Abstract
Background and Purpose
Notch receptors (1-4) are membrane proteins that, upon ligand stilumation, release their cytoplasmic domains to serve as transcription factors. Notch-2 promotes proliferation both during development and cancer but its role in response to ischemic injury is less well understood. The purpose of this study was to understand whether Notch-2 is induced following neonatal stroke and to investigate its functional relevance.
Methods
P12 CD1 mice were subjected to permanent unilateral (right-sided) double ligation of the common carotid artery.
Results
Neonatal ischemia induces a progressive brain injury with prolonged apoptosis and Notch-2 up-regulation. Notch-2 expression was induced shortly after injury in hippocampal areas with elevated c-fos activation and increased cell death. Long-term induction of Notch-2 also occurred in CA1 and CA3 in and around areas of cell death, and had a distinct pattern of expression as compared to Notch-1. In vitro OGD treatment showed a similar increase in Notch-2 in apoptotic cells. In vitro gain of function experiments, utilizing an active form of Notch-2, show that Notch-2 induction is neurotoxic to a comparable extent as OGD treatment.
Conclusions
These results suggest that Notch-2 up-regulation following neonatal ischemia is detrimental to neuronal survival.
Keywords: Neonatal stroke, hippocampus, apoptosis, c-fos, Notch-2
Introduction
Neonatal stroke impacts roughly 1 per 4000 births and frequently can cause severe neuropathological deficits in humans, including acute seizures, epilepsy, cerebral palsy, and learning disabilities.1-5 Neonatal models of brain ischemia have demonstrated that most agents must be given before, during, or soon after the injury to be neuroprotective.6, 7 Therefore, understanding the molecular basis of the sub-acute and chronic evolution of the injury, in a clinically relevant protocol, will be key to the development of new strategies to optimize recovery.
Previous studies showed that Notch signaling is induced after cerebral ischemia in the adult brain in two distinct cell populations: in cortical neurons, where it affected neuronal survival, 8 and in the progenitors of the subventricular zone (SVZ) of the cerebral hemispheres 9 and subgranular zone (SGZ) of the dentate gyrus, 10 where it induced progenitor proliferation. Additionally, in rats subjected to the pro-convulsant kainic acid, Notch-2 expression levels were shown to be elevated in the granule cell layer of the hippocampus.11 Considering the pleiotropic functions of the Notch signaling pathway, understanding how neonatal stroke affects the Notch cascade is potentially of great interest both with respect to neuroprotection and neuroregeneration strategies.
Here we report that following neonatal ischemic injury 1) Notch-2 activity and expression is induced in the ipsilateral injured-hippocampii, in areas of ongoing post-stroke apoptosis and c-fos expression and 2) Notch-2 activation following OGD damage in vitro may be neurotoxic.
Materials & Methods
Unilateral carotid ligation
All research was approved by the Johns Hopkins University School of Medicine Animal Care and Use Committee (ACUC). All litters were derived from the transgenic TNR mouse line.12 This mouse line is on a CD1 background and is phenotypically comparable to wild-type CD1 mice. Litters of P12 pups were bred at the JHU animal facility and housed in polycarbonate cages on a 12 h light dark cycle; food was provided ad libitum. On P12, mice of both genders received permanent unilateral (right-sided) double ligation of the common carotid artery under isofluorane anesthesia or sham surgery as previously described.13, 14. Seizure activity was scored during the 4 hours following injury as previously described in this model.13
Histological preparation
The brains were perfused with 4% PFA and cryoprotected by sequential immersion in 15% and 30% sucrose for 24 hours each. Coronal brain sections 20 μm thick were cut on a cryostat in serial order to create 10 series of sections that were mounted on super frost plus glass slides and stored at -20 °C
In vitro transfection, DNA constructs
High efficiency Ca2+-phosphate transfection was carried out on 10 days in vitro (d.i.v.) neuronal cultures as previously described.15 Co-transfections of pCAG-GFP and pCLEN2 (Notch-2 intracellular domain, NICD2, CDS: 5350-6684 cloned into pCLE), or pCLE 16 alone as control, were carried out. Transfection efficiency was evaluated by GFP expression and alkaline phosphatase immunostaining was performed on randomized samples within each experiment (a ratio 3:1, pCLE or pCLEN2 to pCAG-GFP, gave a 100% co-labeling).
Oxygen glucose deprivation (OGD) and cell-death scoring
OGD was performed on 12 d.i.v. neuronal cultures as previously described.17 Neuronal death was assessed 6 hours after OGD treatment and determined by nucleus condensation/fragmentation after staining with 1 μg/ml of DAPI (Roche). Dishes were counted by an investigator blinded to the experimental condition. Percent cell-death per dish was calculated as follows: [Number of dead GFP+ cells/ Total number of GFP+ cells] × 100]. Average percent cell-death was then calculated for each condition (n=8 per condition).
Antibodies
Antibodies used to detect Notch-2 were rabbit anti-Notch-2 (intracellular portion, 1:500 Abcam; for immunohistochemistry on sections), goat anti-Notch-2 (1:500, Santa Cruz; for western blot and immunohistochemistry in cell culture), rabbit anti-Notch-1 (1:500 Abcam), rabbit anti-c-fos (1:20,000 Calbiochem), mouse anti-GFAP (1:500, Chemicon), rabbit anti-cleaved caspase-3 (1:1000, Cell Signaling), sheep anti-PLAP (1:1000, American Research Product), mouse anti-Arc/Arg 3.1 (1:1000, gift Worley P.), and mouse anti-β-actin (1:5000, Sigma).
Immunohistochemistry
20 μm thick coronal brain sections and neuronal cultures were fixed in 4% PFA, and post-fixed in ice-cold acetone-methanol (1:1) at −20°C for 10 minutes. The immunostainings with anti-Notch and anti-c-fos antibodies on sections were performed according to the instructions included in the TSA fluorescence amplification kit (Perkin Elmer). For all other applications, primary antibodies were visualized with directly conjugated donkey secondary antibodies (Alexa 488, Alexa 555, Alexa 647, Invitrogen). TUNEL labeling was carried out using DeadEnd™ Fluorimetric TUNEL System (Promega) according to the manufacturer's instructions. Nuclei were counterstained with 1 μg/ml DAPI (Roche). Images were taken using a Zeiss Axioscope microscope connected to an Axiocam, or Zeiss confocal LSM 510. All Images were processed using Adobe Photoshop.
Western Blot analysis
Neuronal cultures were washed in ice cold PBS and harvested using RIPA buffer, and protein concentrations were determined using the BCA method (BioRad). Protein samples were subjected to denaturing SDS-PAGE and then transferred from the gel to an Immuno-Blot PVDF membrane (BioRad). Membranes were probed with primary antibodies and HRP-conjugated secondary antibodies. A chemiluminescent substrate (ECL+, GE Amersham) and film were used to visualize the HRP signal.
Computerized brain asymmetry analysis
Fixed cresyl violet stained mouse brain slices, photographed after calibration using an AxioCamcolor camera and AxioVision 2.05 software, were measured using MCID 7.0 Elite (InterFocusImaging Ltd; Cambridge,UK). Brain asymmetry scores were measured as previously described.14
Fluorescent image analysis
TUNEL labeled cells were counted from images, acquired with a 20× objective from three consecutive sections per animal. C-fos and Notch-2 immunolabeling were quantified, using ImageJ-software (NIH), as pixel counts (pixels were fitted to represent positive c-fos cells) and area fraction, respectively, on images (20× objective) from 3 consecutive sections per animal. Data were normalized to control condition fluorescence. C-fos pixel counts and area fraction of Notch-2 measurements were sampled over the entire picture area, of constant resolution.
Data analysis
ANOVA was used to calculate variance among animals for a given time point, Student's T-test was then used for comparisons between the ipsilateral hemisphere from the ligation-injured group and from the sham group. Correlation analysis was performed between the acute seizure score and the extent of injury. A linear relationship was considered r2> 0.5. For the Student's T-test and correlation analysis a probability below 0.05 was considered significant.
Results
Time-course of Injury and apoptosis following neonatal ischemia
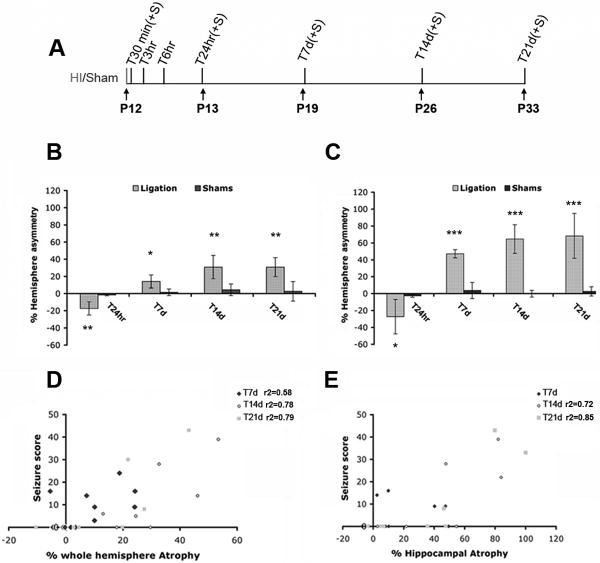
Prior to the 24 hour time-point, neither edema nor evidence of injury was grossly or microscopically visible by cresyl violet (Nissl) staining. 24 hours following unilateral carotid ligation however, edema was detectable in the ipsilateral hippocampus and cortex in 80% of the animals (n=8/10, from 2 litters). Average ipsilateral hemispherical and hippocampal asymmetry scores were significantly different from shams starting from T24hr after ligation (Figure 1, panels B and C). Additionally, starting at T6hr and continuing at T24hr after ligation, we observed an increase in GFAP positive astrocytes in the ipsilateral hemisphere (Figure 4, panels B′ and C′, DG is shown). Hemispheric asymmetry analysis revealed that 7 days after injury there was a decrease in volume of the injured hemisphere and hippocampus as compared with shams (Figure 1, panels B and C). The mean hemispheric and hippocampal volumes progressively decreased over the second week following ligation (P26) as compared with shams and stabilized 21 days after ligation (P33; Figure 1, panels B and C).
Figure 1. Experimental layout of perinatal ischemia in P12 mice, time-course of injury, and correlation of the acute seizure score with the extent of injury following stroke.
(A) For the acute analysis we selected T30 min, T3hr, T6hr and T24hr to detect early molecular changes. T7days, T14days and T21 days were used to monitor the long term effects of neonatal stroke. (For each time-point we utilized n=10 ligated mice. n=4 shams were used at the time points indicated with +S). (B and C) Mean ipsilateral percent hemispheric (B) and hippocampal (C) asymmetry, compared to shams for each time-point analyzed, shows a negative value at T24hr, representing swelling, followed by a progressive brain atrophy following ischemia (see positive percent asymmetry at T7d-T21d). (*: p<0.05, ** : p<0.01, *** : p< 0.001). (D) Plots representing the correlations between acute seizure scores and percent hemispheric atrophy for animals sacrificed at T7d (P19) (r2=0.58, p=0.01), T14d (P26) (r2=0.78, p<0.01) and T21d (P33) (r2=0.79, p<0.01) after ligation. (E) Plots representing the correlations between acute seizure score and severity of hippocampal atrophy at T7d (P19, no linear correlation), T14d (P26; r2=0.72, p=0.01) and at T21d (P33; r2=0.85, p<0.01) following stroke at P12.
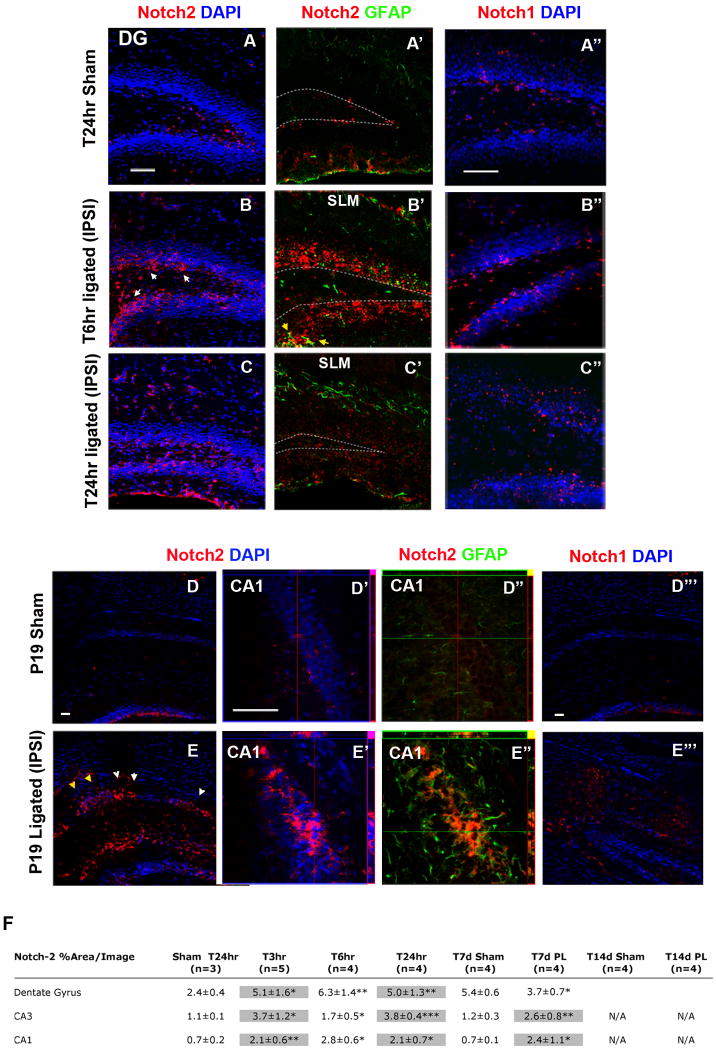
Figure 4. Notch-2 receptor expression and activity in the ipslateral hippocampus, acutely and 7 days following ischemic injury at P12.
(A-A″) Sets of double immunohistochemistry for Notch-2 and Notch-1 show that at T24hr after sham surgery, Notch-2 and Notch-1 expression is restricted to the SGZ. (B-B′) At T6hr after stroke, Notch-2 expression extends to the granule cell layer (GCL) of ipsilateral DG (white arrows). In addition, Notch-2 is expressed only by scattered GFAP+ positive putative astroglia (yellow arrows in B″). (B″) Notch-1 at this time remains restricted to the SGZ. (C-C′) Subsequently, at T24hr after ligation, Notch-2 expression persists ipsilaterally in some cells of the GCL and in the soma of putative astrocytes, invading the stratum lacunosum moleculare (SLM). (C″) Notch-1 expression appears punctuate in the granule cell layer of the DG. (E-E′) 7 days after injury, at P19, Notch-2 is strongly increased as compared to sham control (D-D″) in ectopic niches in the ipsilateral CA1 in and around areas with elevated c-fos expression (yellow arrows, and see Fig. 3, panel A). Orthogonal views of stacked images reveal that Notch-2 is expressed in the granule cell layer in and around condensed nuclei. (E″) Double labeling with Notch-2 and GFAP staining shows that Notch-2 and GFAP expression only modestly co-localize. (E‴) At T7d Notch-1 is only moderately expressed in invading glia and in the granule cell layer. (F) Table summarizing the time course of Notch-2 over-expression in hippocampal region CA1, CA3 and DG. Highlighted boxes indicate regions with elevated apoptosis and c-fos expression. (All scale bars are 50μm; *: p<0.05, ** : p<0.01, *** : p< 0.001).
The acute seizure score (n=38 out 59 ligated animals seized) correlated with the percentage of whole hemisphere asymmetry at T7d, T14d, and T21d (Figure 1, panel D). A strong correlation was also noted between the acute seizure score and the percentage of hippocampal atrophy at T14d and at T21d (Figure 1, panel E).
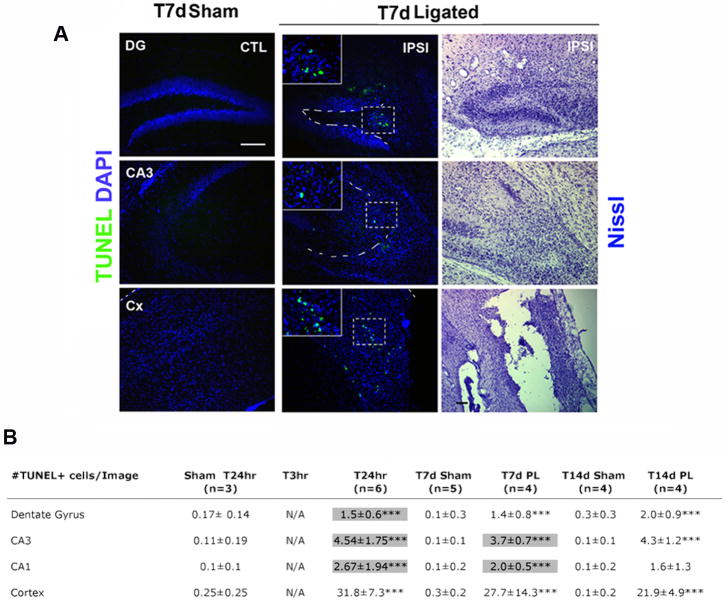
Underlying the progressive cell demise after neonatal ischemia we observed that the number of TUNEL-positive cells significantly increased 24hr after injury in the ipsilateral dorso-medial cortex (cortex), ipsilateral dentate gyrus (DG), CA3, and CA1 regions (Figure 2, table B). Seven days after neonatal stroke (P19), increased apoptosis was still ongoing in injured areas of the ipsilateral hippocampus and in the cortex (Figure 2, panel A and table B). Fourteen days (P26) and twenty-one days (P33) after ligation TUNEL-positive cells were still present but were progressively reduced and localized predominantly in the ipsilateral cortical cyst (Figure 2, table B).
Figure 2. Apoptosis is apparent in ipsilateral hippocampus and neocortex, at acute and later time-points, after ischemic injury at P12.
(A) Tunel /DAPI double-labeling and cresyl violet staining on sections at T7d from sham or ligated mice. At P19, TUNEL (green) and DAPI (blue) shows that in sham and contralateral (CTL) DG, CA3, and dorso-medial cortex there are no apoptotic cells present, whereas in the respective ipsilateral regions several TUNEL-positive cells are visible (see inserts). Cresyl violet staining of the ipsilateral side reveals also that apoptosis occurs in regions with extensive injury and cell demise. (B) Table summarizing the temporal emergence of TUNEL positive cells in the DG, CA3, CA1 regions and dorsolateral cortex. Highlighted boxes indicate regions with elevated c-fos and Notch-2 expression. (Scale bars in A are 50μm; *: p<0.05, ** : p<0.01, *** : p< 0.001).
C-fos expression in the hippocampus acutely and chronically after ischemia
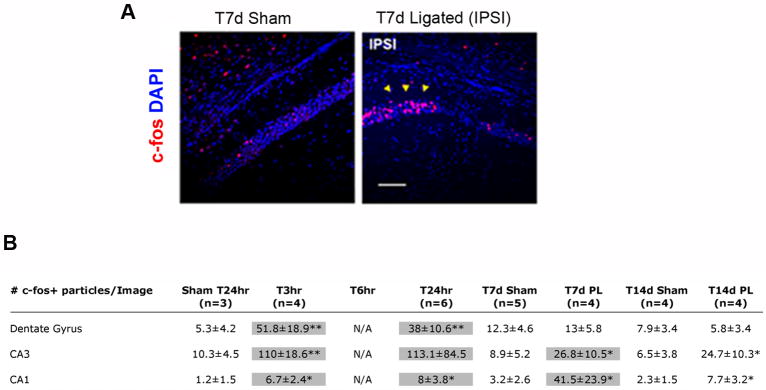
For all the data points and regions analyzed, expression of c-fos protein in the contralateral hemisphere from injured animals was low and comparable to sham animals; for this reason only sham control data are shown (Figure 3, panel A and table B). At T3hr in ligated pups that seized (n=4/4 mice), c-fos expression was significantly increased in the ipsilateral DG, CA3 and CA1 areas (Figure 3, table B). At T24hr c-fos expression was intense in scattered cells in the ipsilateral hippocampus, and neocortex (n= 6/10; Figure 3, table B). Analysis at later time-points revealed that at P19, 83% of the mice with acute post-stroke seizures (n=5/6 mice) had ectopic c-fos expression in ipsilateral CA1 regions adjacent to niches of cell-death (yellow arrows Figure 3, panel A and table B). Fourteen days after ligation, in 100% of the animals with acute post-stroke seizures (n=4/4 mice), c-fos expression was still elevated in scattered pyramidal neurons of the injured hippocampus (Figure 3, table B).
Figure 3. Time course of c-fos expression in ipsilateral hippocampus at acute and later time-points after ischemic injury at P12.
(A) C-fos immunolabeling of T7d (P19) mouse coronal sections reveals that in the ipsilateral hippocampus c-fos expression is ectopically induced in CA1 regions neighboring areas of cell-death (yellow arrows on c-fos/DAPI panel), as compared to P19 sham controls. (B) Table summarizing the time course of appearance of c-fos immunoreactive cells in the hippocampal regions CA1, CA3 and DG. Highlighted boxes indicate regions with elevated apoptosis and Notch-2 expression. (Scale bar in A is 50μm; *: p<0.05, ** : p<0.01, *** : p< 0.001).
Notch-2 is ectopically up-regulated in the hippocampus after neonatal stroke
In control perinatal brains, and contralateral hemispheres of ligated brains, at all time-points analyzed, Notch-2 and Notch-1 expression was restricted to the neurogenic zones of the SVZ and the SGZ (Figure 4, panels A-A″ and D-D‴, and not shown). Acutely at T6hr and T24hr following ischemia, in animals with acute post-stroke seizures (n=4/6 mice per group), Notch-2 expression extended to the granule cell layer (GCL) of the DG (Figure 4, panels B to B′ and table F) and co-localized only in few GFAP+ astroglia (Figure 3 panels B′, putative glia are indicated with yellow arrows). Notch-1 expression on the other hand was elevated in the SGZ (Figure 4, panel B″). At T6hr and T24hr after injury, expression of Notch-2 was also significantly elevated in ipsilateral CA3 and CA1 areas (Figure 4, table F). At T24hr, Notch-1 expression appeared punctuate in the granule cell layer of the DG (Figure 4, panel C″).
At P19, mice with acute seizure scores after injury (n=4/6 mice) showed elevated Notch-2 expression in ectopic foci of the ipsilateral CA1 and CA3 regions where apoptotic cells were detected and c-fos expression was also elevated (Figure 4, panels E-E″ white arrows and Table F grey boxes). Double IHC showed that Notch-2 strongly localized in and around areas of cell-death, (Figure 4, panel E′), and only modestly co-localized with GFAP (Figure 4. panel E″). On the other hand Notch-1 expression was mostly localized in glia (Figure 4, panel E‴). Fourteen days after ischemic injury, in the animals that seized (n=4/6 mice) Notch-2 expression was present in reactive glia (data not shown) and despite being reduced, continued to be present in the partially intact ipsilateral SGZ (Figure 4, Table F).
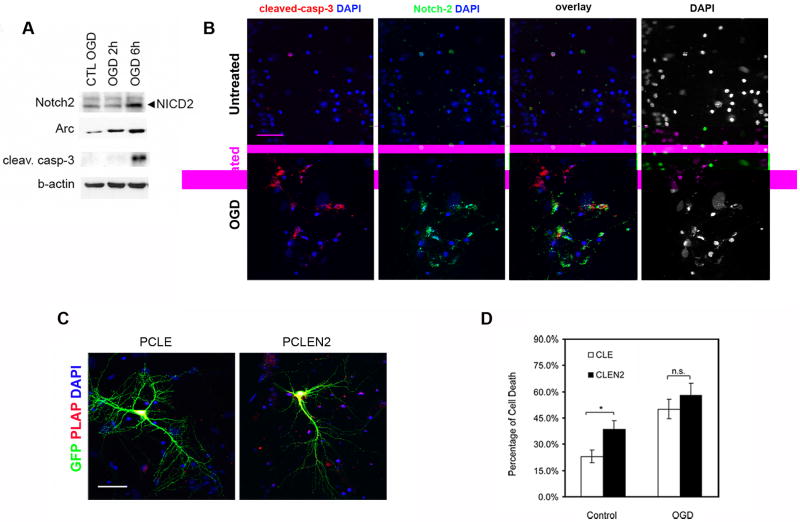
Physiologic Notch-2 expression is critical for neuronal survival
In order to address the effect of Notch-2 expression after hypoxic-ischemic injury, we employed a gain-of-function approach using oxygen glucose deprivation (OGD) in hippocampal primary neuronal cell culture, as an in vitro hypoxia-ischemia model.17 Six hours after OGD Notch-2 processing was induced (NICD2) in the neuronal cultures (Figure 5, Panel A, n=3 experiments), when neuronal activity was elevated (as indicated by the presence of the activity regulated gene Arc/Arg 3.1) and cell-death was ongoing, as shown by the presence of cleaved caspase-3 (Figure 5, Panel A). In addition, 6 hours after OGD, Notch-2 was up-regulated and co-localized with cleaved-caspase-3 in the majority of the cells (Figure 5, Panel B). Transfection with pCLEN2 led to a six-fold increase in Notch-2 expression (data not shown). When we over-expressed NICD2 for 72 hours we observed a significant increase in cell-death in untreated cultures (no OGD) as compared with the control (pCLE) transfected cells (38%±12 versus 23%±9, p<0.05, n=6). Six [when a number starts a sentence it needs to be spelled out] hours after OGD treatment neurons transfected with either pCLE or pCLEN2 had similar levels of cell-death (Figure 5, panel D; n=6). However, interestingly, over-expression of NICD2 in neurons subjected to OGD did not increase cell death compared to control-transfected neurons (Figure 5, Panel D).
Figure 5. Notch-2 over-activation is associated with cell death in vitro.
(A) Western blot from hippocampal neuronal cultures shows that Notch-2 activation, (NICD2, arrow), occurs at 6 hours following OGD when activity, as measured by Arc/Arg 3.1, is elevated and when apoptosis, as measured by the presence of cleaved casp-3, is ongoing. (B) Immunohistochemistry with Notch-2 specific and cleaved casp-3 antibodies show that 6 hours following OGD the level of Notch-2 increases in culture and that the majority of the Notch-2 labeled cells also express cleaved casp-3, a hallmark of apoptosis. DAPI panels visualize condensed nuclei. (C) Immunostaining using a PLAP specific antibody shows that transfected neurons with GFP are also positive for PLAP, indicating the presence of pCLE and pCLEN2. (D) Cell-death scoring on transfected cells indicates that NICD2 over-expression by pCLEN2 is toxic under basal conditions (*: p< 0.05). Under OGD conditions there is an overall increase in cell-death and pCLE and pCLEN2 show similar cell-death scores. (All scale bars are 50μm).
Discussion
As reported in other immature animal hypoxia-ischemia models, the evolution of the neonatal stroke injury is quite prolonged.18, 19 In this model, we observed progressive atrophy in the ipsilateral hemisphere over the three weeks following neonatal stroke at P12. Acutely, the majority of cells died in an environment where edema developed; later, over the following weeks increased apoptosis was visible in the injured hippocampus and neocortex. These results suggest that neonatal stroke has long lasting effects on neuronal viability and supports the existence of a prolonged potential therapeutic-window for alleviating the progression of cell-death after such an injury.
In order to better understand how ischemia affects neuronal activity, we monitored the temporal profile of c-fos expression. In this context, c-fos expression can serve as a marker of several processes, including neuronal hyper-activity following seizure, 20 excessive glutamate response following cerebral ischemia 21 and cell-death.22 A clear up-regulation in c-fos expression was observed 3 hours after stroke in all regions of the ipsilateral hippocampus. The CA3 and CA1 areas are the regions most susceptible to excitotoxic cell-death in the immature brain, 23 whereas the DG remains partially preserved. At one week after ligation (P19), ectopic foci of c-fos expression in CA1 were noted in and around areas of cell-death. At T14d (P26) and T21d (P33) after ligation, when hippocampal atrophy peaked, we observed c-fos expression restricted to scattered pyramidal cells of the ipsilateral hippocampus. This delayed and abnormal c-fos expression can be interpreted as resulting from hyper- or abnormal activity following neonatal ischemia that can contribute to the brain injury, as it has been proposed by others working with rat perinatal models of hypoxia ischemia.24-27
Several recent papers have reported that Notch-1 activation occurs in response to cerebral ischemia in very different cell types: in the germinal zones of the SVZ 9 and SGZ 10 where it has been shown to contribute to the maintenance of the progenitor pool 28, and in the neurons of the cortex 8 where it is thought to contribute to neuronal damage.8 Neonatal mice with acute seizures following double unilateral carotid ligation showed a strong increase in Notch-2 receptor expression in the granule and pyramidal layers of the ipsilateral hippocampus, specifically in regions with ectopic c-fos expression, which subsequently became atrophic. Interestingly Hes5, a conical target of the Notch pathway, was also up-regulated in the hippocampus following ischemia (data not shown), indicating that up-regulation of Notch-2 led to pathway activation.
The widespread induction of Notch-2 in the GCL acutely after neonatal stroke injury, was distinct from Notch-1 which remained largely restricted to the SGZ.10 Interestingly, one week following injury, Notch-1 and Notch-2 still had very different cellular patterns of expression in the ipsilateral hippocampus; Notch-2 was aberrantly increased in injured hippocampal neurons, whereas Notch-1 was localized to reactive glia. This finding suggests a differential role for the two receptors in response to neonatal ischemia.
We have shown that OGD challenge in vitro induced Notch-2 activation in primary hippocampal neurons, similarly to what has been seen with Notch-1 in cortical cultures, 8 and we have shown that most of the cells that had aberrant Notch-2 activation were also positive for the apoptotic marker cleaved-caspase-3. Utilizing a gain of function experiment, we demonstrated that over-expression of the transcriptionally active form of Notch-2 (NICD2) was neurotoxic under basal conditions to a comparable level as after OGD treatment alone. Furthermore, under OGD conditions the control transfected and NICD2 transfected cultures had similar levels of cell-death.
In conclusion this work demonstrates that neonatal ischemia induced by unilateral carotid ligation in P12 mice is a clinically relevant model that produces long lasting anatomical and molecular changes in the hippocampus and cortex. The prolonged and ectopic c-fos expression in regions of ongoing cell death is of particular interest for the possible identification of sites with prolonged abnormal neuronal activity and/or cell demise. Future research using this model may link these sites to the process of post-ischemic epileptogenesis, or alternatively to the focus of new regenerative strategies. In addition, Notch-2 appears to be rapidly and persistently induced in postmitotic neurons by ischemic injury. The work reported here suggests that this aberrant induction of Notch-2 may be neurotoxic. We anticipate that identifying the downstream effectors of Notch-2 after ischemic brain injury could lead to the development of better therapeutic agents, which might help contain the neuronal damage resulting from Notch-2 over-activation.
Acknowledgments
This work was supported by NIH NINDS grants NS052166 and NS061969 to AMC, American Heart Association Predoctoral Award to ZC, and USPHS NS40809 to VLD. We thank K. Schreck for providing the pCLEN2 construct.
References
- 1.Ho SS, Kuzniecky RI, Gilliam F, Faught E, Bebin M, Morawetz R. Congenital porencephaly and hippocampal sclerosis. Clinical features and epileptic spectrum. Neurology. 1997;49:1382–1388. doi: 10.1212/wnl.49.5.1382. [DOI] [PubMed] [Google Scholar]
- 2.Ho SS, Kuzniecky RI, Gilliam F, Faught E, Bebin M, Morawetz R. Congenital porencephaly: Mr features and relationship to hippocampal sclerosis. AJNR Am J Neuroradiol. 1998;19:135–141. [PMC free article] [PubMed] [Google Scholar]
- 3.Burneo JG, Faught E, Knowlton RC, Martin RC, Bebin M, Morawetz R, Kuzniecky R. Temporal lobectomy in congenital porencephaly associated with hippocampal sclerosis. Arch Neurol. 2003;60:830–834. doi: 10.1001/archneur.60.6.830. [DOI] [PubMed] [Google Scholar]
- 4.Koelfen W, Freund M, Varnholt V. Neonatal stroke involving the middle cerebral artery in term infants: Clinical presentation, eeg and imaging studies, and outcome. Dev Med Child Neurol. 1995;37:204–212. doi: 10.1111/j.1469-8749.1995.tb11993.x. [DOI] [PubMed] [Google Scholar]
- 5.Delsing BJ, Catsman-Berrevoets CE, Appel IM. Early prognostic indicators of outcome in ischemic childhood stroke. Pediatr Neurol. 2001;24:283–289. doi: 10.1016/s0887-8994(01)00245-4. [DOI] [PubMed] [Google Scholar]
- 6.Laroia N, McBride L, Baggs R, Guillet R. Dextromethorphan ameliorates effects of neonatal hypoxia on brain morphology and seizure threshold in rats. Brain Res Dev Brain Res. 1997;100:29–34. doi: 10.1016/s0165-3806(97)00018-7. [DOI] [PubMed] [Google Scholar]
- 7.Olsson T, Wieloch T, Smith ML. Brain damage in a mouse model of global cerebral ischemia. Effect of nmda receptor blockade. Brain Res. 2003;982:260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- 8.Arumugam TV, Chan SL, Jo DG, Yilmaz G, Tang SC, Cheng A, Gleichmann M, Okun E, Dixit VD, Chigurupati S, Mughal MR, Ouyang X, Miele L, Magnus T, Poosala S, Granger DN, Mattson MP. Gamma secretase-mediated notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med. 2006;12:621–623. doi: 10.1038/nm1403. [DOI] [PubMed] [Google Scholar]
- 9.Givogri MI, de Planell M, Galbiati F, Superchi D, Gritti A, Vescovi A, de Vellis J, Bongarzone ER. Notch signaling in astrocytes and neuroblasts of the adult subventricular zone in health and after cortical injury. Dev Neurosci. 2006;28:81–91. doi: 10.1159/000090755. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Mao X, Xie L, Greenberg DA, Jin K. Involvement of notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J Cereb Blood Flow Metab. 2009 doi: 10.1038/jcbfm.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari Toninelli G, Bernardi C, Quarto M, Lozza G, Memo M, Grilli M. Long-lasting induction of notch2 in the hippocampus of kainate-treated adult mice. Neuroreport. 2003;14:917–921. doi: 10.1097/01.wnr.0000069962.11849.e6. [DOI] [PubMed] [Google Scholar]
- 12.Mizutani K, Yoon K, Dang L, Tokunaga A, Gaiano N. Differential notch signalling distinguishes neural stem cells from intermediate progenitors. Nature. 2007;449:351–355. doi: 10.1038/nature06090. [DOI] [PubMed] [Google Scholar]
- 13.Comi AM, Weisz CJ, Highet BH, Johnston MV, Wilson MA. A new model of stroke and ischemic seizures in the immature mouse. Pediatr Neurol. 2004;31:254–257. doi: 10.1016/j.pediatrneurol.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 14.Kadam SD, Mulholland JD, McDonald JW, Comi AM. Neurogenesis and neuronal commitment following ischemia in a new mouse model for neonatal stroke. Brain Res. 2008;1208:35–45. doi: 10.1016/j.brainres.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang M, Chen G. High ca2+-phosphate transfection efficiency in low-density neuronal cultures. Nat Protoc. 2006;1:695–700. doi: 10.1038/nprot.2006.86. [DOI] [PubMed] [Google Scholar]
- 16.Gaiano N, Kohtz JD, Turnbull DH, Fishell G. A method for rapid gain-of-function studies in the mouse embryonic nervous system. Nat Neurosci. 1999;2:812–819. doi: 10.1038/12186. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Zulueta M, Feldman AB, Klesse LJ, Kalb RG, Dillman JF, Parada LF, Dawson TM, Dawson VL. Requirement for nitric oxide activation of p21(ras)/extracellular regulated kinase in neuronal ischemic preconditioning. Proc Natl Acad Sci U S A. 2000;97:436–441. doi: 10.1073/pnas.97.1.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston MV, Trescher WH, Ishida A, Nakajima W. Neurobiology of hypoxic-ischemic injury in the developing brain. Pediatr Res. 2001;49:735–741. doi: 10.1203/00006450-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000;20:7994–8004. doi: 10.1523/JNEUROSCI.20-21-07994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng Z, Houser CR. Temporal patterns of fos expression in the dentate gyrus after spontaneous seizures in a mouse model of temporal lobe epilepsy. J Neurosci. 2005;25:7210–7220. doi: 10.1523/JNEUROSCI.0838-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hughes PE, Alexi T, Walton M, Williams CE, Dragunow M, Clark RG, Gluckman PD. Activity and injury-dependent expression of inducible transcription factors, growth factors and apoptosis-related genes within the central nervous system. Prog Neurobiol. 1999;57:421–450. doi: 10.1016/s0301-0082(98)00057-4. [DOI] [PubMed] [Google Scholar]
- 22.Oorschot DE, Black MJ, Rangi F, Scarr E. Is fos protein expressed by dying striatal neurons after immature hypoxic-ischemic brain injury? Exp Neurol. 2000;161:227–233. doi: 10.1006/exnr.1999.7248. [DOI] [PubMed] [Google Scholar]
- 23.Franck JE, Roberts DL. Combined kainate and ischemia produces ‘mesial temporal sclerosis’. Neurosci Lett. 1990;118:159–163. doi: 10.1016/0304-3940(90)90616-h. [DOI] [PubMed] [Google Scholar]
- 24.Williams PA, Dou P, Dudek FE. Epilepsy and synaptic reorganization in a perinatal rat model of hypoxia-ischemia. Epilepsia. 2004;45:1210–1218. doi: 10.1111/j.0013-9580.2004.60403.x. [DOI] [PubMed] [Google Scholar]
- 25.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- 26.McDonald JW, Johnston MV. Physiological and pathophysiological roles of excitatory amino acids during central nervous system development. Brain Res Brain Res Rev. 1990;15:41–70. doi: 10.1016/0165-0173(90)90011-c. [DOI] [PubMed] [Google Scholar]
- 27.Macaya A, Munell F, Ferrer I, de Torres C, Reventos J. Cell death and associated c-jun induction in perinatal hypoxia-ischemia. Effect of the neuroprotective drug dexamethasone. Brain Res Mol Brain Res. 1998;56:29–37. doi: 10.1016/s0169-328x(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, Ruas JL, Poellinger L, Lendahl U, Bondesson M. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]