Abstract
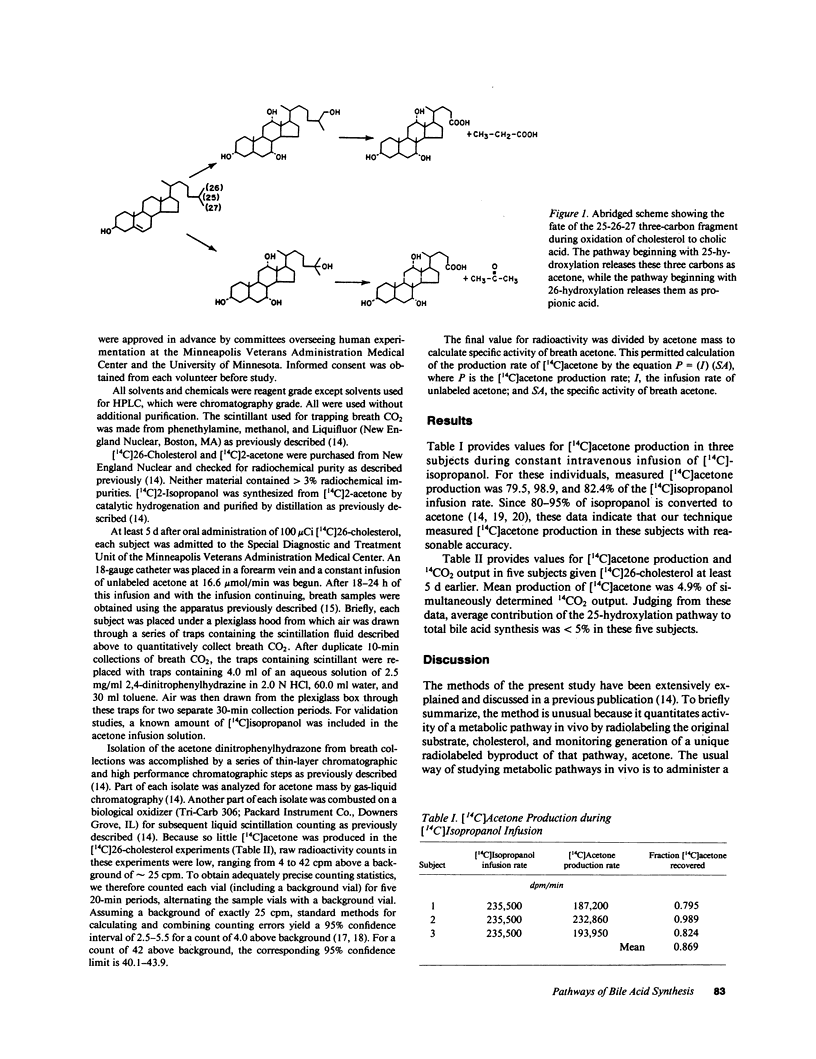
During biosynthesis of bile acid, carbons 25-26-27 are removed from the cholesterol side-chain. Side-chain oxidation begins either with hydroxylation at the 26-position, in which case the three-carbon fragment is released as propionic acid, or with hydroxylation at the 25-position, in which case the three-carbon fragment is released as acetone. We have previously shown in the rat that the contribution of the 25-hydroxylation pathway can be quantitated in vivo by measuring production of [14C]acetone from [14C]26-cholesterol. In the present study, we adapted this method to human subjects. 4 d after oral administration of 100 microCi of [14C]26-cholesterol and 1 d after beginning a constant infusion of 16.6 mumol/min unlabeled acetone, three men and two women underwent breath collections. Expired acetone was trapped and purified as the 2,4 dinitrophenylhydrazine derivative. 14CO2 was trapped quantitatively using phenethylamine. Specific activity of breath acetone was multiplied by the acetone infusion rate to calculate production of [14C]acetone. [14C]Acetone production averaged 4.9% of total release of 14C from [14C]26-cholesterol, estimated by 14CO2 output. The method was validated by showing that [14C]acetone production from [14C]isopropanol averaged 86.9% of the [14C]-isopropanol infusion rate. We conclude that in man, as in the rat, the 25-hydroxylation pathway accounts for less than 5% of bile acid synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björkhem I., Gustafsson J., Johansson G., Persson B. Biosynthesis of bile acids in man. Hydroxylation of the C27-steroid side chain. J Clin Invest. 1975 Mar;55(3):478–486. doi: 10.1172/JCI107954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronholm T., Johansson G. Oxidation of 5 beta-cholestane-3alpha, 7alpha, 12alpha-triol by rat liver microsomes. Eur J Biochem. 1970 Oct;16(2):373–381. doi: 10.1111/j.1432-1033.1970.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Duane W. C., Björkhem I., Hamilton J. N., Mueller S. M. Quantitative importance of the 25-hydroxylation pathway for bile acid biosynthesis in the rat. Hepatology. 1988 May-Jun;8(3):613–618. doi: 10.1002/hep.1840080329. [DOI] [PubMed] [Google Scholar]
- Duane W. C., Levitt D. G., Mueller S. M., Behrens J. C. Regulation of bile acid synthesis in man. Presence of a diurnal rhythm. J Clin Invest. 1983 Dec;72(6):1930–1936. doi: 10.1172/JCI111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duane W. C. Simulation of the defect of bile acid metabolism associated with cholesterol cholelithiasis by sorbitol ingestion in man. J Lab Clin Med. 1978 Jun;91(6):969–978. [PubMed] [Google Scholar]
- Elliott W. H., Hyde P. M. Metabolic pathways of bile acid synthesis. Am J Med. 1971 Nov;51(5):568–579. doi: 10.1016/0002-9343(71)90281-6. [DOI] [PubMed] [Google Scholar]
- Hanson R. F., Isenberg J. N., Williams G. C., Hachey D., Szczepanik P., Klein P. D., Sharp H. L. The metabolism of 3alpha, 7alpha, 12alpha-trihydorxy-5beta-cholestan-26-oic acid in two siblings with cholestasis due to intrahepatic bile duct anomalies. An apparent inborn error of cholic acid synthesis. J Clin Invest. 1975 Sep;56(3):577–587. doi: 10.1172/JCI108127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. F., Staples A. B., Williams G. C. Metabolism of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 26-tetrol and 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha, 25-tetrol into cholic acid in normal human subjects. J Lipid Res. 1979 May;20(4):489–493. [PubMed] [Google Scholar]
- Kase B. F., Pedersen J. I., Strandvik B., Björkhem I. In vivo and vitro studies on formation of bile acids in patients with Zellweger syndrome. Evidence that peroxisomes are of importance in the normal biosynthesis of both cholic and chenodeoxycholic acid. J Clin Invest. 1985 Dec;76(6):2393–2402. doi: 10.1172/JCI112252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki S., Shimazu K., Kuwabara M., Une M., Kihira K., Kuramoto T., Hoshita T. Identification of bile alcohols in human bile. J Lipid Res. 1985 Feb;26(2):230–240. [PubMed] [Google Scholar]
- Lacouture P. G., Wason S., Abrams A., Lovejoy F. H., Jr Acute isopropyl alcohol intoxication. Diagnosis and management. Am J Med. 1983 Oct;75(4):680–686. doi: 10.1016/0002-9343(83)90456-4. [DOI] [PubMed] [Google Scholar]
- Oftebro H., Björkhem I., Skrede S., Schreiner A., Pederson J. I. Cerebrotendinous xanthomatosis: a defect in mitochondrial 26-hydroxylation required for normal biosynthesis of cholic acid. J Clin Invest. 1980 Jun;65(6):1418–1430. doi: 10.1172/JCI109806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard G. A., Jr, Haff A. C., Skutches C. L., Paul P., Holroyde C. P., Owen O. E. Plasma acetone metabolism in the fasting human. J Clin Invest. 1979 Apr;63(4):619–626. doi: 10.1172/JCI109344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salen G., Shefer S., Cheng F. W., Dayal B., Batta A. K., Tint G. S. Cholic acid biosynthesis: the enzymatic defect in cerebrotendinous xanthomatosis. J Clin Invest. 1979 Jan;63(1):38–44. doi: 10.1172/JCI109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefer S., Dayal B., Tint G. S., Salen G., Mosbach E. H. Identification of pentahydroxy bile alcohols in cerebrotendinous xanthomatosis: characterization of 5beta-cholestane-3alpha, 7alpha, 12alpha, 24xi, 25-pentol and 5beta-cholestane-3alpha, 7alpha, 12alpha, 23xi, 25-pentol. J Lipid Res. 1975 Jul;16(4):280–286. [PubMed] [Google Scholar]


