Abstract
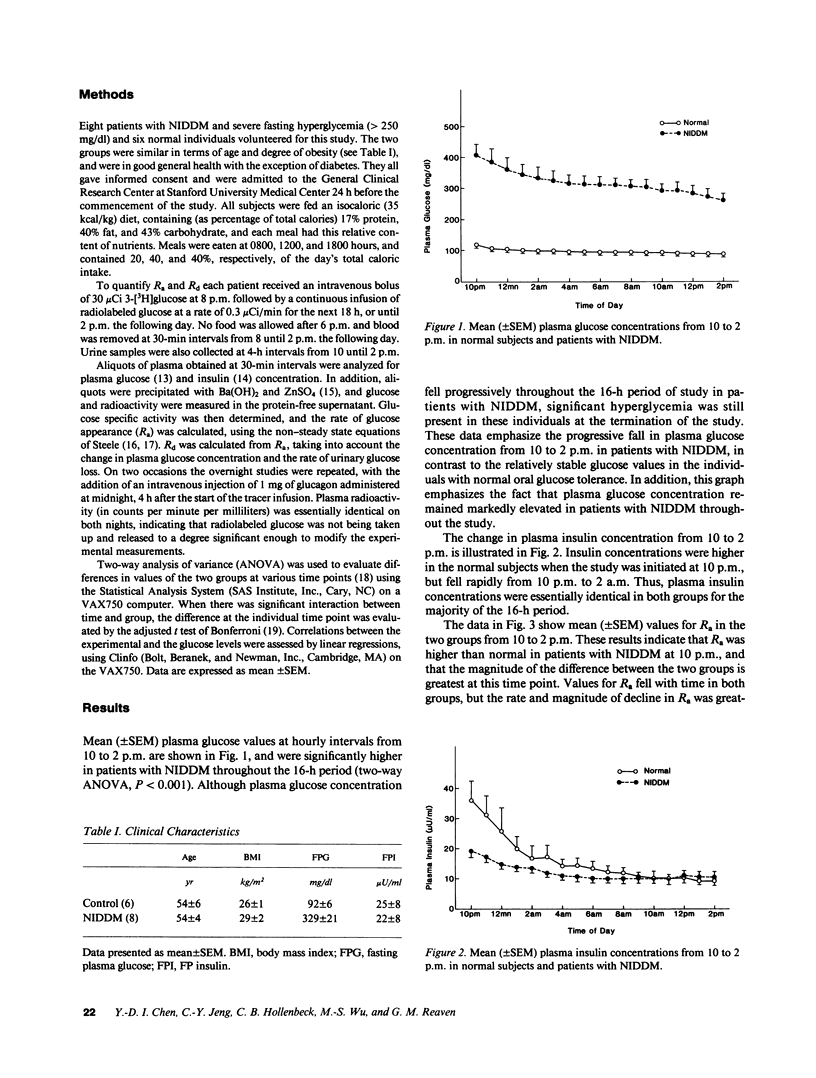
The changes in hepatic glucose production (Ra), tissue glucose disposal (Rd), and plasma glucose and insulin concentration that took place over a 16-h period from 10 to 2 p.m. were documented in 14 individuals; 8 with non-insulin-dependent diabetes mellitus (NIDDM) and 6 with normal glucose tolerance. Values for Ra were higher than normal in patients with NIDDM at 10 p.m. (4.73 +/- 0.41 vs. 3.51 +/- 0.36 mg/kg per min, P less than 0.001), but fell at a much faster rate throughout the night than that seen in normal subjects. As a consequence, the difference between Ra in normal individuals and patients with NIDDM progressively narrowed, and by 2 p.m., had ceased to exist (1.75 +/- 0.61 vs. 1.67 +/- 0.47 mg/kg per min, P = NS). Plasma glucose concentration also declined in patients with NIDDM over the same period of time, but they remained quite hyperglycemic, and the value of 245 +/- 27 mg/dl at 2 p.m. was about three times greater than in normal individuals. Plasma insulin concentrations also fell progressively from 10 to 2 p.m., and were similar in both groups throughout most of the 16-h study period. Thus, the progressive decline in Ra in patients with NIDDM occurred despite concomitant falls in both plasma glucose and insulin concentration. Glucose disposal rates also fell progressively in both groups, but the magnitude of the fall was greater in patients with NIDDM. Consequently, Rd in patients with NIDDM was higher at 10 p.m. (3.97 +/- 0.48 vs. 3.25 +/- 0.13 mg/kg per min, P less than 0.001) and lower the following day at 2 p.m. (1.64 +/- 0.21 vs. 1.97 +/- 0.35 mg/kg per min, P less than 0.01). These results indicate that a greatly expanded pool size can exist in patients with NIDDM at a time when values for Ra are identical to those in normal subjects studied under comparable conditions, which suggests that fasting hyperglycemia in NIDDM is not simply a function of an increase in Ra.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best J. D., Judzewitsch R. G., Pfeifer M. A., Beard J. C., Halter J. B., Porte D., Jr The effect of chronic sulfonylurea therapy on hepatic glucose production in non-insulin-dependent diabetes. Diabetes. 1982 Apr;31(4 Pt 1):333–338. doi: 10.2337/diab.31.4.333. [DOI] [PubMed] [Google Scholar]
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen H. F., Moorhouse J. A. Glucose turnover and disposal in maturity-onset diabetes. J Clin Invest. 1973 Dec;52(12):3033–3045. doi: 10.1172/JCI107502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Desbuquois B., Aurbach G. D. Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab. 1971 Nov;33(5):732–738. doi: 10.1210/jcem-33-5-732. [DOI] [PubMed] [Google Scholar]
- Doberne L., Greenfield M. S., Rosenthal M., Widstrom A., Reaven G. Effect of variations in basal plasma glucose concentration on glucose utilization (M) and metabolic clearance (MCR) rates during insulin clamp studies in patients with non-insulin-dependent diabetes mellitus. Diabetes. 1982 May;31(5 Pt 1):396–400. doi: 10.2337/diab.31.5.396. [DOI] [PubMed] [Google Scholar]
- Ferrannini E., Barrett E. J., Bevilacqua S., DeFronzo R. A. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983 Nov;72(5):1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth R. G., Bell P. M., Marsh H. M., Hansen I., Rizza R. A. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986 May;77(5):1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbath N., Hetenyi G., Jr Glucose dynamics in normal subjects and diabetic patients before and after a glucose load. Diabetes. 1966 Nov;15(11):778–789. doi: 10.2337/diab.15.11.778. [DOI] [PubMed] [Google Scholar]
- Fraze E., Donner C. C., Swislocki A. L., Chiou Y. A., Chen Y. D., Reaven G. M. Ambient plasma free fatty acid concentrations in noninsulin-dependent diabetes mellitus: evidence for insulin resistance. J Clin Endocrinol Metab. 1985 Nov;61(5):807–811. doi: 10.1210/jcem-61-5-807. [DOI] [PubMed] [Google Scholar]
- Godfrey K. Statistics in practice. Comparing the means of several groups. N Engl J Med. 1985 Dec 5;313(23):1450–1456. doi: 10.1056/NEJM198512053132305. [DOI] [PubMed] [Google Scholar]
- Golay A., Swislocki A. L., Chen Y. D., Reaven G. M. Relationships between plasma-free fatty acid concentration, endogenous glucose production, and fasting hyperglycemia in normal and non-insulin-dependent diabetic individuals. Metabolism. 1987 Jul;36(7):692–696. doi: 10.1016/0026-0495(87)90156-9. [DOI] [PubMed] [Google Scholar]
- Greenfield M., Kolterman O., Olefsky J. M., Reaven G. M. The effect of ten days of fasting on various aspects of carbohydrate metabolism in obese diabetic subjects with significant fasting hyperglycemia. Metabolism. 1978 Dec;27(12 Suppl 2):1839–1852. doi: 10.1016/s0026-0495(78)80003-1. [DOI] [PubMed] [Google Scholar]
- JACOBS G., REICHARD G., GOODMAN E. H., Jr, FRIEDMANN B., WEINHOUSE S. Action of insulin and tolbutamide on blood glucose entry and removal. Diabetes. 1958 Sep-Oct;7(5):358–364. doi: 10.2337/diab.7.5.358. [DOI] [PubMed] [Google Scholar]
- Reaven G. M., Chen Y. D., Donner C. C., Fraze E., Hollenbeck C. B. How insulin resistant are patients with noninsulin-dependent diabetes mellitus? J Clin Endocrinol Metab. 1985 Jul;61(1):32–36. doi: 10.1210/jcem-61-1-32. [DOI] [PubMed] [Google Scholar]
- Reaven G. M. Insulin resistance in noninsulin-dependent diabetes mellitus. Does it exist and can it be measured? Am J Med. 1983 Jan 17;74(1A):3–17. doi: 10.1016/0002-9343(83)90650-2. [DOI] [PubMed] [Google Scholar]
- Revers R. R., Fink R., Griffin J., Olefsky J. M., Kolterman O. G. Influence of hyperglycemia on insulin's in vivo effects in type II diabetes. J Clin Invest. 1984 Mar;73(3):664–672. doi: 10.1172/JCI111258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R., WALL J. S., DE BODO R. C., ALTSZULER N. Measurement of size and turnover rate of body glucose pool by the isotope dilution method. Am J Physiol. 1956 Sep;187(1):15–24. doi: 10.1152/ajplegacy.1956.187.1.15. [DOI] [PubMed] [Google Scholar]


