Abstract
Objective
Here we demonstrate that, in human erythrocytes, increases in cAMP that are not localized to a specific receptor-mediated signaling pathway for ATP release can activate effector proteins resulting in inhibition of ATP release. Specifically we sought to establish that exchange proteins activated by cAMP (EPACs) inhibit ATP release via activation of protein kinase C (PKC).
Methods
ATP release stimulated by iloprost (ILO), or isoproterenol (ISO), was determined in the absence and presence of selective phosphodiesterase inhibitors and/or the EPAC activator, 8CPT2OMecAMP (8CPT). To determine whether EPACs inhibit ATP release via activation of PKC, erythrocytes were incubated with phorbol 12-myristate 13-acetate (PMA) prior to either forskolin or ILO in the absence and presence of a PKC inhibitor, calphostin C (CALC).
Results
Selective inhibition of PDEs in one pathway inhibited ATP release in response to activation of the other cAMP-dependent pathway. 8CPT and PMA inhibited both ILO- and ISO-induced ATP release. Inhibition of ATP release with 8CPT was rescued by CALC.
Conclusion
These results support the hypothesis that cAMP not localized to a specific signaling pathway can activate EPACs which inhibit ATP release via activation of PKC and suggest a novel role for EPACs in erythrocytes.
INTRODUCTION
The basic physiological role of the erythrocyte is to transport oxygen (O2). The erythrocyte has also been shown to release ATP (47) which enables that cell to participate in the control of vascular caliber via activation of endothelial purinergic receptors and subsequent production and release of vasodilators (11, 13, 45). Signal transduction pathways for low O2- and receptor-mediated ATP release from erythrocytes that involve activation of the heterotrimeric G proteins Gi and Gs, respectively, have been described (34, 35). In these pathways, activation of either Gi or Gs stimulates adenylyl cyclase (AC) activity resulting in an increase in cAMP, a critical second messenger (34, 35). cAMP then activates protein kinase A (PKA) and, subsequently, the cystic fibrosis transmembrane conductance regulator (CFTR) resulting in controlled rlease of ATP from the erythrocyte (46). In human erythrocytes, both β adrenergic (βAR) and prostacyclin (IPR) receptors are present and are coupled to Gs. Receptor-mediated activation of either receptor results in increases in cAMP and ATP release (1, 43, 44).
Although PKA is the primary mediator of cAMP-dependent effects in cells, cAMP can also interact with other proteins including phosphodiesterases (PDEs) (3, 21) and cAMP-gated ion channels (40). Recently another family of proteins activated by cAMP, exchange proteins activated by cAMP (EPACs), was described (4, 29). The EPACs are guanine nucleotide exchange factors (GEFs) for the Ras family of small GTPases, Rap1 and Rap 2 (9). Two subtypes of EPAC have been described, EPAC1 and EPAC2, each of which is activated by cAMP and exhibits PKA-independent effects (5). Recent evidence suggests that activation of EPACs represents an important mechanism by which cAMP can regulate cellular function independent of PKA (4, 7). Such reports add complexity to the understanding of cAMP-dependent signaling events in cells.
Within erythrocytes, two serine/threonine kinases have been identified, PKA and protein kinase C (PKC), (12, 19, 36). PKA is activated by cAMP and is the main effector protein of that cyclic nucleotide. In contrast, PKC is not activated by cAMP (32). Recently, it was discovered that cAMP-mediated signaling that is independent of PKA is the result of activation of PKC, possible via the activity of EPACs (22). Although EPACs have not been identified in healthy human erythrocytes, their identification in sickle cells would suggest that they are also present in healthy cells (31).
cAMP is a second messenger in multiple signaling pathways that initiate diverse cellular responses. Therefore, if the response to activation of a specific receptor is to result in a discrete response, increases in cAMP must be localized to that specific signaling pathway. A major mechanism for the localization of cAMP signaling is via the activity of PDEs that hydrolyze the cyclic nucleotide preventing the activation of proteins in other signaling pathways (53).
Previously, we reported that PDEs 2, 3 and 4 are present in rabbit and human erythrocytes. More importantly, we established that PDE3 regulates increases in cAMP and ATP release resulting from activation of the IPR while PDEs 2 and 4 hydrolyze cAMP and regulate ATP release in response to activation of the βAR (1). Prior to these reports, segregation of PDEs to any signaling pathway in erythrocytes had not been shown. Here, we investigate the hypothesis that, in human erythrocytes, increases in cAMP not localized/confined to a specific receptor-activated signaling pathway (IPR or βAR) activate EPACs which inhibit release of ATP. We also explored the possibility that PKC contributes to inhibition of ATP release via EPAC activation.
METHODS
Isolation of Erythrocytes
Human blood was obtained by venipuncture using a syringe containing heparin (500 units). Immediately after collection of blood, erythrocytes were isolated by centrifugation at 500 ×g at 4°C for 10 min with the supernatant and buffy coat removed by aspiration. Packed erythrocytes were re- suspended and washed 3 times in a physiological salt solution containing, in mM; 4.7 KCl, 2.0 CaCl2, 1.2 MgSO4, 140.5 NaCl, 21.0 Tris-base and 5.5 dextrose with 0.5% bovine serum albumin, pH adjusted to 7.4. Erythrocytes were prepared on the day of use. The protocol for blood collection was approved by the Institutional Review Board of Saint Louis University.
Incubation of erythrocytes with pharmacological agents
Washed erythrocytes were diluted to a 20% hematocrit (1 ml) and were preincubated with a PDE inhibitor, an activator of EPAC or its vehicle for 30 minutes. Following pre-incubation, erythrocytes were stimulated with either isoproterenol (ISO, 1 μM, Sigma-Aldrich) or iloprost (ILO, 10 nM, Cayman). ATP was determined at 5, 10 and 15 min following addition of each receptor agonist. Values reported are the maximal response with these incubations. The inhibitors used were rolipram, a selective PDE4 inhibitor (Tocris), erythro-9-(2-hydroxy-3-nonyl)adenine, EHNA (Biomol), a selective PDE2 inhibitor, cilostazol, a selective PDE3 inhibitor (Sigma-Aldrich) and calphostin C (Biomol), a selective inhibitor of PKC. The selective activator of EPAC used was 8CPT2OMecAMP (8CPT, Alexis Biochemicals). The concentrations of inhibitors chosen were based on the IC50 values of each inhibitor in other cell types and previous studies using these inhibitors with erythrocytes (1, 26–28). Importantly, at the concentrations used, these inhibitors had no effect on either baseline or total ATP levels (Table 1). The vehicle for rolipram, cilostazol and calphostin C was N’, N-dimethylformamide (DMF, Sigma-Aldrich) and the vehicle for EHNA, 8CPT, ISO and ILO was saline.
Table 1.
Effect of 8CPT2OMecAMP (8CPT), phorbol-myristate-acetate (PMA) and calphostin C (CALC) on total intracellular ATP levels.
| ATP (mM per erythrocyte) | |||
|---|---|---|---|
| CONTROL | 8CPT | PMA | 8CPT+CALC |
| 3.59 ± 0.356 (n=5) | 3.91 ± 0.394 (n=7) | 3.12 ± 0.326 (n=8) | 3.90 ± 0.331 (n=8) |
Values are means ± SE. Values are not statistically different.
Measurement of ATP
ATP was measured using the luciferin-luciferase technique (48). A 200 μl sample of an erythrocyte suspension was injected into a cuvette containing 100 μl of 10 mg/ml crude firefly tail extract (Sigma) and 100 μl of 0.5 mg/ml D-Luciferin (Sigma). The light emitted from the reaction was detected using a luminometer (Turner Designs, TD 20/20). A standard curve was obtained for each experiment. Cell counts were obtained from the suspension of erythrocytes and amounts of ATP measured were normalized to 4 ×108 cells/mL. The peak ATP value was compared to an ATP standard curve generated on the day of use.
Measurement of Total Intracellular ATP
A 50 μl sample of erythrocytes (20% hematocrit) was lysed in distilled water at room temperature. ATP in the lysate diluted 8,000 fold, was measured using the ATP assay. The values were normalized to the ATP concentration per erythrocyte as determined by direct cell counting.
Measurement of Hemoglobin
Erythrocyte suspensions used to measure ATP were centrifuged at 500 × g for 10 minutes at 4° C. The amount of hemoglobin present in the supernatant was determined by measurement of absorbance at 405 nm (oxyhemoglobin). If any increase in free hemoglobin was detected, the data were excluded from analysis.
Data Analysis
Statistical significance was determined using an analysis of variance (ANOVA). In the event that the F-ratio indicated a change had occurred, a Fisher’s LSD test was performed to identify individual differences between groups. Results are reported as means ± the standard error of the mean (SEM).
RESULTS
Effect of a PDE3 inhibitor, cilostazol (CILO), on isoproterenol (ISO)-induced ATP release
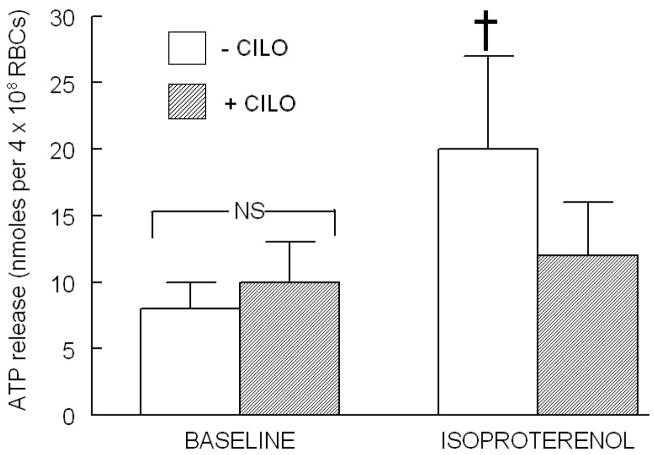
We have shown previously that inhibitors of both PDE2 and PDE4 potentiate both the increases in cAMP and ATP release in response to ISO (1), whereas inhibition of PDE3 with CILO (10 μM), had no effect on ISO-induced increases in cAMP. However, in the latter studies, the effect on ATP release was not investigated. Therefore, in order to determine the effect of inhibition of PDE3 on ISO-induced ATP release, erythrocytes were incubated with the selective PDE3 inhibitor, CILO (10 μM), prior to stimulation with ISO (1 μM). Here we report that incubation of human erythrocytes with CILO inhibits ISO-induced release of ATP (Figure 1). This concentration of CILO had no effect on basal ATP release or on total ATP content of erythrocytes as previously reported (1).
Figure 1.
Effect of cilostazol (CILO, 10 μM) on isoproterenol (ISO, 1 μM)-induced ATP release from human erythrocytes (n=4). Erythrocytes were incubated in the absence (open bars) and presence (shaded bars) of CILO for 30 min prior to stimulation with ISO. Values are the means ± SE. † = different from all other values (P<0.01); NS=not statistically different.
Effect of inhibitors of PDE2, erythro-9-(2-hydroxy-3nonoyl)adenine (EHNA), or PDE4, rolipram (ROL), on iloprost (ILO)-induced ATP release
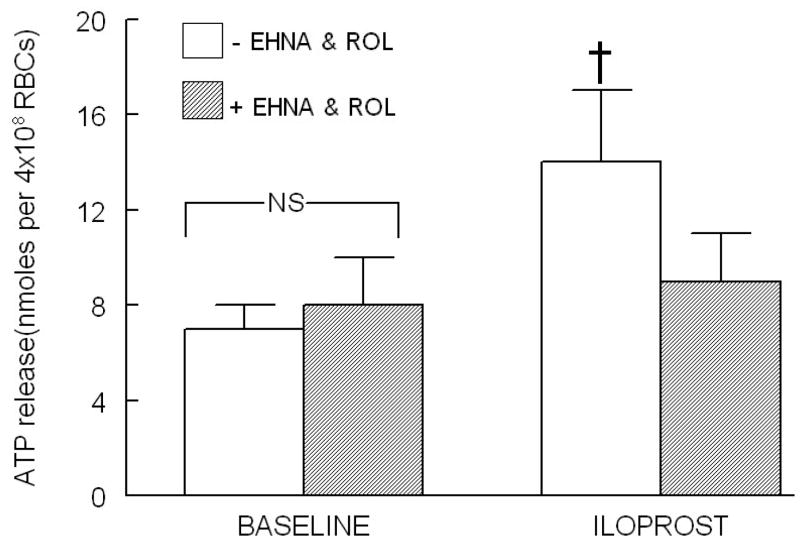
CILO has been shown to potentiate increases in cAMP and ATP release in response to ILO-induced activation of the IPR (1, 17). However, inhibitors of either PDE2 or PDE4 had no effect on increases in cAMP following ILO administration. In contrast, pretreatment of erythrocytes with selective inhibitors of PDE2 and PDE4 simultaneously, EHNA (30 μM) and ROL (20 μM), respectively, inhibited ILO (10 nM)-induced ATP release (Figure 2). EHNA and ROL, at the concentrations used, had no effect on basal ATP release (Figure 2) or on total ATP content of erythrocytes as previously reported (1).
Figure 2.
Effect of EHNA (30 μM) and rolipram (ROL, 20 μM) on iloprost (ILO, 10 nM)-induced ATP release from human erythrocytes (n=6). Erythrocytes were incubated in absence (open bars) and presence (shaded bars) of EHNA and ROL for 30 min prior to stimulation with ILO. Values are the means ± SE. † = different from all other values (P<0.01); NS=not statistically different.
Effect of a direct activator of EPAC, 8CPT2OMecAMP (8CPT), on ISO- and ILO-induced ATP release
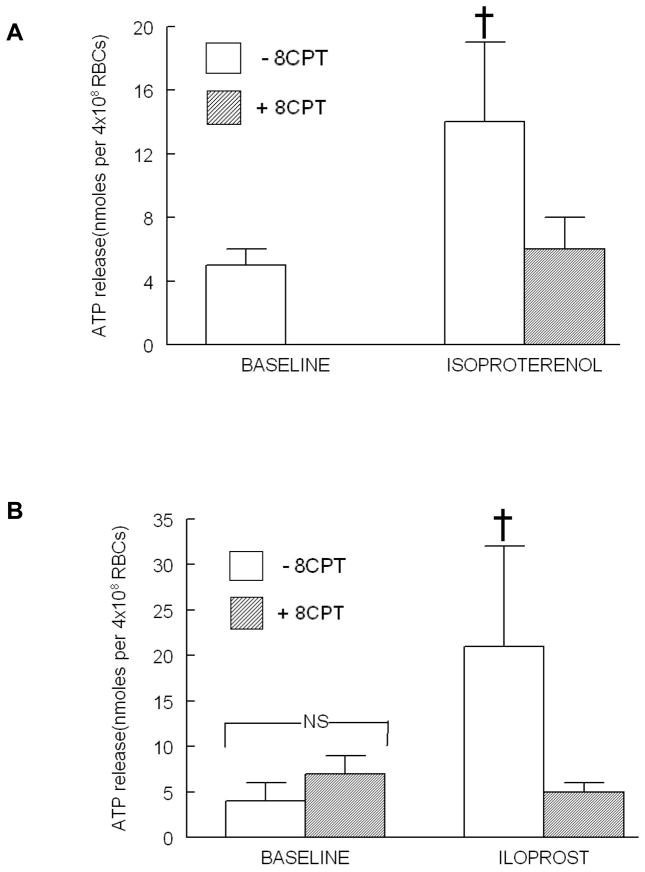
Increases in cAMP can activate several effector proteins in addition to PKA including EPACs (7). Therefore, to determine the contribution of the activation of EPACs to ATP release from human erythrocytes, cells were incubated with 8CPT (10 μM), a cell permeable cAMP analog, that directly activates EPACs prior to stimulation with either ISO (1 μM) or ILO (10 nM). At this concentration 8CPT does not activate PKA (14, 15, 18). As depicted in Figure 3, both the ISO- and ILO-induced ATP release were inhibited by 8CPT. Importantly, 8CPT had no effect on baseline ATP release (Figure 3B) or total ATP content of erythrocytes (Table 1).
Figure 3.
Effect of 8CPT2OMEcAMP (8CPT, 10μM) on isoproterenol (ISO, 1μM)-and iloprost (ILO, 10 nM)-induced ATP release from human erythrocytes. (A) Erythrocytes were incubated in the absence (open bars) and presence (shaded bars) of 8CPT for 30min prior to stimulation with ISO (n=6). Since 8CPT had no effect on baseline ATP levels as shown in (B) the baseline values were not determined in this set of experiments. (B) Erythrocytes were incubated in the absence (open bars) and presence (shaded bars) of 8CPT for 30 min prior to stimulation with ILO (n=6). Values are the means ± SE. † = different from all other values (P<0.01).
Effect of phorbol 12-myristate 13-acetate (PMA) on forskolin (FSK)-and ILO-induced ATP release
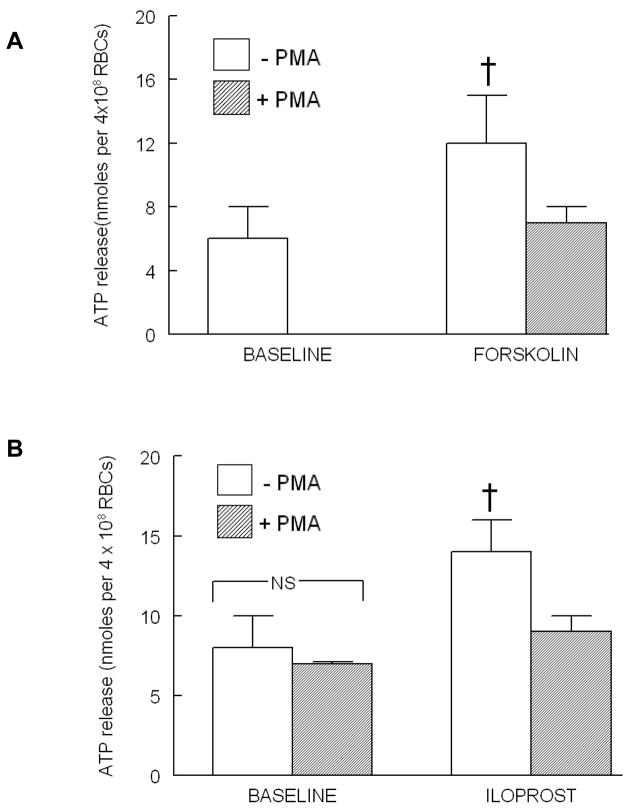
In other cell types, EPACs have been reported to activate PKC, a protein kinase present in erythrocytes (22, 33). Therefore, to determine the effect of activation of PKC on ATP release from erythrocytes, cells were incubated with a non-selective activator of PKC, PMA (1 μM), followed by FSK (10μM) which activates all isoforms of adenylyl cyclase and increases cAMP throughout the cell. PMA inhibited FSK-induced ATP release from erythrocytes (Figure 4). In a separate set of experiments, cells were incubated with PMA (1 μM) prior to incubation with ILO (10 nM). PMA inhibited ILO-induced ATP release from these cells (Figure 4B). The concentration of PMA used had no effect on baseline ATP release (Figure 4B) or total ATP content of erythrocytes (Table 1).
Figure 4.
Effect of PMA (100 nM) on forskolin (FSK, 10 μM)-and iloprost (ILO, 10 nM)-induced ATP release from human erythrocytes. (A) Erythrocytes were incubated in the absence (open bars) and presence (shaded bars) of PMA for 30 min prior to stimulation with FSK (n=11). Since PMA had no effect on baseline ATP levels as shown in (B) the baseline values were not determined in this set of experiments. (B) Erythrocytes were incubated in the absence (open bars) and presence (shaded bars) of PMA for 30 min prior to stimulation with ILO (n=5). Values are the means ± SE. † = different from all other values (P<0.05); NS=not statistically different.
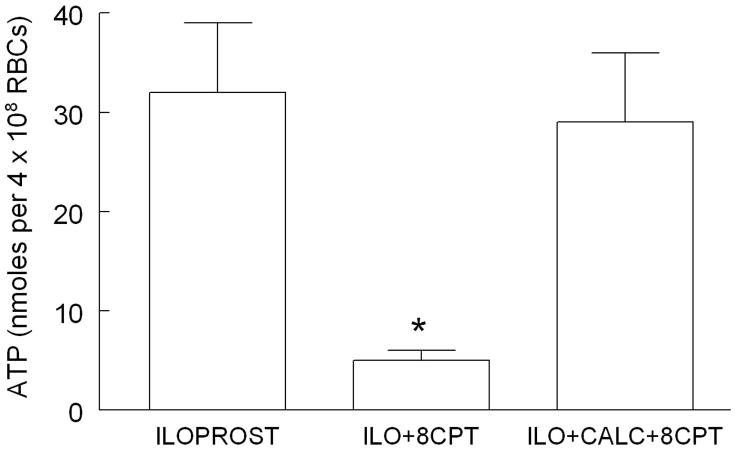
Effect of calphostin C (CALC) and 8CPT on ILO-induced ATP release
To determine if the mechanism of EPAC-induced inhibition of ATP release is via activation of PKC, erythrocytes were stimulated with ILO (10nM) after pretreatment with either 8CPT (10μM) or 8CPT in the presence of CALC (1μM), an inhibitor of PKC. Treatment of erythrocytes with CALC prevented the inhibition of ATP mediated by 8CPT (Figure 5). Baseline ATP levels were not different among the treatment groups and were 13 ± 3, 7 ± 2 and 19 ± 4 nmoles per 4 × 108 erythrocytes for control, 8CPT and 8CPT+CALC groups, respectively. As shown in Table 1, 8CPT and CALC had no effect on total ATP levels.
Figure 5.
Effect of 8CPT2OMEcAMP (8CPT, 10μM) and calphostin C (CALC, 1μM) on iloprost (ILO, 10 nM)-induced ATP release from human erythrocytes. Erythrocytes were incubated in the absence (n=16) or presence of 8CPT (n=8) or 8CPT and CALC (n-8) for 30 min prior to stimulation with ILO. Values are the means ± SE. * = different from all other values (P<0.01).
DISCUSSION
Within cells, activation of signaling pathways that share common proteins and second messengers, yet elicit specific responses, requires that there be mechanisms which localize the signal and thereby isolate these pathways (2, 6, 10, 20, 39). cAMP is a second messenger in diverse signaling pathways regulating many physiological processes. Therefore, if activation of a selective receptor is to produce a specific response, increases in cAMP must be tightly regulated and localized to the activated pathway (25, 41, 52).
The magnitude and duration of increases in cAMP generated when a signaling pathway is activated are regulated by PDEs localized to that pathway (49, 51, 53). Previously, we reported that PDEs 2 and 4 hydrolyze cAMP produced in response to activation of the βAR while PDE3 hydrolyzes cAMP produced following activation of the IPR (1). In the present study, we demonstrate that the administration of selective inhibitors of PDEs associated with one signaling pathways inhibits ATP release in response to activation of the other pathway (Figures 1 and 2). One interpretation of this result is that inhibition of PDE activity not compartmentalized with the activated pathway resulted in non-localized increases in cAMP (Figure 6). However, these studies do not reveal a mechanism by which non-localized increases in cAMP inhibited receptor-mediated ATP release.
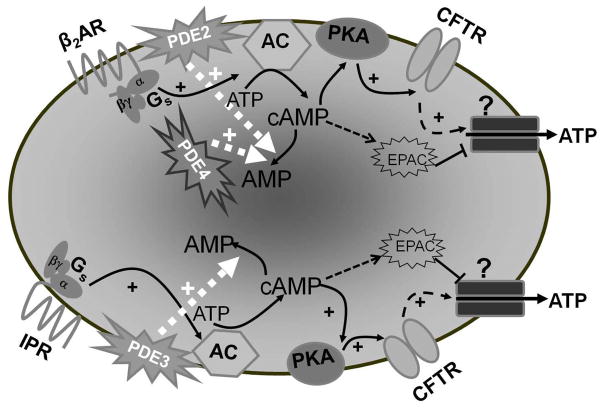
Figure 6.
Activation of the β2AR with isoproterenol increases cAMP with subsequent PKA activation. PKA then phosphorylates and activates CFTR, which controls the activity of an ATP conduit in the erythrocyte membrane. Increases in cAMP associated with activation of the β2AR are regulated by the activities of both PDE2 and PDE4. Similarly, activation of the IPR by iloprost results in increases in cAMP, which activate ATP release from the erythrocyte. However, in the case of the IPR signaling pathway, increases in cAMP are regulated by the activity of PDE3. Increases in cAMP not associated with activation of either the β2AR or IPR activate EPACs which inhibit ATP release from the erythrocyte. Abbreviations: β2AR – β2 adrenergic receptor; Gs – the heterotrimeric G protein, Gs; α, β and γ – G protein subunits; PDE – phosphodiesterase; ATP – adenosine triphosphate; cAMP cyclic adenosine monophosphate; AMP – adenosine monophosphate; AC – adenylyl cyclase; PKA – protein kinase A; CFTR – cystic fibrosis transmembrane conductance regulator; ? – unknown ATP conduit; + – activation; IPR – prostacyclin receptor; EPACs -exchange proteins activated by cAMP
Although most signaling associated with increases in cAMP involve activation of PKA, recently another family of cAMP effector proteins has been identified, the exchange proteins activated by cAMP (EPACs), (7). Both PKA and EPACs are present in erythrocytes (12, 19, 31). It has been established that activation of PKA is required for ATP release from these cells (46), but no role for EPACs has previously been identified in these cells. We hypothesized that EPACs could inhibit ATP release since they can oppose the effects of PKA in other cells (4, 7, 29). Since there are currently, no inhibitors of EPACs and because erythrocytes are anucleated cells with no protein synthetic capability, we used pharmacological methods to determine the role of EPAC in these cells. Erythrocytes were incubated with the EPAC activator, 8CPT, prior to stimulation with either ILO (IPR) or ISO (βAR). This EPAC activator inhibited ATP release in response to both agonists (Figure 3A and B). One interpretation of these results is that increases in cAMP not associated with activation of the targeted receptor resulted in stimulation of EPAC activity in erythrocytes (Figure 6).
EPACs are found in the cytosol or associated with the plasma membrane (29, 38) and can be activated by G-protein coupled receptors including the βAR (23, 30). There are several proposed mechanisms by which EPACs can participate in cellular signaling pathways. In non-erythroid cells, the actions of EPACs can be mediated by either activation of the small G protein, Rap (50) or phosphoinositide 3 kinase and, subsequently, PKB (8). In addition, EPACs can increase the activity of phospholipase Cε leading to increased intracellular Ca2+ and generation of diacylglyerol (DAG), both of which can activate isoforms of protein kinase C (PKC) (24, 37, 42), a protein present in erythrocytes (16). To begin to elucidate the mechanism by which EPACs exert their inhibitory effects on receptor-mediated ATP release from erythrocytes, we evaluated the role of PKC. Administration of PMA, a direct activator of PKC, resulted in inhibition of ATP release from human erythrocytes in response to either direct activation of all adenylyl cyclase activity with forskolin (Figure 4A) or in response to selective IPR activation with iloprost (Figure 4B). Importantly, inhibition of PKC with CALC prevented the effects of EPAC activation with 8CPT (Figure 5). The latter studies are consistent with the hypothesis that EPACs inhibit receptor-mediated ATP release from erythrocytes via activation of PKC.
In conclusion, we have identified a novel role for EPACs in the regulation of receptor-mediated signaling pathways in human erythrocytes. Previously, we demonstrated the importance of increases in cAMP for ATP release from these cells (1, 46). Here we present evidence in support of the hypothesis that not only are increases in cAMP necessary for ATP release, but that this release also requires tight control/localization of that cAMP to the signaling pathway that is activated. Consequently, when increases in cAMP are not localized, EPACs are activated and, via a different signaling pathway, inhibit ATP release. These results suggest that EPACs can contribute to a negative regulatory mechanism to control the release of ATP from human erythrocytes (Figure 6).
Acknowledgments
The authors thank J.L. Sprague for inspiration. This work is supported by NIH grants HL-64180, HL-89094 and ADA grant RA-133.
References
- 1.Adderley SP, Dufaux EA, Sridharan M, Bowles EA, Hanson MS, Stephenson AH, Ellsworth ML, Sprague RS. Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans. Am J Physiol Heart Circ Physiol. 2009;296:H1617–1624. doi: 10.1152/ajpheart.01226.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baillie GS. Compartmentalized signalling: spatial regulation of cAMP by the action of compartmentalized phosphodiesterases. FEBS J. 2009;276:1790–1799. doi: 10.1111/j.1742-4658.2009.06926.x. [DOI] [PubMed] [Google Scholar]
- 3.Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 4.Borland G, Smith BO, Yarwood SJ. EPAC proteins transduce diverse cellular actions of cAMP. Br J Pharmacol. 2009;158:70–86. doi: 10.1111/j.1476-5381.2008.00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bos JL. Epac: a new cAMP target and new avenues in cAMP research. Nat Rev Mol Cell Biol. 2003;4:733–738. doi: 10.1038/nrm1197. [DOI] [PubMed] [Google Scholar]
- 6.Bruss MD, Richter W, Horner K, Jin SL, Conti M. Critical role of PDE4D in beta2-adrenoceptor-dependent cAMP signaling in mouse embryonic fibroblasts. J Biol Chem. 2008;283:22430–22442. doi: 10.1074/jbc.M803306200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng X, Ji Z, Tsalkova T, Mei F. Epac and PKA: a tale of two intracellular cAMP receptors. Acta biochimica et biophysica Sinica. 2008;40:651–662. doi: 10.1111/j.1745-7270.2008.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cullen KA, McCool J, Anwer MS, Webster CR. Activation of cAMP-guanine exchange factor confers PKA-independent protection from hepatocyte apoptosis. Am J Physiol Gastrointest Liver Physiol. 2004;287:G334–343. doi: 10.1152/ajpgi.00517.2003. [DOI] [PubMed] [Google Scholar]
- 9.de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 10.Di Benedetto G, Zoccarato A, Lissandron V, Terrin A, Li X, Houslay MD, Baillie GS, Zaccolo M. Protein kinase A type I and type II define distinct intracellular signaling compartments. Circ Res. 2008;103:836–844. doi: 10.1161/CIRCRESAHA.108.174813. [DOI] [PubMed] [Google Scholar]
- 11.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 12.Dreyfuss G, Schwartz KJ, Blout ER. Compartmentalization of cyclic AMP-dependent protein kinases in human erythrocytes. Proc Natl Acad Sci U S A. 1978;75:5926–5930. doi: 10.1073/pnas.75.12.5926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ellsworth ML, Ellis CG, Goldman D, Stephenson AH, Dietrich HH, Sprague RS. Erythrocytes: oxygen sensors and modulators of vascular tone. Physiology (Bethesda) 2009;24:107–116. doi: 10.1152/physiol.00038.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enserink JM, Christensen AE, de Rooij J, van Triest M, Schwede F, Genieser HG, Doskeland SO, Blank JL, Bos JL. A novel Epac-specific cAMP analogue demonstrates independent regulation of Rap1 and ERK. Nat Cell Biol. 2002;4:901–906. doi: 10.1038/ncb874. [DOI] [PubMed] [Google Scholar]
- 15.Gloerich M, Bos JL. Epac: defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- 16.Govekar RB, Zingde SM. Protein kinase C isoforms in human erythrocytes. Ann Hematol. 2001;80:531–534. doi: 10.1007/s002770100352. [DOI] [PubMed] [Google Scholar]
- 17.Hanson MS, Stephenson AH, Bowles EA, Sridharan M, Adderley S, Sprague RS. Phosphodiesterase 3 is present in rabbit and human erythrocytes and its inhibition potentiates iloprost-induced increases in cAMP. Am J Physiol Heart Circ Physiol. 2008;295:H786–793. doi: 10.1152/ajpheart.00349.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: new tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal. 2008;20:10–20. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosey MM, Tao M. Phosphorylation of rabbit and human erythrocyte membranes by soluble adenosine 3′:5′-monophosphate-dependent and -independent protein kinases. J Biol Chem. 1977;252:102–109. [PubMed] [Google Scholar]
- 20.Houslay MD. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem Sci. 2010;35:91–100. doi: 10.1016/j.tibs.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Houslay MD, Milligan G. Tailoring cAMP-signalling responses through isoform multiplicity. Trends in biochemical sciences. 1997;22:217–224. doi: 10.1016/s0968-0004(97)01050-5. [DOI] [PubMed] [Google Scholar]
- 22.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. J Neurosci. 2005;25:6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kassel KM, Wyatt TA, Panettieri RA, Jr, Toews ML. Inhibition of human airway smooth muscle cell proliferation by beta 2-adrenergic receptors and cAMP is PKA independent: evidence for EPAC involvement. Am J Physiol Lung Cell Mol Physiol. 2008;294:L131–138. doi: 10.1152/ajplung.00381.2007. [DOI] [PubMed] [Google Scholar]
- 24.Keiper M, Stope MB, Szatkowski D, Bohm A, Tysack K, Vom Dorp F, Saur O, Oude Weernink PA, Evellin S, Jakobs KH, Schmidt M. Epac- and Ca2+-controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J Biol Chem. 2004;279:46497–46508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- 25.Leroy J, Abi-Gerges A, Nikolaev VO, Richter W, Lechene P, Mazet JL, Conti M, Fischmeister R, Vandecasteele G. Spatiotemporal dynamics of beta-adrenergic cAMP signals and L-type Ca2+ channel regulation in adult rat ventricular myocytes: role of phosphodiesterases. Circ Res. 2008;102:1091–1100. doi: 10.1161/CIRCRESAHA.107.167817. [DOI] [PubMed] [Google Scholar]
- 26.Lugnier C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: a new target for the development of specific therapeutic agents. Pharmacol Ther. 2006;109:366–398. doi: 10.1016/j.pharmthera.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Maurice DH, Palmer D, Tilley DG, Dunkerley HA, Netherton SJ, Raymond DR, Elbatarny HS, Jimmo SL. Cyclic nucleotide phosphodiesterase activity, expression, and targeting in cells of the cardiovascular system. Mol Pharmacol. 2003;64:533–546. doi: 10.1124/mol.64.3.533. [DOI] [PubMed] [Google Scholar]
- 28.Mehats C, Andersen CB, Filopanti M, Jin SL, Conti M. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends in endocrinology and metabolism: TEM. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- 29.Metrich M, Berthouze M, Morel E, Crozatier B, Gomez AM, Lezoualc’h F. Role of the cAMP-binding protein Epac in cardiovascular physiology and pathophysiology. Pflugers Arch. 459:535–546. doi: 10.1007/s00424-009-0747-y. [DOI] [PubMed] [Google Scholar]
- 30.Metrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc’h F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102:959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 31.Murphy MM, Zayed MA, Evans A, Parker CE, Ataga KI, Telen MJ, Parise LV. Role of Rap1 in promoting sickle red blood cell adhesion to laminin via BCAM/LU. Blood. 2005;105:3322–3329. doi: 10.1182/blood-2004-07-2881. [DOI] [PubMed] [Google Scholar]
- 32.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 33.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Heterotrimeric G protein Gi is involved in a signal transduction pathway for ATP release from erythrocytes. Am J Physiol Heart Circ Physiol. 2004;286:H940–945. doi: 10.1152/ajpheart.00677.2003. [DOI] [PubMed] [Google Scholar]
- 35.Olearczyk JJ, Stephenson AH, Lonigro AJ, Sprague RS. Receptor-mediated activation of the heterotrimeric G-protein Gs results in ATP release from erythrocytes. Med Sci Monit. 2001;7:669–674. [PubMed] [Google Scholar]
- 36.Palfrey HC, Waseem A. Protein kinase C in the human erythrocyte. Translocation to the plasma membrane and phosphorylation of bands 4.1 and 4.9 and other membrane proteins. J Biol Chem. 1985;260:16021–16029. [PubMed] [Google Scholar]
- 37.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. Embo J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponsioen B, Gloerich M, Ritsma L, Rehmann H, Bos JL, Jalink K. Direct spatial control of Epac1 by cyclic AMP. Mol Cell Biol. 2009;29:2521–2531. doi: 10.1128/MCB.01630-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rampersad SN, Ovens JD, Huston E, Umana MB, Wilson LS, Netherton SJ, Lynch MJ, Baillie GS, Houslay MD, Maurice DH. Cyclic AMP phosphodiesterase 4D (PDE4D) Tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J Biol Chem. 2010;285:33614–33622. doi: 10.1074/jbc.M110.140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rich TC, Fagan KA, Nakata H, Schaack J, Cooper DM, Karpen JW. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J Gen Physiol. 2000;116:147–161. doi: 10.1085/jgp.116.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richter W, Day P, Agrawal R, Bruss MD, Granier S, Wang YL, Rasmussen SG, Horner K, Wang P, Lei T, Patterson AJ, Kobilka B, Conti M. Signaling from beta1- and beta2-adrenergic receptors is defined by differential interactions with PDE4. EMBO J. 2008;27:384–393. doi: 10.1038/sj.emboj.7601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nature cell biology. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- 43.Sprague R, Bowles E, Stumpf M, Ricketts G, Freidman A, Hou WH, Stephenson A, Lonigro A. Rabbit erythrocytes possess adenylyl cyclase type II that is activated by the heterotrimeric G proteins Gs and Gi. Pharmacol Rep. 2005;57(Suppl):222–228. [PubMed] [Google Scholar]
- 44.Sprague RS, Bowles EA, Hanson MS, DuFaux EA, Sridharan M, Adderley S, Ellsworth ML, Stephenson AH. Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes. Microcirculation. 2008;15:461–471. doi: 10.1080/10739680701833804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol. 1996;271:H2717–2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 46.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 47.Sprague RS, Hanson MS, Achilleus D, Bowles EA, Stephenson AH, Sridharan M, Adderley S, Procknow J, Ellsworth ML. Rabbit erythrocytes release ATP and dilate skeletal muscle arterioles in the presence of reduced oxygen tension. Pharmacol Rep. 2009;61:183–190. doi: 10.1016/s1734-1140(09)70020-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strehler BL. Bioluminescence assay: principles and practice. Methods of biochemical analysis. 1968;16:99–181. doi: 10.1002/9780470110348.ch2. [DOI] [PubMed] [Google Scholar]
- 49.Terrin A, Di Benedetto G, Pertegato V, Cheung YF, Baillie G, Lynch MJ, Elvassore N, Prinz A, Herberg FW, Houslay MD, Zaccolo M. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: role of compartmentalized phosphodiesterases. J Cell Biol. 2006;175:441–451. doi: 10.1083/jcb.200605050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, Stork PJ. Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation. Mol Cell Biol. 2006;26:2130–2145. doi: 10.1128/MCB.26.6.2130-2145.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Willoughby D, Baillie GS, Lynch MJ, Ciruela A, Houslay MD, Cooper DM. Dynamic regulation, desensitization, and cross-talk in discrete subcellular microdomains during beta2-adrenoceptor and prostanoid receptor cAMP signaling. J Biol Chem. 2007;282:34235–34249. doi: 10.1074/jbc.M706765200. [DOI] [PubMed] [Google Scholar]
- 52.Zaccolo M. cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br J Pharmacol. 2009;158:50–60. doi: 10.1111/j.1476-5381.2009.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zaccolo M. Phosphodiesterases and compartmentalized cAMP signalling in the heart. Eur J Cell Biol. 2006;85:693–697. doi: 10.1016/j.ejcb.2006.01.002. [DOI] [PubMed] [Google Scholar]