001;32:438–441. doi: 10.1161/01.str.32.2.438. [DOI] [PubMed] [Google Scholar]
- 5.Wahlgren N, Ahmed N, Davalos A, Ford GA, Grond M, Hacke W, Hennerici MG, Kaste M, Kuelkens S, Larrue V, Lees KR, Roine RO, Soinne L, Toni D, Vanhooren G. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SIST-MOST): an observational study. Lancet. 2007;369:275–282. doi: 10.1016/S0140-6736(07)60149-4. [DOI] [PubMed] [Google Scholar]
- 6.Berger C, Fiorelli M, Steiner T, Schabitz W-R, Bozzao L, Bluhmki E, Hacke W, von Kummer R. Hemorrhagic transformation of ischemic brain tissue: Asymptomatic or symptomatic? Stroke. 2001;32:1330–1335. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 7.Madden KP, Karanjia PN, Adams HP, Jr, Clarke WR. Accuracy of initial stroke subtype diagnosis in the TOAST study. Trial of ORG 10172 in acute stroke treatment. Neurology. 1995;45:1975–1979. doi: 10.1212/wnl.45.11.1975. [DOI] [PubMed] [Google Scholar]
- 8.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the Northern Manhattan Stroke Study experience. Neurology. 1997;48:1204–1211. doi: 10.1212/wnl.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 9.Toni D, Iweins F, von Kummer R, Busse O, Bogousslavsky J, Falcou A, Lesaffre E, Lenzi GL. Identification of lacunar infarcts before thrombolysis in the ECASS I study. Neurology. 2000;54:684–688. doi: 10.1212/wnl.54.3.684. [DOI] [PubMed] [Google Scholar]
- 10.Toni D, Del Duca R, Fiorelli M, Sacchetti ML, Bastianello S, Giubilei F, Martinazzo C, Argentino C. Pure motor hemiparesis and sensorimotor stroke. Accuracy of very early clinical diagnosis of lacunar strokes. Stroke. 1994;25:92–96. doi: 10.1161/01.str.25.1.92. [DOI] [PubMed] [Google Scholar]
- 11.Cocho D, Belvis R, Marti-Fabregas J, Bravo Y, Aleu A, Pagonabarraga J, Molina-Porcel L, Diaz-Manera J, San Roman L, Martinez-Lage M, Martinez A, Moreno M, Marti-Vilalta JL. Does thrombolysis benefit patients with lacunar syndrome? Eur Neurol. 2006;55:70–73. doi: 10.1159/000091982. [DOI] [PubMed] [Google Scholar]
- 12.Hwang YH, Seo JG, Lee HW, Park SP, Suh CK. Early neurological deterioration following intravenous recombinant tissue plasminogen activator therapy in patients with acute lacunar stroke. Cerebrovasc Dis. 2008;26:355–359. doi: 10.1159/000151638. [DOI] [PubMed] [Google Scholar]
- 13.The NINDS t-PA Stroke Study Group. Intracerebral hemorrhage after intravenous t-PA therapy for ischemic stroke. Stroke. 1997;28:2109–2118. doi: 10.1161/01.str.28.11.2109. [DOI] [PubMed] [Google Scholar]
- 14.Barber PA, Demchuk AM, Zhang J, Buchan AM ASPECT study group. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


